Improving the Quality and Production of Philodendron Plants Using Nanoparticles and Humic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Experimental Design
- Control.
- 1 ml/L humic acid (HA)
- 2 ml/L humic acid (HA)
- 1.5 ml/L nano fertilizer NPK (NPK NPs)
- 2 ml/L nano fertilizer NPK (NPK NPs)
- 100 mg/L iron nanoparticles (FeNPs)
- 150 mg/L iron nanoparticles (FeNPs)
- 150 mg/L zinc nanoparticles (ZnNPs)
- 200 mg/L zinc nanoparticles (ZnNPs)
- 20 mg/L Silver nanoparticles (SNPs)
- 40 mg/L Silver nanoparticles (SNPs)
- 60 mg/L Silver nanoparticles (SNPs)
2.2. The Growth Parameters
2.3. Physiological Characters
- -
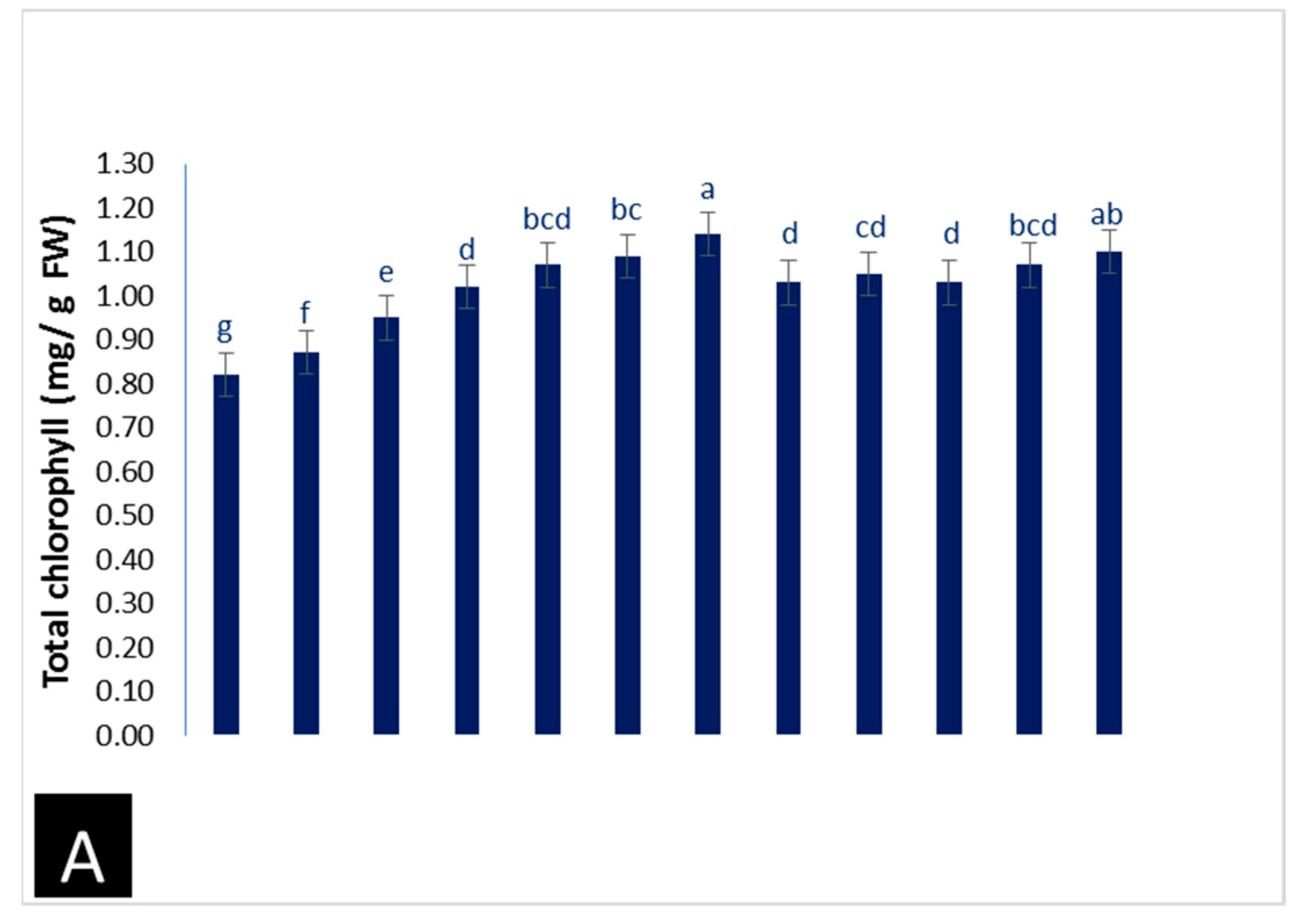
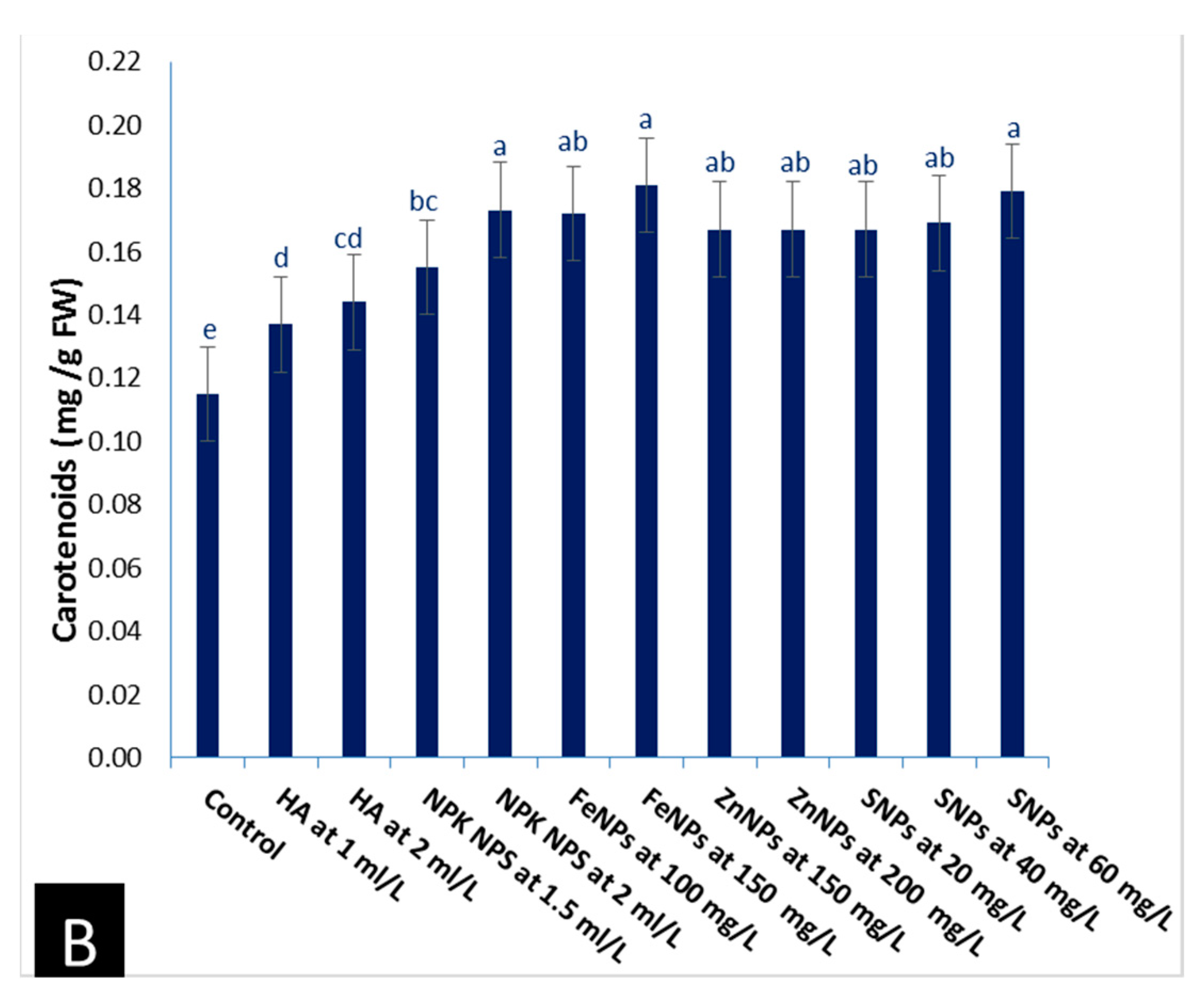
- Total chlorophyll and carotenoid contents in leaves were determined according to Allel et al. [35].
