Effects of Supplemental UV-A LEDs on the Nutritional Quality of Lettuce: Accumulation of Protein and Other Essential Nutrients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growing Conditions
2.2. Growth Characteristics
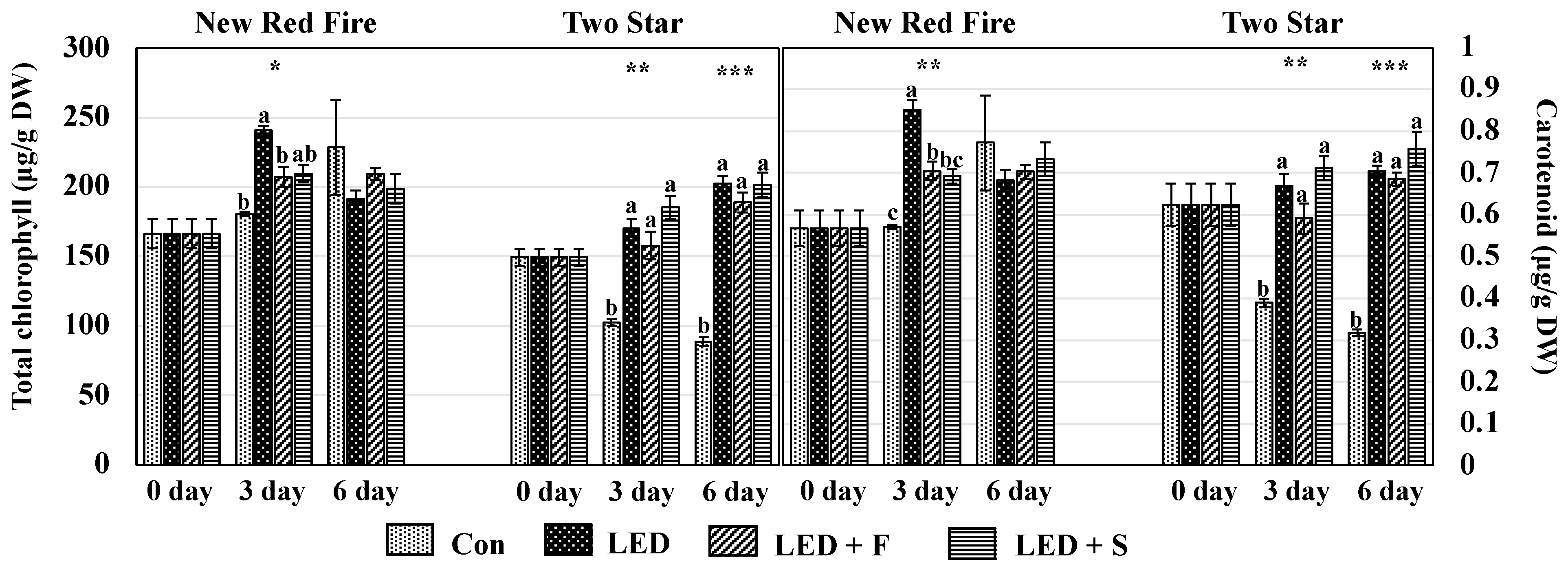
2.3. Chlorophyll and Carotenoids
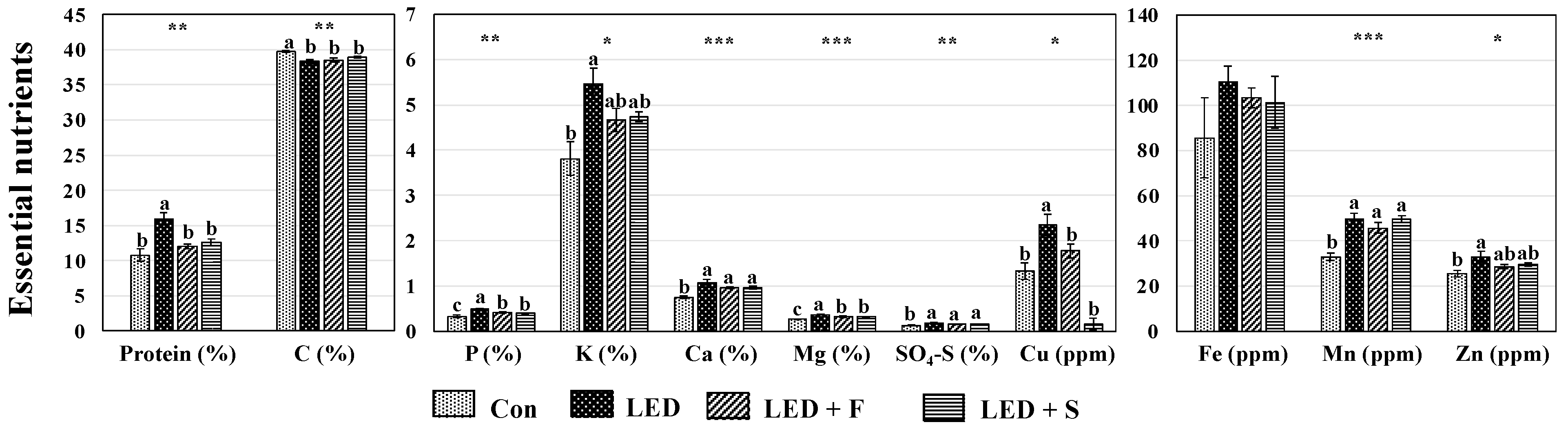
2.4. Essential Nutrients
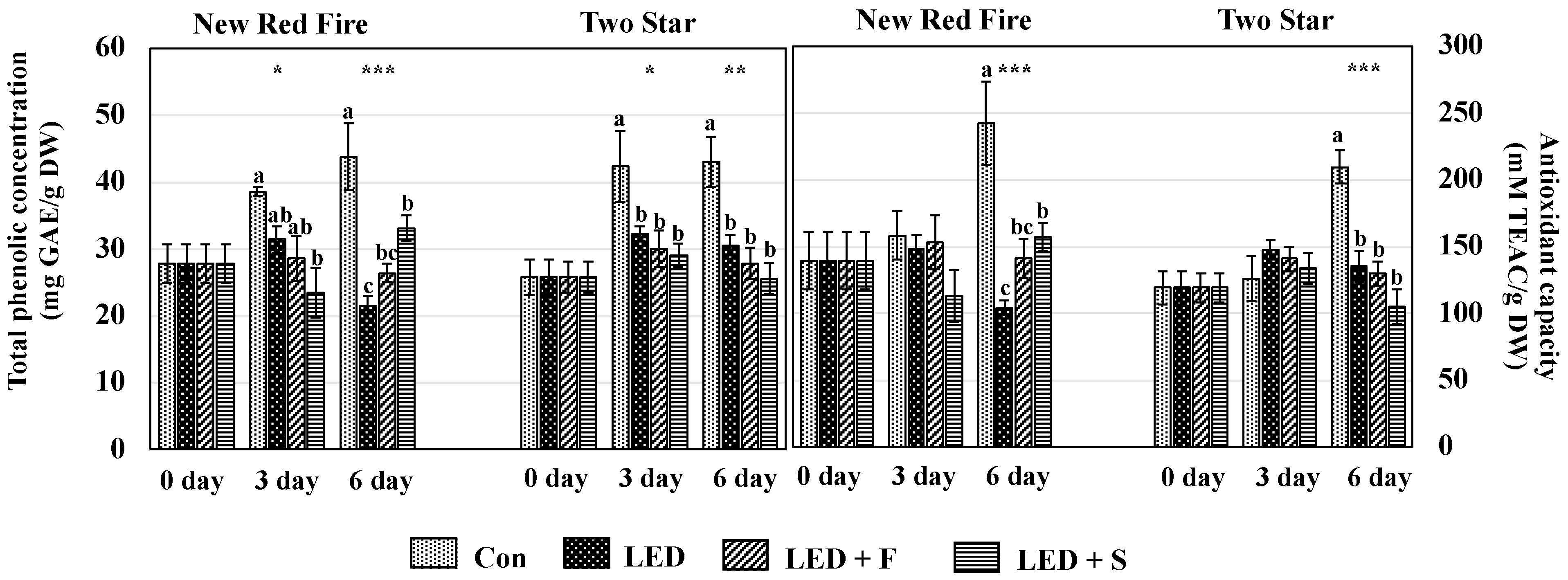
2.5. Total Phenolic Compounds and Antioxidant Capacity
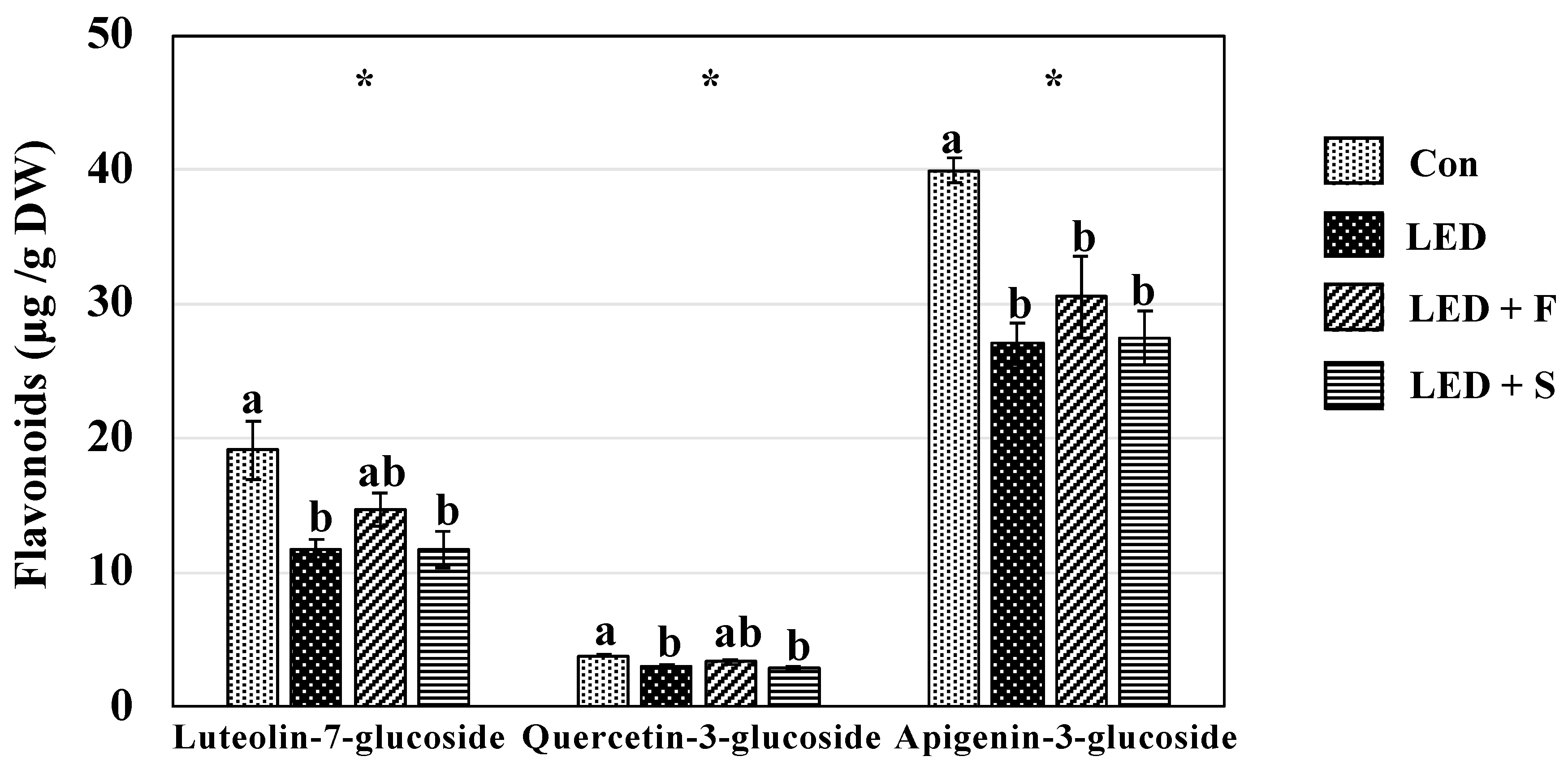
2.6. Individual Phenolic Compounds
2.7. Statistical Analyses
3. Results and Discussion
3.1. Growth Characteristics
3.2. Chlorophyll and Carotenoids
3.3. Protein and Other Essential Nutrients
3.4. Total Phenolic Concentration and Antioxidant Capacity
3.5. Individual Phenolic Compounds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Wu, G.; Bazer, F.W.; Cross, H.R. Land-based production of animal protein: Impacts, efficiency, and sustainability. Ann. N. Y. Academy Sci. 2014, 1328, 18–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.; Xu, J.; Wang, W.; Rajashekar, C.B. The effect of supplemental blue, red and far-red light on the growth and the nutritional quality of red and green leaf lettuce. Am. J. Plant Sci. 2019, 10, 2219–2235. [Google Scholar] [CrossRef] [Green Version]
- Krizek, D.T.; Mirecki, R.M.; Britz, S.J. Inhibitory effects of ambient levels of solar UV-A and UV-B radiation on growth of cucumber. Physiol. Plant. 1997, 100, 886–893. [Google Scholar] [CrossRef]
- Luthria, D.L.; Mukhopadhyay, S.; Krizek, D.T. Content of total phenolics and phenolic acids in tomato (Lycopersicon esculentum Mill.) fruits as influenced by cultivar and solar UV radiation. J. Food Compos. Anal. 2006, 19, 771–777. [Google Scholar] [CrossRef]
- Li, Q.; Kubota, C. Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ. Exp. Bot. 2009, 67, 59–64. [Google Scholar] [CrossRef]
