Maintaining Canopy Density under Summer Stress Conditions Retains PSII Efficiency and Modulates Must Quality in Cabernet Franc
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Treatment Applications
2.3. Canopy Parameters and Productivity
2.4. Point Quadrat Analysis
2.5. Chlorophyll Fluorescence Analysis and Leaf and Berry Temperature
2.6. Must Quality Analysis
2.7. Statistical Analysis
3. Results
3.1. Environmental Conditions
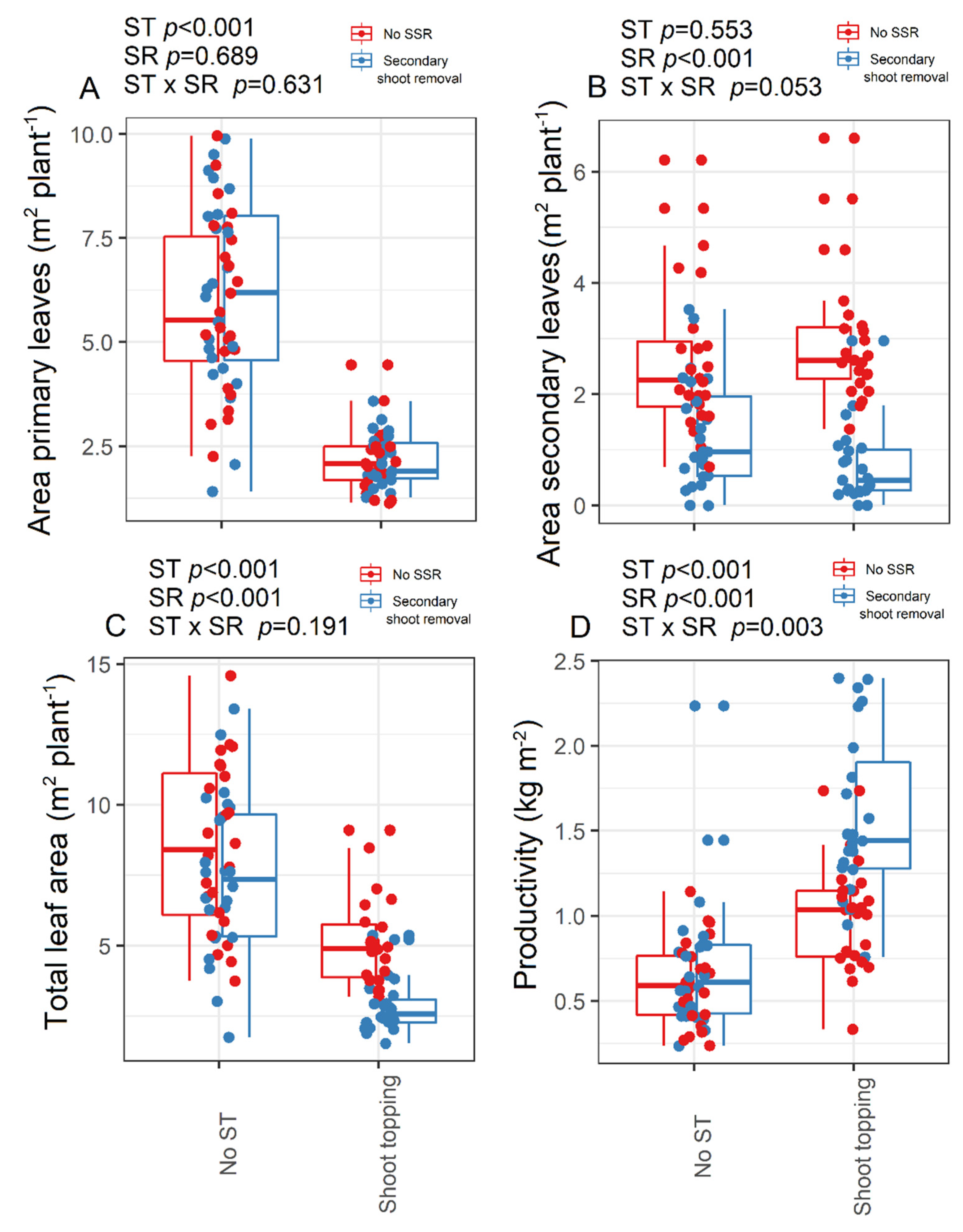
3.2. Canopy, Vegetative, and Productivity Traits
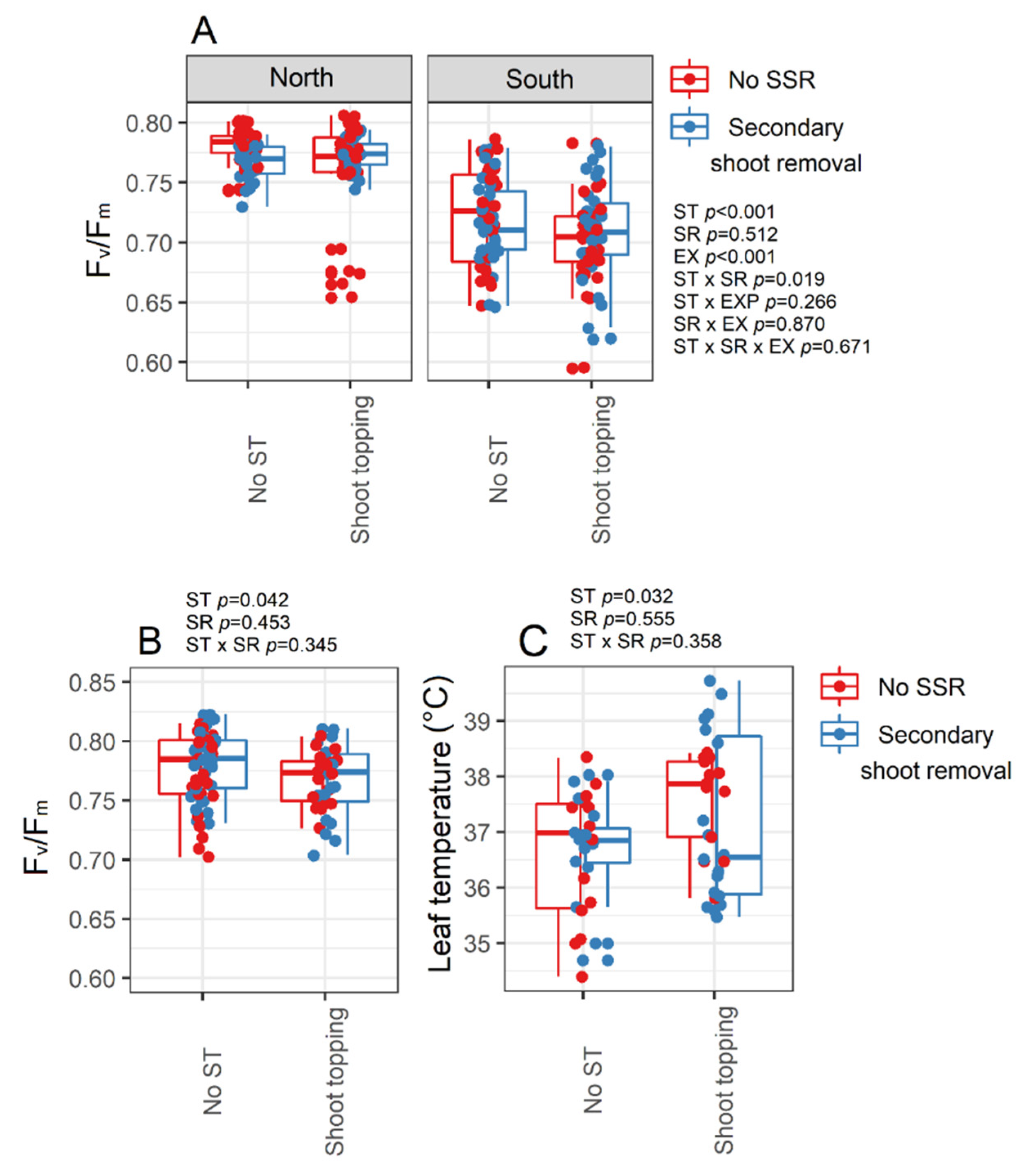
3.3. Chlorophyll Fluorescence Analysis
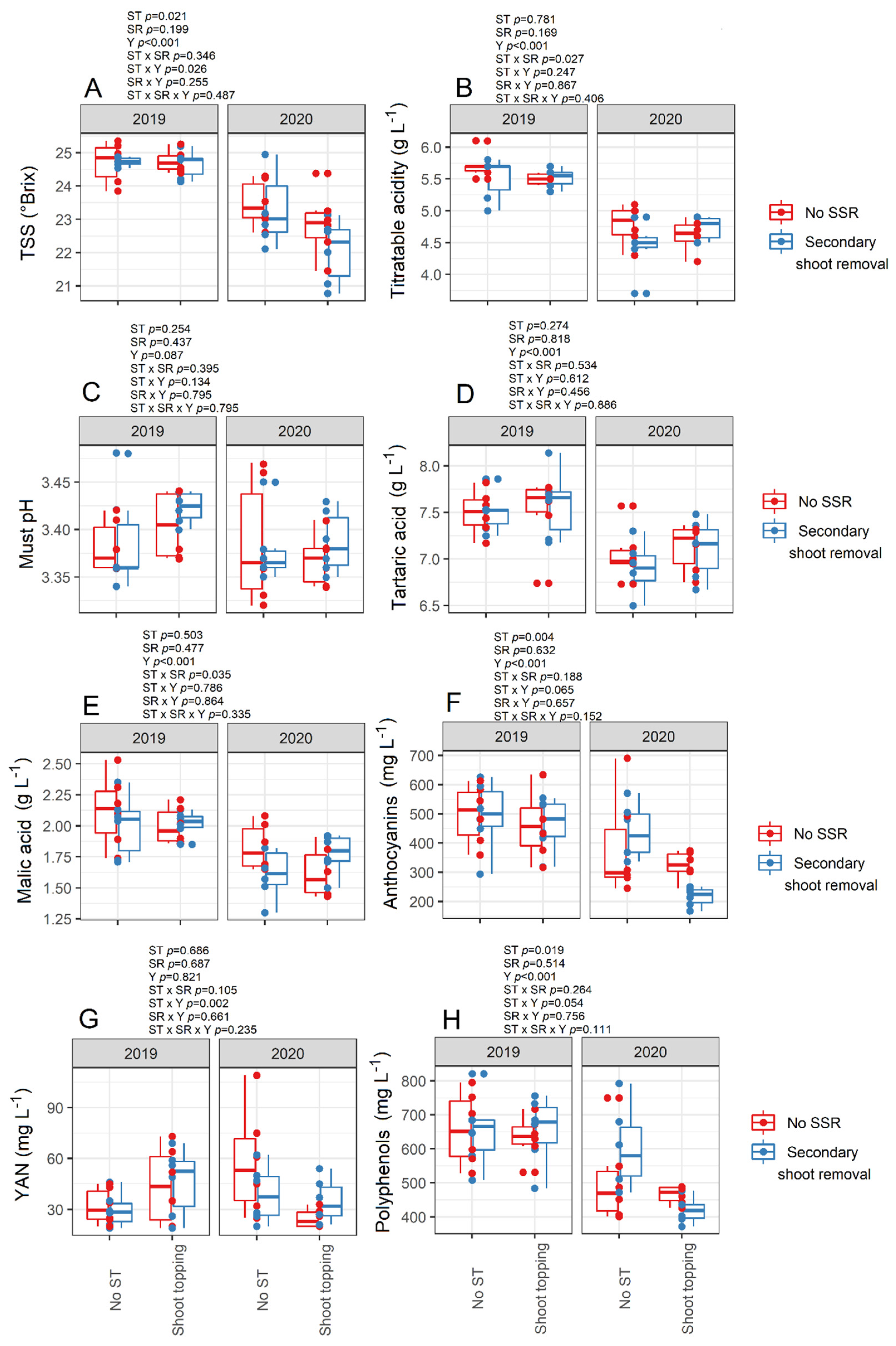
3.4. Must Quality and Berry Temperature
4. Discussion
4.1. Can Shoot Bundling Be Effective at Reducing Photosynthetic Impairment under Multifactorial Summer Stresses?
4.2. Dense Canopies Reduce Malic Acid Breakdown and Anthocyanins Degradation under Hot Summer Conditions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, X.; De Bei, R.; Fuentes, S.; Collins, C. Influence of Canopy Management Practices on Canopy Architecture and Reproductive Performance of Semillon and Shiraz Grapevines in a Hot Climate. Am. J. Enol. Vitic. 2019, 70, 360–372. [Google Scholar] [CrossRef]
- Palliotti, A.; Tombesi, S.; Silvestroni, O.; Lanari, V.; Gatti, M.; Poni, S. Changes in vineyard establishment and canopy management urged by earlier climate-related grape ripening: A review. Sci. Hortic. 2014, 178, 43–54. [Google Scholar] [CrossRef]
- Dry, P.R. Canopy management for fruitfulness. Aust. J. Grape Wine Res. 2000, 6, 109–115. [Google Scholar] [CrossRef]
- Geller, J.P.; Kurtural, S.K. Mechanical Canopy and Crop-Load Management of Pinot gris in a Warm Climate. Am. J. Enol. Vitic. 2013, 64, 65–73. [Google Scholar] [CrossRef]
- Verdenal, T.; Zufferey, V.; Dienes-Nagy, A.; Bourdin, G.; Gindro, K.; Viret, O.; Spring, J.-L. Timing and Intensity of Grapevine Defoliation: An Extensive Overview on Five Cultivars in Switzerland. Am. J. Enol. Vitic. 2019, 70, 427–434. [Google Scholar] [CrossRef]