- -
- -
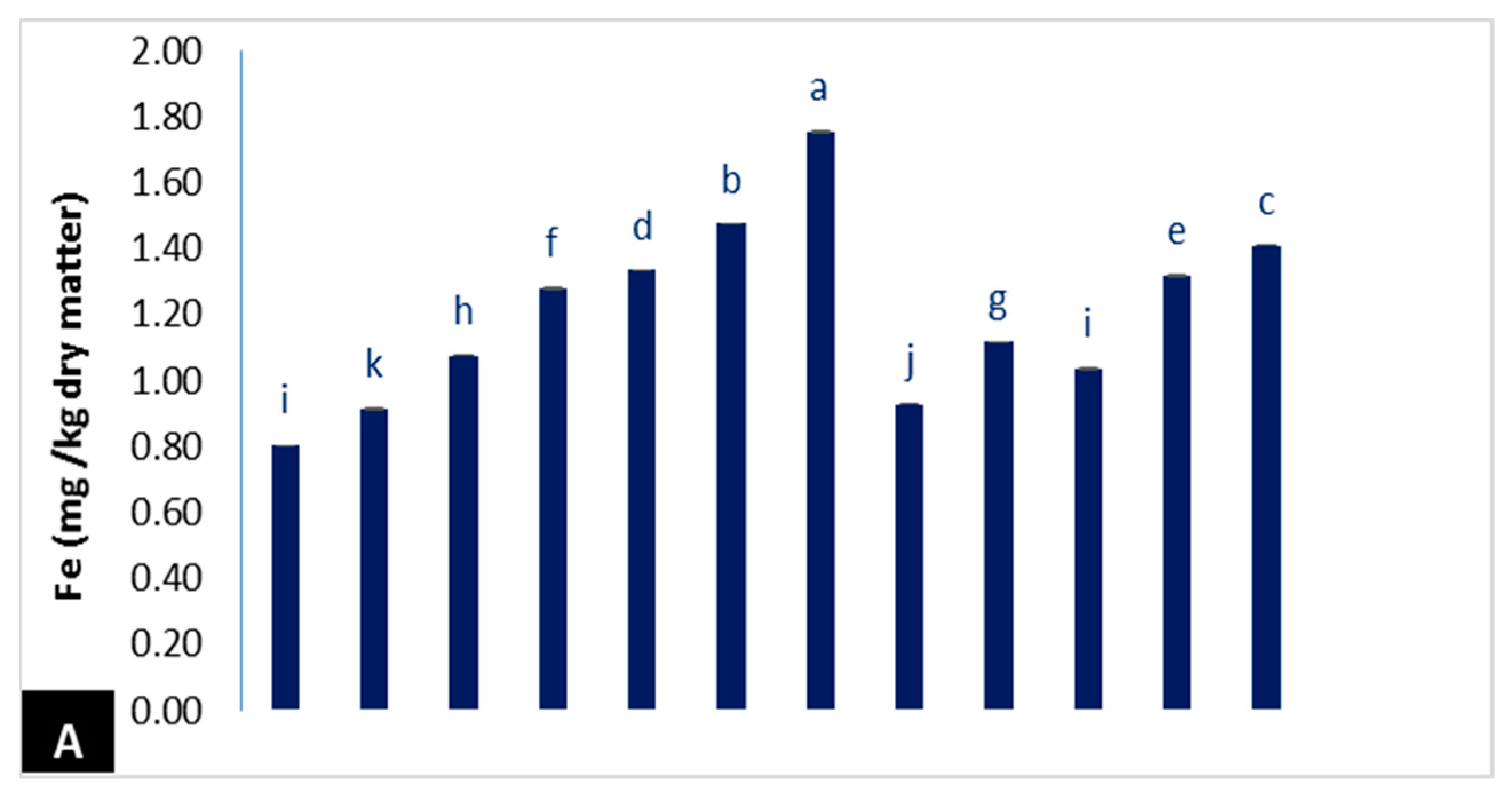
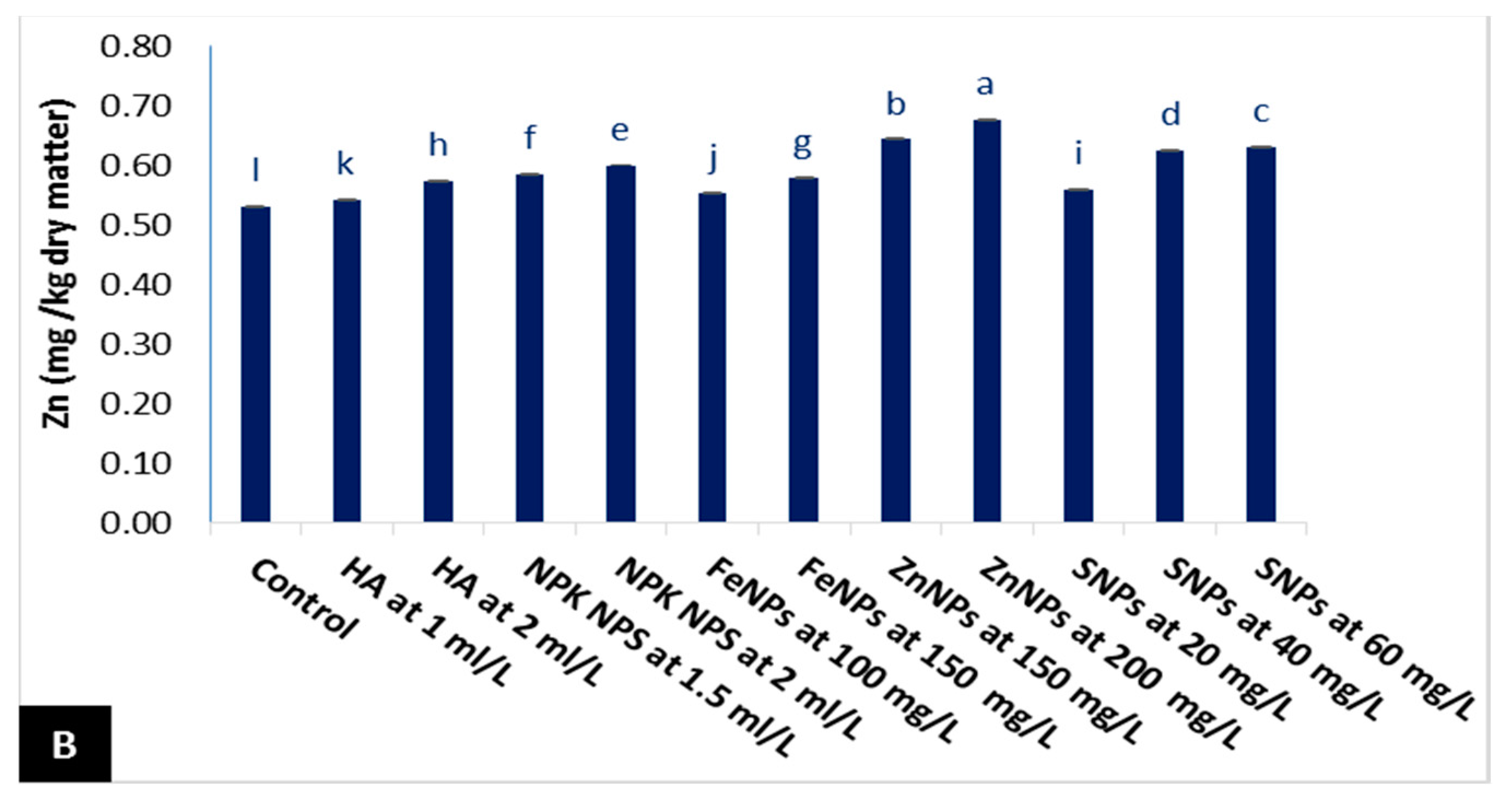
- Nitrogen (N) (%) was determined by micro Kjeldahl as per the method described by the A.O.A.C. [39]. Phosphorus (P) (%) was determined by spectrophotometer as per the method described by Jackson [40]. Potassium (K) (%) was determined by flame emission spectrometry according to Page et al. [41]. Iron (Fe mg/kg) and zinc (Zn mg/kg) were specified by using inductively coupled spectrometry plasma (ICP) Model Ultimate-Jobin Yvonzz.e.