- Steinmetz, K.A.; Potter, J.D. Vegetables, fruit, and cancer prevention: A review. J. Am. Diet. Assoc. 1996, 96, 1027–1039. [Google Scholar] [CrossRef]
- Prior, R.L.; Cao, G. Antioxidant phytochemicals in fruits and vegetables: Diet and health implications. HortScience 2000, 35, 588–592. [Google Scholar] [CrossRef] [Green Version]
- Rajashekar, C.B.; Carey, E.E.; Zhao, X.; Oh, M.M. Health-promoting phytochemicals in Fruits and vegetables: Impact of abiotic stresses and crop production practices. Funct. Plant Sci. Biotechnol. 2009, 3, 30–38. [Google Scholar]
- Tsormpatsidis, E.; Henbest, R.G.C.; Davis, F.J.; Battey, N.H.; Hadley, P.; Wagstaffe, A. UV irradiance as a major influence on growth, development and secondary products of commercial importance in Lollo Rosso lettuce ‘Revolution’ grown under polyethylene films. Environ. Exp. Bot. 2008, 63, 232–239. [Google Scholar] [CrossRef]
- Bantis, F.; Ouzounis, T.; Radoglou, K. Artificial LED lighting enhances growth characteristics and total phenolic content of Ocimum basilicum, but variably affects transplant success. Sci. Hortic. 2016, 198, 277–283. [Google Scholar] [CrossRef] [Green Version]
- Taulavuori, E.; Taulavuori, K.; Holopainen, J.K.; Julkunen-Tiitto, R.; Acar, C.; Dincer, I. Targeted use of LEDs in improvement of production efficiency through phytochemical enrichment: LEDs improve production efficiency. J. Sci. Food Agric. 2017, 97, 5059–5064. [Google Scholar] [CrossRef] [PubMed]
- Tulchinsky, T.H. Micronutrient deficiency conditions: Global health issues. Public Health Rev. 2010, 32, 243–255. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Guo, Z.; Jiang, X.; Ahammed, G.J.; Zhou, Y. Light regulation of horticultural crop nutrient uptake and utilization. Horticultural. Plant J. 2021, 7, 367–379. [Google Scholar] [CrossRef]