- Poni, S.; Giachino, E. Growth, photosynthesis and cropping of potted grapevines (Vitis vinifera L. cv. Cabernet Sauvignon) in relation to shoot trimming. Aust. J. Grape Wine Res. 2000, 6, 216–226. [Google Scholar] [CrossRef]
- Petrie, P.R.; Trought, M.C.T.; Howell, G.S.; Buchan, G.D. The effect of leaf removal and canopy height on whole-vine gas exchange and fruit development of Vitis vinifera L. Sauvignon Blanc. Funct. Plant Biol. 2003, 30, 711–717. [Google Scholar] [CrossRef]
- Hunter, J.; De Villiers, Q.; Watts, J. The effect of partial defoliation on quality characteristics of Vitis vinifera L. cv. Cabernet Sauvignon grapes. II. Skin color, skin sugar, and wine quality. Am. J. Enol. Vitic. 1991, 42, 13–18. [Google Scholar]
- Vandeleur, R.; Sullivan, W.; Athman, A.; Jordans, C.; Gilliham, M.; Kaiser, B.; Tyerman, S.D. Rapid shoot-to-root signalling regulates root hydraulic conductance via aquaporins. Plant Cell Environ. 2014, 37, 520–538. [Google Scholar] [CrossRef]
- Hunter, J.; Visser, J. The Effect of Partial Defoliation, Leaf Position and Developmental Stage of the Vine on the Photosynthetic Activity of Vitis vinifera L. cv Cabernet Sauvignon. S. Afr. J. Enol. Vitic. 1988, 9. [Google Scholar] [CrossRef] [Green Version]
- Hunter, J.; Volschenk, C.; Mania, E.; Castro, A.V.; Booyse, M.; Guidoni, S.; Pisciotta, A.; Di Lorenzo, R.; Novello, V.; Zorer, R. Grapevine row orientation mediated temporal and cumulative microclimatic effects on grape berry temperature and composition. Agric. For. Meteorol. 2021, 310, 108660. [Google Scholar] [CrossRef]
- Cohen, S.D.; Tarara, J.M.; Gambetta, G.A.; Matthews, M.A.; Kennedy, J.A. Impact of diurnal temperature variation on grape berry development, proanthocyanidin accumulation, and the expression of flavonoid pathway genes. J. Exp. Bot. 2012, 63, 2655–2665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarara, J.M.; Lee, J.; Spayd, S.E.; Scagel, C.F. Berry temperature and solar radiation alter acylation, proportion, and concentration of anthocyanin in Merlot grapes. Am. J. Enol. Vitic. 2008, 59, 235–247. [Google Scholar]