2.4. Anatomical Studies
2.5. Statistical Analysis
3. Results
3.1. Morphological Growth Characters
3.1.1. Plant Height, Plant Width and Number of Leaves/Plants
3.1.2. The Leaf Dimensions (Length and Width of Leaf Blades, Length of Petioles)
3.1.3. Leaf Area, Stem Diameter, and Root Length
3.1.4. The Fresh and Dry Weights of Leaves, Stems, Roots, and the Whole Plant
3.2. Physiological Characters
3.2.1. Photosynthetic Pigments
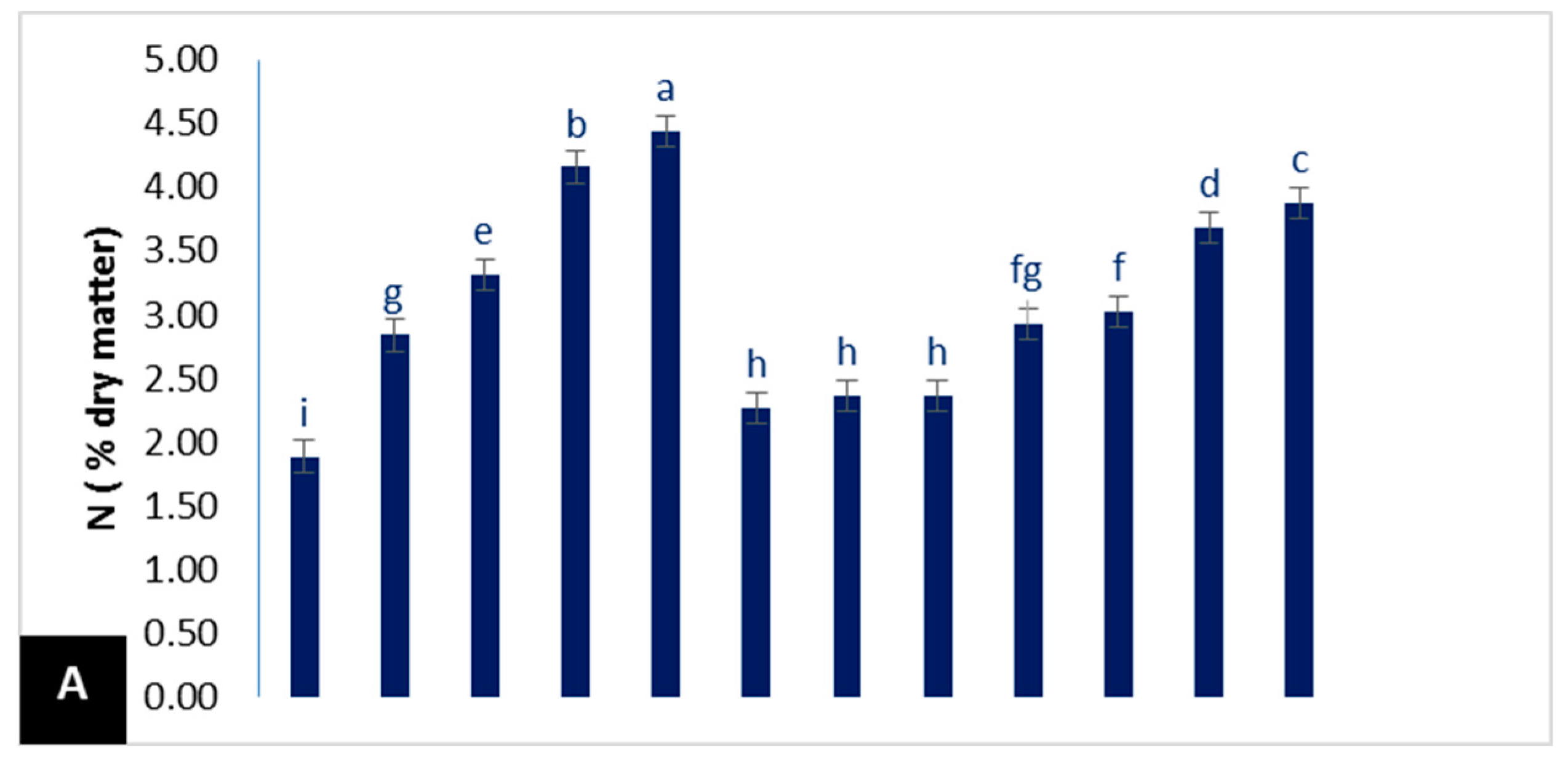
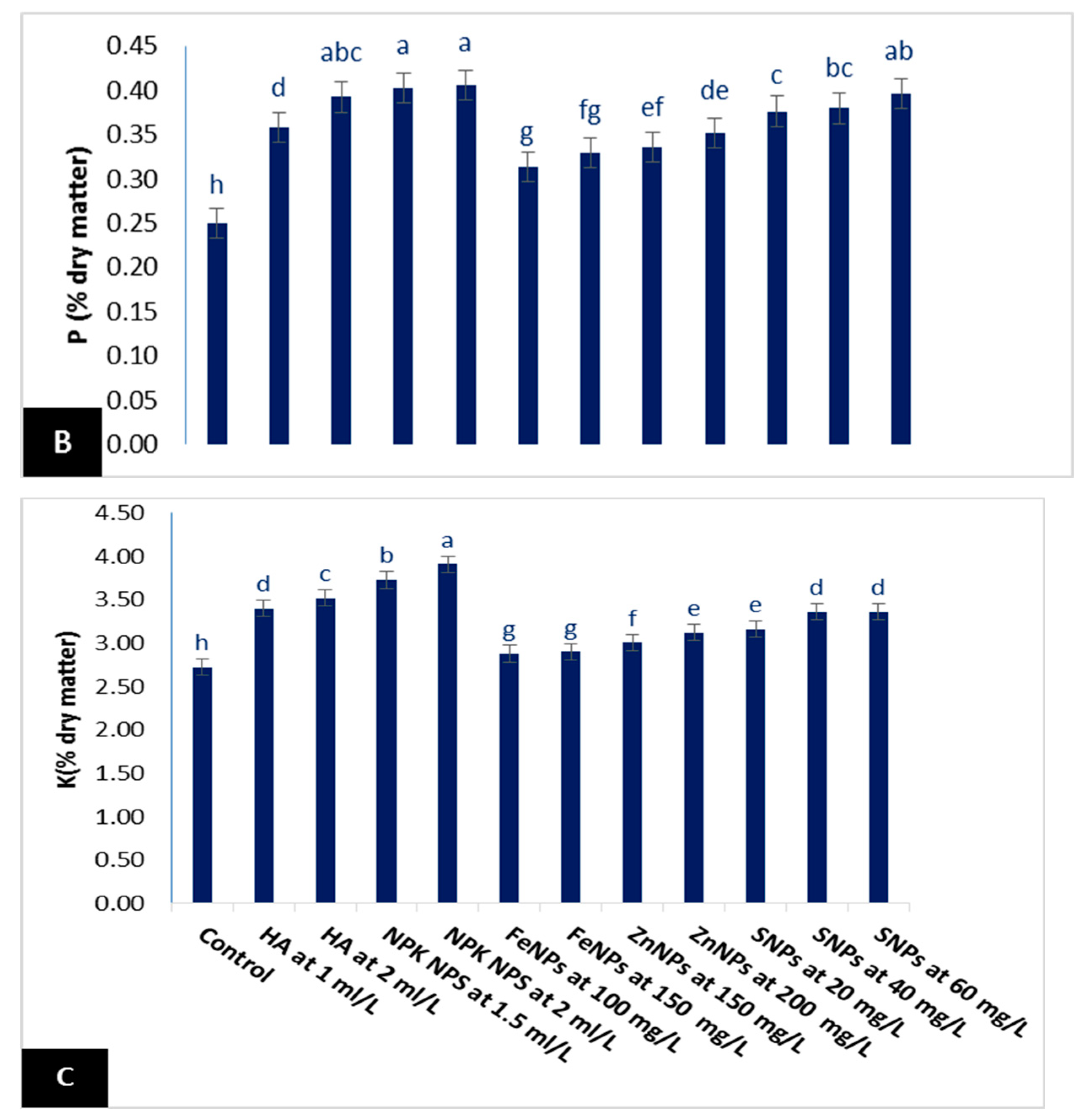
3.2.2. Mineral Contents
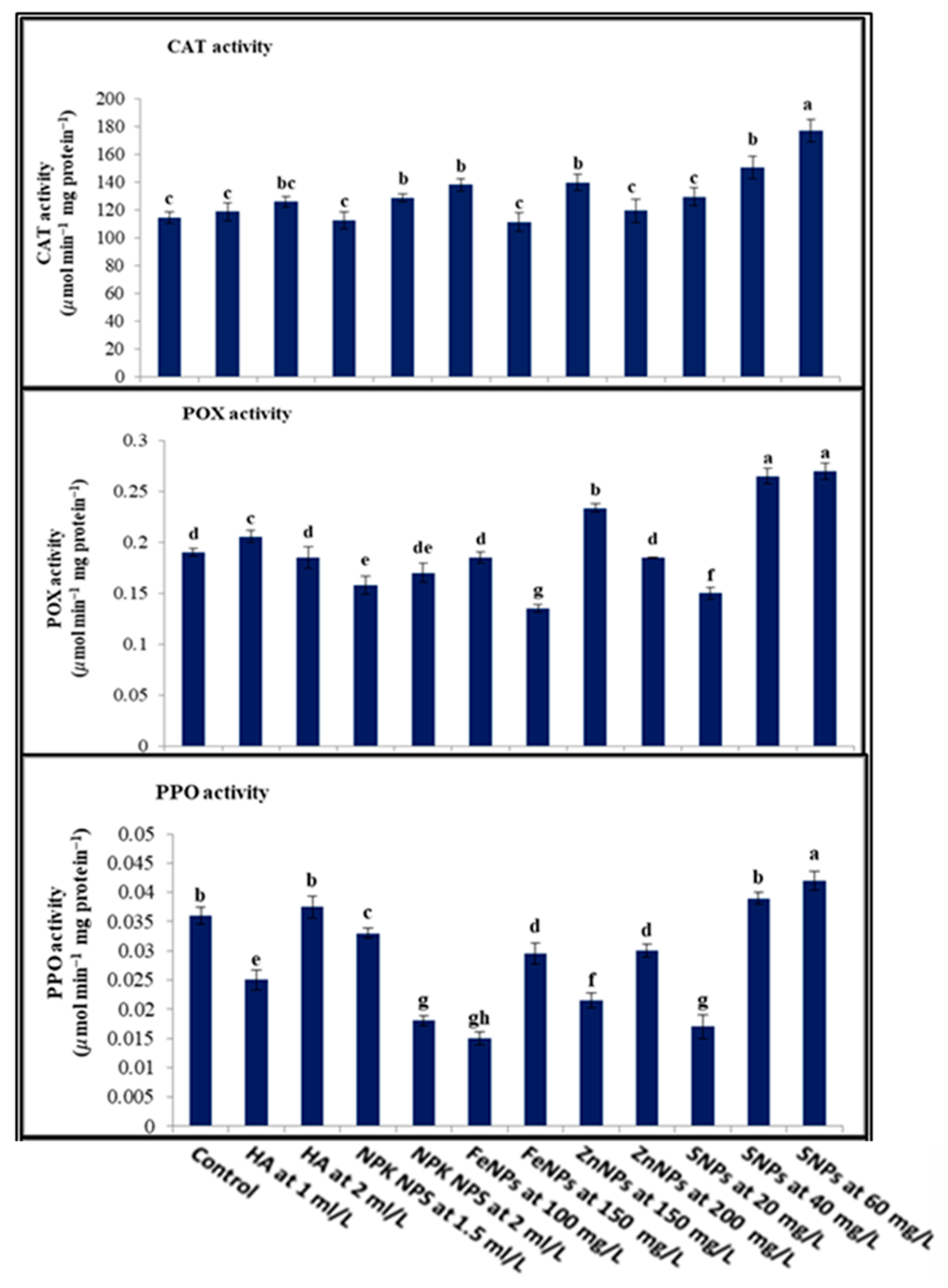
3.2.3. Enzymes Activities
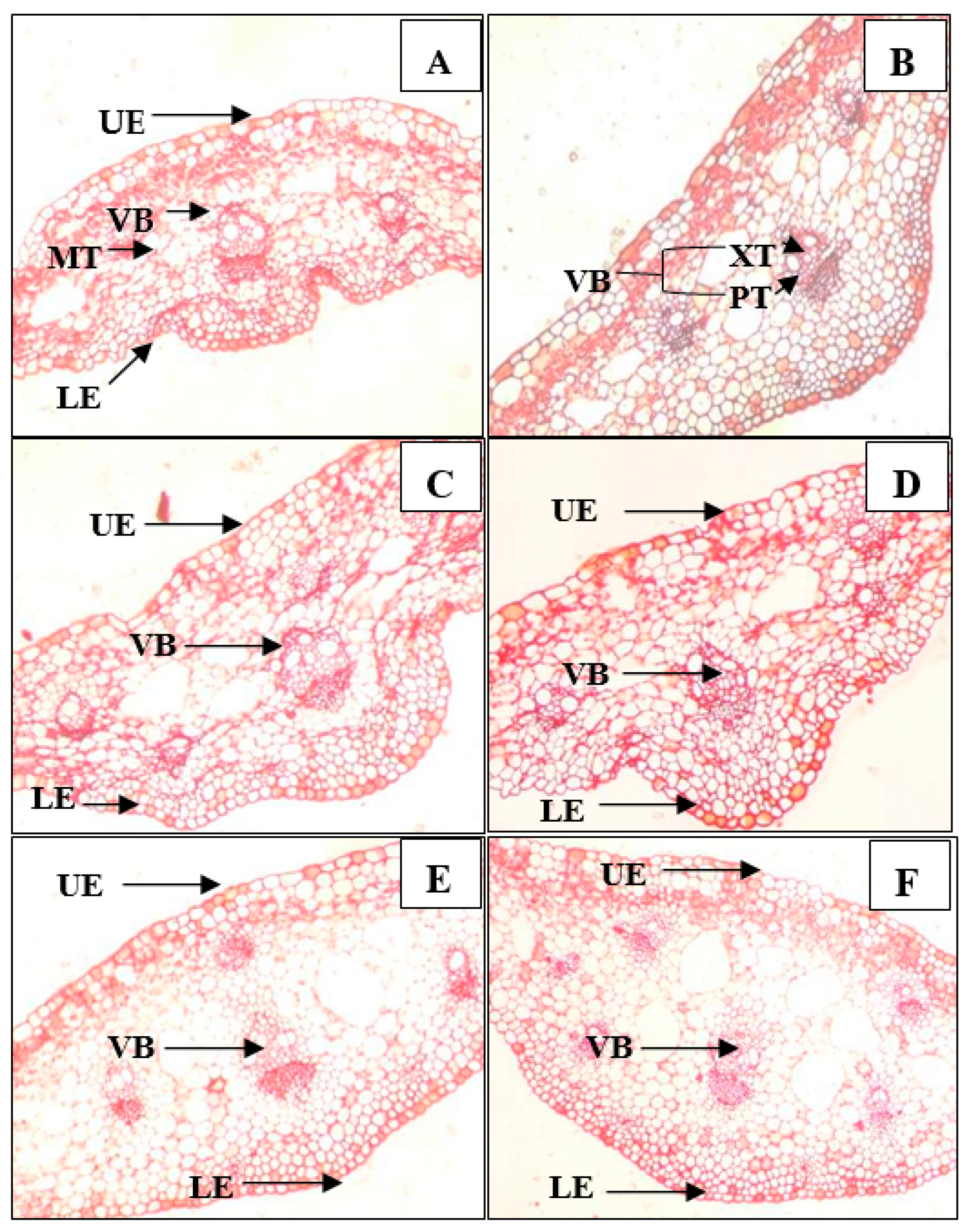
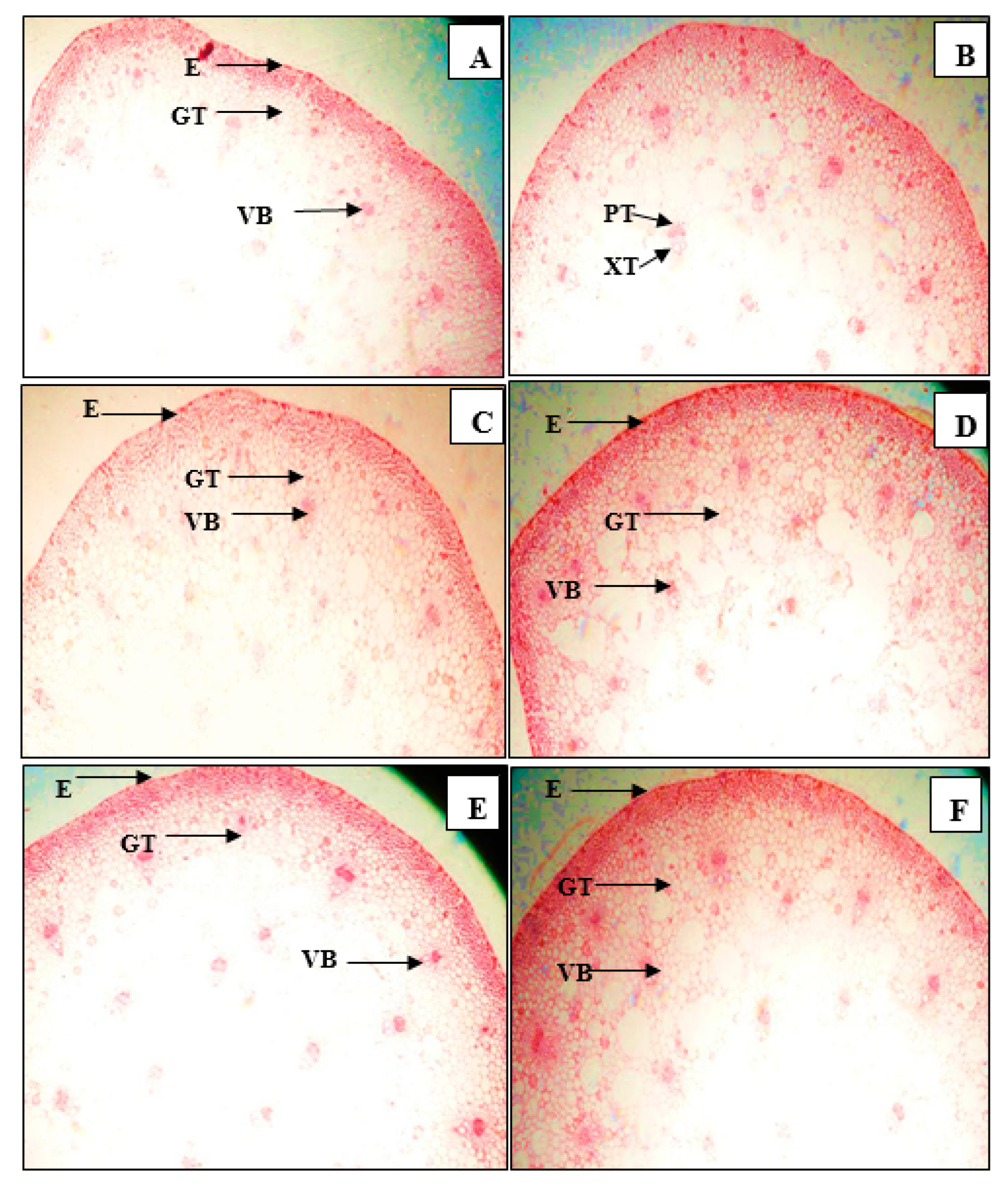
3.3. Anatomical Structure of Leaves and Stems
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salachna, P.; Byczy, A.Z.; Zawadzi, A.; Piechocki, R.; Mizieli, M. Stimulatory effect of silver nanoparticles on the growth and flowering of potted oriental lilies. Agronomy 2019, 9, 610. [Google Scholar] [CrossRef]
- Manzoor, A.; Bashir, M.A.; Hashmi, M.M. Nanoparticles as a preservative solution can enhance postharvest attributes of cut flowers. Italus Hortus 2020, 27, 1–14. [Google Scholar] [CrossRef]
- Kamiab, F.; Fahreji, S.S.; Bahramabadi, E.Z. Antimicrobial and Physiological Effects of Silver and Silicon Nanoparticles on Vase Life of Lisianthus (Eustoma grandiflora cv. Echo) Flowers. Int. J. Hort. Sci. Technol. 2017, 4, 135–144. [Google Scholar] [CrossRef]
- Ali, S.; Mehmood, A.; Khan, N. Uptake, translocation, and consequences of nanomaterials on plant growth and stress adaptation. J. Nanomater. 2021, 2021, 6677616. [Google Scholar] [CrossRef]
- Harsinia, M.G.; Habibib, H.; Talaeic, G.H. Study the effects of iron nano chelated fertilizers foliar application on yield and yield components of new line of wheat cold region of kermanshah provence. Agric. Adv. 2014, 3, 95–102. [Google Scholar] [CrossRef]
- Salama, H.M.H. Effects of silver nanoparticles in some crop plants, Common bean (Phaseolus vulgaris L.) and corn (Zea mays L.). Int. Res. J. Biotechnol. 2012, 3, 190–197. [Google Scholar]
- Zaghloul, M.; El-Fattah, Y.M.A.; Mohamed, M.; Khedr, M.M.; Elsadek, M.A. Effect of different ratios nano-Fertilizer and gibberellic acid on the vegetative growth and chemical compositions of Codiaeum variegatum (L.) cv. Gold Dust. Hort. J. Suez Canal Univ. 2020, 9, 31–44. [Google Scholar]
- El-Hefnawy, S.F.M. Nano NPK and growth regulator promoting changes in growth and mitotic index of pea plants under salinity stress. J. Agric. Chem. Biotechnol. Mansoura Univ. 2020, 11, 263–269. [Google Scholar] [CrossRef]
- Ekinci, M.; Dursun, A.; Yildirim, E.; Parlakova, F. Effects of nanotechnology liquid fertilizers on the plant growth and yield of cucumber (Cucumis sativus L.). Acta Sci. Pol. Hortorum Cultus 2014, 13, 135–141. [Google Scholar]
- Alloway, B.J. Soil factors associated with zinc deficiency in crops and humans. Environ. Geochem. Health 2009, 31, 537–554. [Google Scholar] [CrossRef]
- Wannoussa, W.; Masy, T.; Lambert, S.D.; Heinrichs, B.; Tasseroul, L.; Al-Ahmad, A.; Weekers, F.; Thonart, P.; Hiligsmann, S. Effect of Iron Nanoparticles Synthesized by a Sol-Gel Process on Rhodococcus erythropolis T902.1 for Biphenyl Degradation. J. Water Resour. Prot. 2005, 7, 264–277. [Google Scholar] [CrossRef]
- Elfeky, S.A.; Mohammed, M.A.; Khater, M.S.; Osman, Y.A.H.; Elsherbini, E. Effect of magnetite Nano-Fertilizer on Growth and yield of Ocimum basilicum L. Int. J. Indig. Med. Plants 2013, 46, 1286–1293. [Google Scholar]
- Brittenham, G.M. New advances in iron metabolism, iron deficiency, and iron overload. Curr. Opin. Hematol. 1994, 1, 101–106. [Google Scholar]
- Rui1, M.; Ma, C.; Hao, Y.; Guo, J.; Rui, Y.; Tang, X.; Zhao, Q.; Fan, X.; Zhang, Z.; Hou, T.; et al. Iron oxide nanoparticles as a potential iron fertilizer for peanut (Arachis hypogaea). Front. Plant Sci. 2016, 7, 815. [Google Scholar] [CrossRef]
- Li, J.; Chang, P.R.; Huang, J.; Wang, Y.; Yuan, H.; Ren, H. Physiological effects of magnetic iron oxide nanoparticles towards watermelon. J. Nanosci. Nanotechnol. 2013, 13, 5561–5567. [Google Scholar] [CrossRef] [PubMed]