- Muller, O.; Krawinkel, M. Malnutrition and health in developing countries. Can. Med. Assoc. J. 2005, 173, 79–286. [Google Scholar] [CrossRef] [Green Version]
- Morales, L.O.; Tegelberg, R.; Brosche, M.; Keinanen, M.; Lindfors, A.; Aphalo, P.J. Effects of solar UV-A and UV-B radiation on gene expression and phenolic accumulation in Betula pendula leaves. Tree Physiol. 2010, 30, 923–934. [Google Scholar] [CrossRef] [Green Version]
- Taulavuori, K.; Pyysalo, A.; Taulavuori, E.; Julkunen-Tiitto, R. Responses of phenolic acid and flavonoid synthesis to blue and blue-violet light depends on plant species. Environ. Exp. Bot. 2018, 150, 183–187. [Google Scholar] [CrossRef] [Green Version]
- Kopsell, D.A.; Sams, C.E.; Morrow, R.C. Blue wavelengths from LED lighting increase nutritionally important metabolites in specialty crops. HortScience 2015, 50, 1285–1288. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Wang, L.; Li, T.; Yang, Q.; Guo, W. Sugar accumulation and growth of lettuce exposed to different lighting modes of red and blue LED light. Sci. Rep. 2019, 9, 6926. [Google Scholar] [CrossRef] [Green Version]
- Gieseking, J.E.; Snider, H.J.; Getz, C.A. Destruction of organic matter in plant material by the use of nitric and perchloric acids. Ind. Eng. Chem. Anal. Ed. 1935, 7, 185–186. [Google Scholar] [CrossRef]
- Milton, K.; Dintzis, F.R. Nitrogen-to-protein conversion factors for tropical plant samples. Biotropica 1981, 13, 177–181. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using folin-ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.J.; Rice-Evans, C.A. Spectrophotometric determination of antioxidant activity. Redox Rep. 1996, 2, 161–171. [Google Scholar] [CrossRef]