- Rienth, M.; Torregrosa, L.; Sarah, G.; Ardisson, M.; Brillouet, J.-M.; Romieu, C. Temperature desynchronizes sugar and organic acid metabolism in ripening grapevine fruits and remodels their transcriptome. BMC Plant Biol. 2016, 16, 164. [Google Scholar] [CrossRef]
- Mori, K.; Goto-Yamamoto, N.; Kitayama, M.; Hashizume, K. Loss of anthocyanins in red-wine grape under high temperature. J. Exp. Bot. 2007, 58, 1935–1945. [Google Scholar] [CrossRef]
- Greer, D.H.; Weedon, M.M. The impact of high temperatures on Vitis vinifera cv. Semillon grapevine performance and berry ripening. Front. Plant Sci. 2013, 4, 491. [Google Scholar] [CrossRef] [Green Version]
- Richard, E.; Smart, R.E. Principles of Grapevine Canopy Microclimate Manipulation with Implications for Yield and Quality. A Review. Am. J. Enol. Vitic. 1985, 36, 230–239. [Google Scholar]
- Mattivi, F.; Guzzon, R.; Vrhovsek, U.; Stefanini, A.M.; Velasco, R. Metabolite Profiling of Grape: Flavonols and Anthocyanins. J. Agric. Food Chem. 2006, 54, 7692–7702. [Google Scholar] [CrossRef]
- Poni, S.; Gatti, M.; Palliotti, A.; Dai, Z.; Duchêne, E.; Truong, T.-T.; Ferrara, G.; Matarrese, A.M.S.; Gallotta, A.; Bellincontro, A.; et al. Grapevine quality: A multiple choice issue. Sci. Hortic. 2018, 234, 445–462. [Google Scholar] [CrossRef] [Green Version]
- Austin, C.N.; Wilcox, W.F. Effects of Fruit-Zone Leaf Removal, Training Systems, and Irrigation on the Development of Grapevine Powdery Mildew. Am. J. Enol. Vitic. 2011, 62, 193–198. [Google Scholar] [CrossRef]
- Bertamini, M.; Faralli, M.; Varotto, C.; Grando, M.; Cappellin, L. Leaf Monoterpene Emission Limits Photosynthetic Downregulation under Heat Stress in Field-Grown Grapevine. Plants 2021, 10, 181. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Mittler, R. Plant responses to multifactorial stress combination. New Phytol. 