- El-Desouky Heba, S.; Islam, K.R.; Bergefurd, B.; Gary, G.; Harker, T.; Abd-El-Dayem, H.; Ismail, F.; Mady, M.; Zewail, R.M.Y. Nano iron fertilization significantly increases tomato yield by increasing plants’ vegetable growth and photosyntciency. J. Plant Nutr. 2021, 44, 1649–1663. [Google Scholar] [CrossRef]
- Cakmak, I. Enrichment of cereal grains with zinc: Agronomic or genetic biofortification? Plant Soil 2008, 302, 1–17. [Google Scholar] [CrossRef]
- Hafez, Y.; Elkohby, W.; Mazrou, Y.S.A.; Ghazy, M.; Elgamal, A.; Abdelaal, K.A. Alleviating the detrimental impacts of salt stress on morpho-hpysiological and yield characters of rice plants (Oryza sativa L.) using actosol, Nano-Zn and Nano-Si. Fresenius Environ. Bull. 2020, 29, 6882–6897. [Google Scholar]
- Khan, H.R.; McDonald, G.K.; Rengel, Z. Zinc fertilization and water stress affects plant water relations, stomatal conductance and osmotic adjustment in chickpea (Cicer arientinum L.). Plant Soil 2004, 267, 271–284. [Google Scholar] [CrossRef]
- Ghidan, A.Y.; Al Antary, T.M. Applications of Nanotechnology in Agriculture. In Applications of Nanobiotechnology; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Alabdallah, N.M.; Alzahrani, H.S. Impact of ZnO nanoparticles on growth of cowpea and okra plants under salt stress conditions. Biosci. Biotechnol. Res. Asia 2020, 17, 329–340. [Google Scholar] [CrossRef]
- Hatami, M.; Ghorbanpour, M. Effect of nanosilver on physiological performance of pelargonium plants exposed to dark storage. J. Horti. Res. 2013, 21, 15–20. [Google Scholar] [CrossRef]
- Lü, P.; Caoa, J.; Hea, S.; Liua, J.; Li, H.; Chenga, G.; Dinga, Y.; Joycec, D.C. Nano-silver pulse treatments improve water relations of cut rose cv. Movie Star flowers. Postharvest Biol. Technol. 2010, 57, 196–202. [Google Scholar] [CrossRef]
- Lok, C.N.; Ho, C.M.; Chen, R.; He, Q.Y.; Yu, W.Y.; Sun, H.; Tam, P.K.H.; Chiu, J.F.; Ch, C.M. Silver nanoparticles: Partial oxidation and antibacterial activities. J. Biol. Inorg. Chem. 2007, 12, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Abou-Attia, F.A.M.; Abdelaal, K.A.A. Effect of Bio and Mineral fertilization on the main insect pests and some characters of sugar beet plants. J. Agric. Sci. Mansoura Univ. 2007, 32, 1471–1485. [Google Scholar] [CrossRef]
- Abdelaal, K.A.A.; Badawy, S.A.; Abdel Aziz, R.M.; Neana, S.M.M. Effect of mineral nitrogen levels and biofertilizer on morphophysiological characters of three sweet sorghum varieties (Sorghum bicolor L. Moench). J. Plant Prod. Mansoura Univ. 2015, 6, 189–203. [Google Scholar]
- Abdelaal, K.A.A.; Tawfik, S.F. Response of Sugar Beet Plant (Beta vulgaris L.) to Mineral Nitrogen Fertilization and Bio-Fertilizers. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 677–688. [Google Scholar]
- Abdelaal, K.A. Pivotal Role of Bio and Mineral Fertilizer Combinations on Morphological, Anatomical and Yield Characters of Sugar Beet Plant (Beta vulgaris L.). Middle East J. Agric. Res. 2015, 4, 717–734. [Google Scholar]
- Mohamed, A.A.; Mazrou, Y.; El-Henawy, A.S.; Awad, N.M.; Abdelaal, K.; Hafez, Y.; Hantash, R.M.A. Effect of sulfur applied and foliar spray of yeast on growth and yield of sugar beet under irrigation deprivation conditions. Fresenius Environ. Bull. 2022, 31, 4199–4209. [Google Scholar]
- Hayes, M.; Malcolm, R.L. Considerations of Compositions and Aspects of the Structures of Humic Substances. In Humic Substances and Chemical Contaminats; Clapp, C.E., Hayes, M.H.B., Senesi, N., Bloom, P.R., Jardine, P.M., Eds.; Soil Science Society of America, Inc.: Madison, WI, USA, 2001; pp. 3–40. [Google Scholar] [CrossRef]
- MacCarthy, P.; Clapp, C.E.; Malcolm, R.L.; Bloom, P.R. Humic Substances in Soil and Crop Sciences: An Overview. In Humic Substances in Soil and Crop Sciences: Selected Readings; American Society of Agronomy, Crop Science Society of America, Soil Science Society of America: Madison, WI, USA, 1990; pp. 1–12. [Google Scholar] [CrossRef]
- Cavalcante, Í.H.L.; Silva-Matos, R.R.S.; Albano, F.G.; Silva, G.B., Jr.; Silva, A.M.; Costa, L.S. Foliar spray of humic substances on seedling production of yellow passion fruit. J. Food Agric. Environ. 2013, 11, 301–304. [Google Scholar]