- Pennycooke, J.C.; Cox, S.; Stushnoff, C. Relationship of cold acclimation, total phenolic content and antioxidant capacity with chilling tolerance in petunia (Petunia hybrida). Environ. Exp. Bot. 2005, 53, 225–232. [Google Scholar] [CrossRef]
- Woolley, A.; Sumpter, S.; Lee, M.; Xu, J.; Barry, S.; Wang, W.; Rajashekar, C.B. Accumulation of mineral nutrients and phytochemicals in lettuce and tomato grown in high tunnel and open field. Am. J. Plant Sci. 2019, 10, 125–138. [Google Scholar] [CrossRef] [Green Version]
- Tezuka, T.; Yamaguchi, F.; Ando, Y. Physiological activation in radish plants by UV-A radiation. J. Photochem. Photobiol. B Biol. 1994, 24, 33–40. [Google Scholar] [CrossRef]
- Brazaitytė, A.; Viršilė, A.; Jankauskienė, J.; Sakalauskienė, S.; Samuolienė, G.; Sirtautas, R.; Novičkovas, A.; Dabašinskas, L.; Miliauskienė, J.; Vaštakaitė, V.; et al. Effect of supplemental UV-A irradiation in solid-state lighting on the growth and phytochemical content of microgreens. Int. Agrophysics 2015, 29, 13–22. [Google Scholar] [CrossRef]
- Krizek, D.T.; Britz, S.J.; Mirecki, R.M. Inhibitory effects of ambient levels of solar UV-A and UV-B radiation on growth of cv. New Red Fire lettuce. Physiol. Plant. 1998, 103, 1–7. [Google Scholar] [CrossRef]
- Cooley, N.M.; Higgins, J.T.; Holmes, M.G.; Attridge, T.H. Ecotypic differences in responses of Arabidopsis thaliana L. elevated polychromatic UV-A and UV-B+A radiation in the natural environment: A positive correlation between UV-B+A inhibition and growth rate. J. Photochem. Photobiol. B Biol. 2001, 60, 143–150. [Google Scholar] [CrossRef]
- Kataria, S.; Guruprasad, K.N.; Ahuja, S.; Singh, B. Enhancement of growth, photosynthetic performance and yield by exclusion of ambient UV components in C3 and C4 plants. J. Photochem. Photobiol. B Biol. 2013, 127, 140–152. [Google Scholar] [CrossRef]