2022, 234, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Faralli, M.; Lawson, T. Natural genetic variation in photosynthesis: An untapped resource to increase crop yield potential? Plant J. 2020, 101, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Faralli, M.; Bontempo, L.; Bianchedi, P.L.; Moser, C.; Bertamini, M.; Lawson, T.; Camin, F.; Stefanini, M.; Varotto, C. Natural variation in stomatal dynamics drives divergence in heat stress tolerance and contributes to the seasonal intrinsic water-use efficiency in Vitis vinifera (subsp. sativa and sylvestris). J. Exp. Bot. 2021, 73, 3238–3250. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.M.; Ortuño, M.F.; Lopes, C.M.; Chaves, M.M. Grapevine varieties exhibiting differences in stomatal response to water deficit. Funct. Plant Biol. 2012, 39, 179–189. [Google Scholar] [CrossRef]
- Palliotti, A.; Silvestroni, O.; Petoumenou, D. Photosynthetic and photoinhibition behavior of two field-grown grapevine cultivars under multiple summer stresses. Am. J. Enol. Vitic. 2009, 60, 189–198. [Google Scholar]
- Bertamini, M.; Muthuchelian, K.; Nedunchezhian, N. Photoinhibition of Photosynthesis in Sun and Shade Grown Leaves of Grapevine (Vitis vinifera L.). Photosynthetica 2004, 42, 7–14. [Google Scholar] [CrossRef]
- Serra, I.; Strever, A.; Myburgh, P.A.; Deloire, A. The interaction between rootstocks and cultivars (Vitis vinifera L.) to enhance drought tolerance in grapevine. Aust. J. Grape Wine Res. 2014, 20, 1–14. [Google Scholar] [CrossRef]
- Faralli, M.; Bianchedi, P.L.; Bertamini, M.; Varotto, C. Rootstock Genotypes Shape the Response of cv. Pinot gris to Water Deficit. Agronomy 2020, 11, 75. [Google Scholar] [CrossRef]
- Kraus, C.; Pennington, T.; Herzog, K.; Hecht, A.; Fischer, M.; Voegele, R.T.; Hoffmann, C.; Töpfer, R.; Kicherer, A. Effects of canopy architecture and microclimate on grapevine health in two training systems. Vitis 2018, 57, 53–60. [Google Scholar]