- Piccolo, A.; Nardi, S.; Concheri, G. Structural characteristics of humic substances as regulated tonitrate uptake and growth regulation in plant systems. Soil Biol. Biochem. 1992, 24, 373–380. [Google Scholar] [CrossRef]
- Hebeish, A.; El-Rafie, M.H.; El-Sheikh, M.A.; El-Naggar, M.E. Nanostructural features of SNP powder synthesized through concurrent formation of the nanosized particles of both starch and silver. J. Nanotechnol. 2013, 2013, 201057. [Google Scholar] [CrossRef]
- Matthew, E.O.; Douglas, A.L.; Isaacs, R. An inexpensive accurate method for measuring leaf area and defoliation through digital image analysis. J. Econ. Entomol. 2002, 95, 1190–1194. [Google Scholar]
- Allel, D.; Ben-Amar, A.; Abdelly, C. Leaf photosynthesis, chlorophyll fluorescence and ion content of barley (Hordeum vulgare) in response to salinity. J. Plant Nutr. 2017, 41, 497–508. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Hammerschmidt, R.; Nuckles, E.M.; Kuc, J. Association of enhanced peroxidase activity with induced systemic resistance ofcucumber to Colletotrichum lagenarium. Physiol. Plant Pathol. 1982, 20, 73–82. [Google Scholar] [CrossRef]
- Malik, C.P.; Singh, M.B. Plant Enzymology and Histoenzymology; Kalyani Publishers: Delhi, India, 1980; pp. 54–56. [Google Scholar]
- Association of Official Analytical Chemists. Official Methods of Analysis of the Association of Official Agricultural Chemists, 16th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1995. [Google Scholar]
- Jackson, M.B. Interactions between nitrogen and growth regulators in the control of plant development. In Proceedings of a Meeting/Organized by the British Plant Growth Regulator Group and the Agriculture Group of the Society of Chemical Industry; The SCI Lecture Theatre, Belgrave Square: London, UK, 1983; p. 110. [Google Scholar]
- Page, A.L.; Miller, R.H.; Keeney, D.R. Methods of Soil Analysis. Part 2: Chemical and Microbiological Properties, 2nd ed.; American Society of Agronomy: Madison, WI, USA, 1982. [Google Scholar]
- Gutmann, M. Improved staining procedures for photographic documentation of phenolic deposits in semi thin sections of plant tissue. J. Microsc. 1995, 179, 277–281. [Google Scholar] [CrossRef]
- Ruzin, S.E. Plant Micro Techniques and Microscopy, 1st ed.; Oxford University Press: Oxford, MS, USA, 1999; p. 322. [Google Scholar]
- Duncan, B.D. Multiple ranges and multiple F-test. Biometria 1955, 11, 1–42. [Google Scholar] [CrossRef]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research, 2nd ed.; Wiley Inter Science: New York, NY, USA, 1984; pp. 1–690. [Google Scholar]
- Ghorbanpour, M.; Hatami, M. Changes in growth, antioxidant defense system and major essential oils constituents of Pelargonium graveolens plant exposed to nano-scale silver and thidiazuron. Indian J. Plant Physiol. 2015, 20, 116–123. [Google Scholar] [CrossRef]
- Sadak, M.S. Impact of silver nanoparticles on plant growth, some biochemical aspects, and yield of fenugreek plant (Trigonella foenumgraecum). Bull. Natl. Res. Cent. 2019, 43, 38. [Google Scholar] [CrossRef]
- Guzman-Baez, G.A.; Trejo-Tellez, L.I.; Ramırez-Olvera, M.; Salinas-Ruız, J.; Bello-Bello, J.J.; Alcantar-Gonzalez, G.; Hidalgo-Contreras, J.V.; Gómez-Merino, F.C. Silver nanoparticles increase nitrogen, phosphorus, and potassium concentrations in leaves and stimulate root length and number of roots in tomato seedlings in a hormetic manner. Dose-Response Int. J. 2021, 19, 1–15. [Google Scholar] [CrossRef]
- Kedziora, A.; Speruda, M.; Krzy’zewska, E.; Rybka, J.; Łukowiak, A.; Bugla-Płosko, G. Similarities and Differences between Silver Ions and Silver in Nanoforms as Antibacterial Agents. Int. J. Mol. Sci. 2018, 19, 444. [Google Scholar] [CrossRef] [PubMed]
- Syu, Y.-Y.; Hung, J.-H.; Chen, J.-C.; Chuang, H.-W. Impacts of size and shape of silver nanoparticles on Arabidopsis plant growth and gene expression. Plant Physiol. Biochem. 2014, 83, 57–64. [Google Scholar] [CrossRef] [PubMed]
- AL-Gym, A.J.K.; Al-Asady, M.H.S. Effect of the method and level of adding NPK nanoparticles and mineral fertilizers on the growth and yield of yellow corn and the content of mineral nutrient of some plant parts. Plant Arch. 2020, 20, 38–43. [Google Scholar]