- Jeon, Y.M.; Son, K.H.; Kim, S.M.; Oh, M.M. Growth of dropwort plants and their accumulation of bioactive compounds after exposure to UV lamp or LED irradiation. Hortic. Environ. Biotechnol. 2018, 59, 659–670. [Google Scholar] [CrossRef]
- Krizek, D.T. Influence of PAR and UV-A in determining plant sensitivity and photomorphogenic responses to UV-B radiation. Photochem. Photobiol. 2004, 79, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Rivard, C.; Pliakoni, E.; Wang, W.; Rajashekar, C.B. Supplemental UV-A and UV-B affect the nutritional quality of lettuce and tomato: Health-promoting phytochemicals and essential nutrients. Am. J. Plant Sci. 2021, 12, 104–126. [Google Scholar] [CrossRef]
- Lee, M.; Rivard, C.; Wang, W.; Pliakoni, E.; Gude, K.; Rjashekar, C.B. Spectral blocking of solar radiation in high tunnels by poly covers: Its impact on nutritional quality regarding essential nutrients and health-promoting phytochemicals in lettuce and tomato. Horticulturae 2021, 7, 524. [Google Scholar] [CrossRef]
- De-Onis, M.; Monteiro, C.; Akre, J.; Clugston, J. The worldwide magnitude of protein- energy malnutrition: An overview from the WHO global data-base on child growth. Bull. World Health Organ. 1993, 71, 703–712. [Google Scholar] [PubMed]
- Vaštakaitė, V.; Viršilė, A.; Brazaitytė, A.; Samuolienė, G.; Jankauskienė, J.; Sirtautas, R.; Duchovskis, P. The effect of UV-A supplemental lighting on antioxidant properties of Ocimum basilicum L. microgreens in greenhouse. In Proceedings of the 7th International Scientific Conference “Rural Development 2015: Towards the Transfer of Knowledge, Innovations and Social Progress”, Madrid, Spain, 20–22 May 2015. [Google Scholar]
- Verdaguer, D.; Jansen, M.A.K.; Llorens, L.; Morales, L.O.; Neugart, S. UV-A radiation effects on higher plants: Exploring the known unknown. Plant Sci. 2017, 255, 72–81. [Google Scholar] [CrossRef] [PubMed]