- de Orduña, R.M. Climate change associated effects on grape and wine quality and production. Food Res. Int. 2010, 43, 1844–1855. [Google Scholar] [CrossRef]
- Jones, G.V.; White, M.A.; Cooper, O.R.; Storchmann, K. Climate Change and Global Wine Quality. Clim. Change 2005, 73, 319–343. [Google Scholar] [CrossRef]
- Coombe, B.G. Distribution of solutes within the developing grape berry in relation to its morphology. Am. J. Enol. Vitic. 1987, 38, 120–127. [Google Scholar]
- Keller, M. The Science of Grapevines; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Duchêne, É.; Dumas, V.; Butterlin, G.; Jaegli, N.; Rustenholz, C.; Chauveau, A.; Bérard, A.; Le Paslier, M.C.; Gaillard, I.; Merdinoglu, D. Genetic variations of acidity in grape berries are controlled by the interplay between organic acids and potassium. Theor. Appl. Genet. 2020, 133, 993–1008. [Google Scholar] [CrossRef]
- Bergqvist, J.; Dokoozlian, N.; Ebisuda, N. Sunlight exposure and temperature effects on berry growth and composition of Cabernet Sauvignon and Grenache in the Central San Joaquin Valley of California. Am. J. Enol. Vitic. 2001, 52, 1–7. [Google Scholar]


| Shoot Number per Plant | Bunch Number per Plant | Bunch Weight per Plant (kg) | Mean Bunch Weight (g FW) | Pruning Wood (g FW) | Ravaz Index | Fertility | |||
|---|---|---|---|---|---|---|---|---|---|
| 2019 | Shoot topping | SSR | 9.71 | 11.92 b | 3.16 b | 271.92 | 0.72 bc | 4.49 bc | 1.25 b |
| No SSR | 9.54 | 11.08 b | 2.92 b | 272.52 | 0.69 bc | 4.37 bc | 1.18 b | ||
| No shoot topping | SSR | 9.79 | 12.50 b | 3.23 b | 264.32 | 0.77 ab | 4.43 bc | 1.30 b | |
| No SSR | 9.33 | 11.71 b | 3.11 b | 272.04 | 0.85 ab | 3.92 c | 1.29 b | ||
| 2020 | Shoot topping | SSR | 9.70 | 17.39 a | 4.18 a | 252.40 | 0.60 c | 7.17 a | 1.78 a |
| No SSR | 9.61 | 18.70 a | 4.79 a | 256.17 | 0.73 bc | 6.82 a | 1.94 a | ||
| No shoot topping | SSR | 9.71 | 17.92 a | 4.44 a | 249.21 | 0.83 ab | 5.39 b | 1.85 a | |
| No SSR | 9.29 | 17.88 a | 4.66 a | 263.06 | 0.92 a | 5.34 b | 1.94 a | ||