- Mahmoud, A.W.M.; Swaefy, H.M. Comparison between commercial and nano NPK in presence of nano zeolite on sage plant yield and its components under water stress. Agriculture 2020, 66, 24–39. [Google Scholar] [CrossRef]
- Sun, H.; Tao, J.; Liu, S.; Huang, S.; Chen, S.; Xie, X.; Yoneyama, K.; Zhang, Y.; Xu, G. Strigolactones are involved in phosphate- and nitrate-deficiency-induced root development and auxin transport in rice. J. Exp. Bot. 2014, 65, 6735–6746. [Google Scholar] [CrossRef]
- Xu, J.; Zha, M.; Li, Y.; Ding, Y.; Chen, L.; Ding, C.; Wang, S. The interaction between nitrogen availability and auxin, cytokinin, and strigolactone in the control of shoot branching in rice (Oryza sativa L.). Plant Cell Rep. 2015, 34, 1647–1662. [Google Scholar] [CrossRef]
- Elkhoby, W.M.; El-khtyar, A.M.; Hassan, H.M.; Mikhael, B.B.; Abdelaal, K.A.A. Effect of spilt application of nitrogen fertilizer on morpho-physiological attributes and grain yield of broadcast seeded Egyptian hybrid rice (1). J. Plant Prod. Mansoura Univ. 2013, 4, 1259–1280. [Google Scholar]
- Omar, A.; AboYoussef, M.; Shoughy, A.; Abd El-Aty, M.S.; Abdelaal, K.; Hafez, Y.; Kamara, M. Response of Egyptian Yasmin rice cultivar to different seeding number per hill and different nitrogen levees. Fresenius Environ. Bull. 2022, 31, 1258–1265. [Google Scholar]
- Hafez, E.M.; Abdelaal, K.A.A. Impact of Nitrogen fertilization levels on morphophysiological characters and yield quality of some Maize hybrids (Zea mays L.). Egyption J. Agron. 2015, 37, 35–48. [Google Scholar]
- Majid, M.A.; Saiful Islam, M.; EL Sabagh, A.; Hasan, M.K.; Saddam, M.O.; Barutcular, C.; Ratnasekera, D.; Abdelaal, K.A.A.; Islam, M.S. Influence of varying nitrogen levels on growth, yield and nitrogen efficiency of hybrid maize (Zea mays). J. Exp. Biol. Agric. Sci. 2017, 5, 134–142. [Google Scholar]
- Abou Khadrah, S.; Gharib, H.S.; Mohamed, A.A.; Elhosary, M.A.; Abdelaal, K.A.; Hafez, Y.M. Combination of nitrogen and potassium fertilizers improve physiological and yield characters of two wheat cultivars. Fresenius Environ. Bull. 2020, 29, 8998–9004. [Google Scholar]
- Mosalem, M.; Mazrou, Y.; Badawy, S.; Abd Ullah, M.A.; Mubarak, M.G.; Hafez, Y.M.; Abdelaal, K.A. Evaluation of sowing methods and nitrogen levels for grain yield and components of durum wheat under arid regions of Egypt. Rom. Biotechnol. Lett. 2021, 26, 3031–3039. [Google Scholar] [CrossRef]
- Zalat, S.S.; EL-Sayed, A.A.; Elkhoby, R.A.; Hafez, Y.; Ali, E.; Abdelaal, K.A. Effect of method and time of micronutrients application on sugar beet productivity under two nitrogen fertilizer sources. Fresenius Environ. Bull. 2021, 30, 9135–9141. [Google Scholar]
- Apel, K.; Santel, H.-J.; Redlinger, T.E.; Falk, H. The protochlorophyllide holochrome of barley (Hordeum vulgare L.). Isolation and characterization of the NADPH protochlorophyllide oxidoreductase. Eur. J. Biol. Chem. 1980, 111, 251–258. [Google Scholar] [CrossRef]
- Ma, J.; Haldar, S.; Khan, M.A.; Sharma, S.D.; Merrick, W.C.; Elizabeth, C.; Theil, E.C.; Goss, D.J. Fe2þ binds iron responsive element-RNA, selectively changing protein-binding affinities and regulating mRNA repression and activation. Proc. Natl. Acad. Sci. USA 2012, 109, 8417–8422. [Google Scholar] [CrossRef]
- Briat, J.-F.; Curie, C.; Gaymard, F. Iron utilization and metabolism in plants. Curr. Opin. Plant Biol. 2009, 10, 276–282. [Google Scholar] [CrossRef]
- Dawa, K.K.; Zaghloul, M.M.; Ahmed, H.M.I.; Hamad, K.H.M. Impact of foliar application with iron, zinc, silicon nano particles and yeast on growth, yield and water use efficiency of tomato plants under water stress conditions. J. Plant Prod. Mansoura Univ. 2020, 11, 523–530. [Google Scholar] [CrossRef]
- Strader, L.C.; Beisner, E.R.; Bartel, B. Silver ions increase auxin efflux independently of effects on ethylene response. Plant Cell 2009, 21, 3585–3590. [Google Scholar] [CrossRef]
- Purvis, A.C. Sequence of chloroplast degreening in calamondin fruit as influenced by ethylene and AgNO3. Plant Physiol. 1980, 66, 624–627. [Google Scholar] [CrossRef]