| Treatments | ||||
|---|---|---|---|---|
| Control | LED | LED + F | LED + S | |
| PPFD (μmol/m2/sec) | 190 ± 1.9 | 192 ± 3.1 | 197 ± 4.8 | 192 ± 4.4 |
| UV-A (watts/m2) | - | 1.37 ± 0.03 | 0.814 ± 0.1 | 1.15 ± 0.04 |
| New Red Fire | Two Star | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Shoot | Shoot | ||||||||
| Day | Fresh Mass (g) | Dry Mass (g) | Leaf Area (cm2) | Number of Leaves | Fresh Mass (g) | Dry Mass (g) | Leaf Area (cm2) | Number of Leaves | |
| 0 | 43.3 | 2.8 | 1056.8 | 12.7 | 40.2 | 3.8 | 779.32 | 16.0 | |
| 3 | Control | 53.7 | 3.8 | 1198.3 | 14.0 | 48.5 | 5.7a | 941.94 | 18.0 |
| LED | 61.5 | 3.5 | 1492.3 | 16.0 | 58.6 | 4.9ab | 1093.97 | 16.7 | |
| LED + F | 54.7 | 3.2 | 1327.7 | 15.0 | 50.8 | 4.1b | 959.20 | 16.3 | |
| LED + S | 56.3 | 2.8 | 1246.3 | 14.0 | 55.4 | 4.4b | 1034.40 | 15.3 | |
| Significance | ns | ns | ns | ns | ns | * | ns | ns | |
| 6 | Control | 62.5 | 5.1a | 1554.7 | 15.7 | 54.5 | 7.7a | 1027.27 | 19.7 |
| LED | 61.7 | 3.5b | 1478.7 | 14.8 | 60.3 | 5.1b | 1201.00 | 18.5 | |
| LED + F | 59.0 | 3.4b | 1493.3 | 15.0 | 49.6 | 4.3c | 974.61 | 17.3 | |
| Led + S | 67.6 | 3.9b | 1636.0 | 16.0 | 56.0 | 4.4c | 1077.79 | 16.5 | |
| Significance | ns | ** | ns | ns | ns | *** | ns | ns | |
| Light Source | Protein (%) | C (%) | P (%) | K (%) | Ca (%) | SO4-S (%) | Mn (ppm) | |
|---|---|---|---|---|---|---|---|---|
| 3 days | Control | 13.25 | 37.93 | 0.48 b | 5.78 b | 0.81 | 0.166 b | 68.08 |
| LED | 15.75 | 37.31 | 0.56 ab | 6.76 ab | 0.86 | 0.203 ab | 70.78 | |
| LED + F | 16.33 | 36.88 | 0.65 a | 7.35 a | 0.95 | 0.237 a | 70.23 | |
| LED + S | 15.52 | 36.73 | 0.61 a | 7.11 a | 0.96 | 0.216 a | 71.1 | |
| Significance | ns | ns | * | * | ns | * | ns | |
| 6 days | Control | 14.42 c | 38.42 a | 0.52 c | 6.10 b | 0.87 b | 0.19 b | 59.3 b |