| p-value | ST | ns | ns | ns | ns | <0.001 | <0.001 | ns | |
| SR | ns | ns | ns | ns | 0.015 | ns | ns | ||
| Y | ns | <0.001 | <0.001 | ns | ns | <0.001 | <0.001 | ||
| ST × SR | ns | ns | ns | ns | ns | ns | ns | ||
| ST × Y | ns | ns | ns | ns | ns | 0.003 | ns | ||
| SR × Y | ns | ns | ns | ns | ns | ns | ns | ||
| ST × SR × Y | ns | ns | ns | ns | ns | ns | ns |
| Bunch Exposure | Berry Temperature (°C) | TSS (Brix°) | pH | Total Acidity | Tartaric Acid | Malic Acid | Potassium (g L−1) | Yeast-Assimilable N (mg L−1) | Anthocyanins (mg L−1) | Polyphenols (mg L−1) |
|---|---|---|---|---|---|---|---|---|---|---|
| (g L−1) | (g L−1) | (g L−1) | ||||||||
| South | 35.27 | 23.55 | 3.44 | 4.2 | 7.2 | 1.39 | 1.96 | 35.33 | 348.67 | 529.33 |
| North | 29.14 | 22.07 | 3.29 | 4.63 | 7.21 | 1.43 | 1.76 | 21.33 | 481.33 | 645.33 |
| p-value | 0.001 | 0.055 | 0.004 | 0.007 | 0.467 | 0.394 | 0.033 | 0.065 | 0.028 | 0.015 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faralli, M.; Zanzotti, R.; Bertamini, M. Maintaining Canopy Density under Summer Stress Conditions Retains PSII Efficiency and Modulates Must Quality in Cabernet Franc. Horticulturae 2022, 8, 679. https://doi.org/10.3390/horticulturae8080679
Faralli M, Zanzotti R, Bertamini M. Maintaining Canopy Density under Summer Stress Conditions Retains PSII Efficiency and Modulates Must Quality in Cabernet Franc. Horticulturae. 2022; 8(8):679. https://doi.org/10.3390/horticulturae8080679
Chicago/Turabian StyleFaralli, Michele, Roberto Zanzotti, and Massimo Bertamini. 2022. "Maintaining Canopy Density under Summer Stress Conditions Retains PSII Efficiency and Modulates Must Quality in Cabernet Franc" Horticulturae 8, no. 8: 679. https://doi.org/10.3390/horticulturae8080679
APA StyleFaralli, M., Zanzotti, R., & Bertamini, M. (2022). Maintaining Canopy Density under Summer Stress Conditions Retains PSII Efficiency and Modulates Must Quality in Cabernet Franc. Horticulturae, 8(8), 679. https://doi.org/10.3390/horticulturae8080679