- Anzoor, N.; Ahmed, T.; Noman, M.; Shahid, M.; Nazir, M.M.; Ali, L.; Alnusaire, T.S.; Li, B.; Rainer, S.R.; Gang, W.G. Iron oxide nanoparticles ameliorated the cadmium and salinity stresses in wheat plants, facilitating photosynthetic pigments and restricting cadmium uptake. Sci. Total Environ. 2021, 769, 145221. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Ali, B.; Adrees, M.; Arshad, M.; Hussain, A.; Rehman, M.Z.; Abdul Waris, A. Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere 2019, 214, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Munir, T.; Rizwan, M.; Kashif, M.; Shahzad, A.; Ali, S.; Amin, N.; Zahid, R.; Alam, M.F.E.; Imran, M. Effect of zinc oxide nanoparticles on the growth and Zn uptake in wheat (Triticum aestivum L.) by seed priming method. Dig. J. Nanomater. Biostruct. 2018, 13, 315–323. [Google Scholar]
- Liu, X.; Wang, F.; Shi, Z.; Tong, R.; Shi, X. Bioavailability of Zn in ZnO nanoparticle-spiked soil and the implications to maize plants. J. Nanopart. Res. 2015, 17, 175. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C.; Clark, R.B. Micronutrients in crop production. Adv. Agron. 2002, 77, 185–268. [Google Scholar] [CrossRef]
- Tarafdar, J.C.; Sharma, S.; Raliya, R. Nanotechnology: Interdisciplinary science of applications. Afr. J. Biotechnol. 2013, 12, 219–226. [Google Scholar] [CrossRef]
- Solanki, P.; Bhargava, A.; Chhipa, H.; Jain, N.; Panwar, J. Nano-fertilizers and their smart delivery system. In Nanotechnologies in Food and Agriculture; Springer International Publishing: Cham, Switzerland, 2015; pp. 81–101. [Google Scholar] [CrossRef]
- Qureshi, A.; Singh, D.K.; Dwivedi, S. Nano-fertilizers: A novel way for enhancing nutrient use efficiency and crop productivity. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 3325–3335. [Google Scholar] [CrossRef]
- Wasaya, A.; Shabir, M.S.; Hussain, M.; Ansar, M.; Aziz, A.; Hassan, W.; Ahmad, I. Foliar application of zinc and boron improved the productivity and net returns of maize grown under rainfed conditions of Pothwar plateau. J. Soil Sci. Plant Nutr. 2017, 17, 33–45. [Google Scholar] [CrossRef]
- Ahmad, J.; Bashir, H.; Bagheri, R.; Baig, A.; Al-Huqail, A.; Ibrahim, M.M.; Qureshi, M.I. Drought and salinity induced changes inecophysiology and proteomic profile of Parthenium hysterophorus. PLoS ONE 2017, 12, e0185118. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Singh, U.; Bindraban, P.S.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Zinc oxide nanoparticles alleviate drought-induced alterations in sorghum performance, nutrient acquisition, and grain fortification. Sci. Total Environ. 2019, 688, 926–934. [Google Scholar] [CrossRef]
- Khan, I.; Raza, M.; Awan, S.A.; Khalid, M.H.; Raja, N.I.; Min, S.; Zhang, A.; Naeem, M.; Meraj, T.A.; Iqbal, N.; et al. In vitro effect of metallic silver nanoparticles (AgNPs): A novel approach toward the feasible production of biomass and natural antioxidants in pearl millet (Pennisetum glaucum L.). Appl. Ecol. Environ. Res. 2019, 17, 12877–12892. [Google Scholar] [CrossRef]
- Rashwan, E.A.A.; Abdelaal, K.A.A. Effect of Nano Zink-oxide foliar application on some flax cultivars under different irrigation treatments. Egypt. J. Plant Breed. 2019, 23, 119–145. [Google Scholar]
- Ragab, A.Y.; Geries, L.S.M.; Abdelaal, K.A.A.; Hanna, S.A. Growth and productivity of onion plant (Allium cepa L.) as affected by transplanting method and NPK fertilization. Fresenius Environ. Bull. 2019, 28, 7777–7786. [Google Scholar]
- Abdelaal, K.A.; Ragab, A.Y.; El-Hag, D.A.; Osman, M.F. Effect of nitrogen and potassium fertilization on productivity and quality sugar beet plants at North Delta Region, Egypt. Fresenius Environ. Bull. 2019, 28, 7833–7839. [Google Scholar]
- Abdelaal, K.A.; EL-Shawy, E.A.; Hafez, Y.M.; Abdel-Dayem, S.M.; Chidya, R.C.G.; Saneoka, H.; El Sabagh, A. Nano-Silver and non-traditional compounds mitigate the adverse effects of net blotch disease of barley in correlation with up-regulation of antioxidant enzymes. Pak. J. Bot. 2020, 52, 1065–1072. [Google Scholar] [CrossRef]
- Rashwan, E.; Alsohim, A.S.; El-Gammaal, A.; Hafez, Y.; Abdelaal, K.A.A. Foliar application of nano zink-oxide can alleviate the harmful effects of water deficit on some flax cultivars under drought conditions. Fresenius Environ. Bull. 2020, 29, 8889–8904. [Google Scholar]