| LED | 18.92 a | 36.86 b | 0.62 a | 7.26 a | 1.04 a | 0.22 a | 77.1 a | |
| LED + F | 16.81 b | 37.07 b | 0.58 b | 7.16 a | 0.94 ab | 0.21 ab | 70.6 ab | |
| LED + S | 16.81 b | 37.34 b | 0.55 bc | 7.08 a | 0.96 ab | 0.21 ab | 84.1 a | |
| Significance | *** | *** | *** | ** | * | * | * |
| Phenolic Acids (μg/g DW) | ||||
|---|---|---|---|---|
| Light Source | 3 Days after UV Treatments | 6 Days after UV Treatments | ||
| Gallic Acid | Caffeic Acid | Gallic Acid | Caffeic Acid | |
| Control | 10.24 ab | 51.32 a | 4.49 b | 33.07 |
| LED | 14.99 a | 40.45 ab | 5.07 b | 21.24 |
| LED + F | 12.57 ab | 43.05 ab | 3.93 b | 20.61 |
| LED + S | 6.74 b | 33.17 b | 7.85 a | 17.75 |
| Significance | * | ** | * | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.; Kim, J.; Oh, M.-M.; Lee, J.-H.; Rajashekar, C.B. Effects of Supplemental UV-A LEDs on the Nutritional Quality of Lettuce: Accumulation of Protein and Other Essential Nutrients. Horticulturae 2022, 8, 680. https://doi.org/10.3390/horticulturae8080680
Lee M, Kim J, Oh M-M, Lee J-H, Rajashekar CB. Effects of Supplemental UV-A LEDs on the Nutritional Quality of Lettuce: Accumulation of Protein and Other Essential Nutrients. Horticulturae. 2022; 8(8):680. https://doi.org/10.3390/horticulturae8080680
Chicago/Turabian StyleLee, Myungjin, Jungkwun Kim, Myung-Min Oh, Jin-Hui Lee, and Channa B. Rajashekar. 2022. "Effects of Supplemental UV-A LEDs on the Nutritional Quality of Lettuce: Accumulation of Protein and Other Essential Nutrients" Horticulturae 8, no. 8: 680. https://doi.org/10.3390/horticulturae8080680
APA StyleLee, M., Kim, J., Oh, M.-M., Lee, J.-H., & Rajashekar, C. B. (2022). Effects of Supplemental UV-A LEDs on the Nutritional Quality of Lettuce: Accumulation of Protein and Other Essential Nutrients. Horticulturae, 8(8), 680. https://doi.org/10.3390/horticulturae8080680