- El-Flaah, R.F.; El-Said, R.A.R.; Nassar, M.A.; Hassan, M.; Abdelaal, K.A.A. Effect of rhizobium, nano silica and ascorbic acid on morpho-physiological characters and gene expression of POX and PPO in faba bean (Vicia faba L.) Under salinity stress conditions. Fresenius Environ. Bull. 2021, 30, 5751–5764. [Google Scholar]
| Treatments | Plant Height (cm) | Plant Width (cm) | Number of Leaves/Plant | |||
|---|---|---|---|---|---|---|
| 6 Months | 12 Months | 6 Months | 12 Months | 6 Months | 12 Months | |
| Control | 22.63 i | 37.62 f | 24.30 f | 28.67 g | 3.75 d | 6.42 g |
| HA at 1 mL/L | 29.17 defg | 46.25 cd | 30.38 cde | 44.33 cde | 4.17 bcd | 8.00 ef |
| HA at 2 mL/L | 29.42 def | 46.62 cd | 31.25 cde | 47.00 bcde | 4.25 bcd | 8.55 de |
| NPK NPs at 1.5 mL/L | 30.18 cde | 47.03 cd | 31.98 cd | 47.83 bcd | 4.42 abcd | 9.11 cd |
| NPK NPs at 2 mL/L | 32.63 bc | 50.24 bc | 35.73 bc | 51.41 abc | 4.67 ab | 9.67 bc |
| FeNPs at 100 mg/L | 25.97 h | 40.04 ef | 25.83 ef | 35.13 fg | 3.83 cd | 7.17 fg |
| FeNPs at 150 mg/L | 26.67 fgh | 44.93 cde | 30.27 cde | 41.50 def | 4.08 bcd | 7.58 f |
| ZnNPs at 150 mg/L | 26.29 gh | 43.92 de | 29.20 def | 39.21 ef | 3.83 cd | 7.25 fg |
| ZnNPs at 200 mg/L | 27.81 efgh | 45.49 cde | 31.08 cde | 46.68 bcde | 4.33 abcd | 7.67 ef |
| SNPs at 20 mg/L | 31.18 cd | 48.85 bcd | 35.38 bc | 51.33 abc | 4.50 abc | 9.22 cd |
| SNPs at 40 mg/L | 34.22 ab | 53.49 b | 38.33 b | 53.25 ab | 4.75 ab | 10.17 b |
| SNPs at 60 mg/L | 36.63 a | 60.75 a | 51.04 a | 59.25 a | 5.00 a | 11.67 a |
| Treatments | Length of Leaves Blade (cm) | Width of Leaves Blade (cm) | Length of Petiole (cm) | |||
|---|---|---|---|---|---|---|
| 6 Months | 12 Months | 6 Months | 12 Months | 6 Months | 12 Months | |
| Control | 7.67 f | 10.12 f | 6.31 e | 9.76 g | 15.63 f | 23.60 g |
| HA at 1 mL/L | 9.80 de | 13.09 cde | 8.03 d | 13.06 cdef | 18.94 cd | 29.55 de |
| HA at 2 mL/L | 9.99 d | 13.48 cde | 8.10 d | 13.35 cde | 19.68 cd | 31.25 cd |
| NPK NPs at 1.5 mL/L | 12.17 c | 14.12 bcd | 9.03 c | 13.47 cd | 20.45 c | 31.51 cd |
| NPK NPs at 2 mL/L | 13.49 bc | 14.38 bc | 9.89 c | 14.29 bc | 22.66 b | 33.08 c |
| FeNPs at 100 mg/L | 8.46 ef | 10.66 f | 7.18 de | 11.71 f | 15.70 f | 24.34 fg |
| FeNPs at 150 mg/L | 8.68 def | 11.48 ef | 7.64 d | 11.92 ef | 16.71 ef | 28.72 e |
| ZnNPs at 150 mg/L | 8.86 def | 12.08 def | 7.85 d | 12.32 def | 17.98 de | 26.06 f |
| ZnNPs at 200 mg/L | 9.50 de | 13.09 cde | 7.88 d | 12.94 cdef | 18.78 cd | 29.26 de |
| SNPs at 20 mg/L | 13.42 bc | 14.24 bc | 9.22 c | 14.20 bc | 20.75 c | 32.02 c |
| SNPs at 40 mg/L | 13.86 b | 16.14 b | 11.65 b | 15.27 b | 24.65 a | 36.63 b |
| SNPs at 60 mg/L | 15.66 a | 18.17 a | 13.19 a | 17.62 a | 26.51 a | 41.77 a |
| Treatments | Leaf Area (cm2) | Stem Diameter (cm) | Root Length (cm) |
|---|---|---|---|
| Control | 91.06 g | 1.14 g | 32.38 g |
| HA at 1 mL/L | 158.20 cde | 1.55 e | 44.00 cd |
| HA at 2 mL/L | 166.75 cd | 1.59 de | 45.25 c |
| NPK NPs at 1.5 mL/L | 173.18 c | 1.70 de | 46.75 c |
| NPK NPs at 2 mL/L | 187.34 c | 2.00 bc | 51.17 ab |
| FeNPs at 100 mg/L | 117.14 fg | 1.22 g | 34.92 g |
| FeNPs at 150 mg/L | 125.96 ef | 1.28 g | 35.50 fg |
| ZnNPs at 150 mg/L | 137.90 def | 1.32 fg | 39.00 ef |
| ZnNPs at 200 mg/L | 156.37 cde | 1.53 ef | 41.25 de |
| SNPs at 20 mg/L | 186.64 c | 1.80 cd | 47.67 bc |
| SNPs at 40 mg/L | 228.28 b | 2.21 ab | 54.08 a |
| SNPs at 60 mg/L | 295.22 a | 2.30 a | 54.33 a |
| Treatments | Fresh Weight (g/Plant) | Dry Weight (g/Plant) | ||||||
|---|---|---|---|---|---|---|---|---|
| Leaves | Stems | Roots | Total | Leaves | Stems | Roots | Total | |
| Control | 48.94 f | 14.92 f | 13.67 e | 77.47 f | 3.44 g | 0.81 f | 1.40 f | 5.66 h |
| HA at 1 mL/L | 65.50 de | 17.48 cd | 19.86 cd | 102.83 de | 5.01 def | 1.17 def | 2.50 cde | 8.83 def |
| HA at 2 mL/L | 71.61 cd | 18.98 c | 22.55 bc | 113.14 d | 5.75 cde | 1.21 cde | 2.60 bcde | 9.40 de |
| NPK NPs at 1.5 mL/L | 78.83 c | 27.25 b | 26.66 ab | 132.74 c | 6.30 cd | 1.25 cde | 2.79 bcd | 10.33 cd |
| NPK NPs at 2 mL/L | 92.67 b | 28.00 b | 28.42 a | 149.08 b | 6.91 bc | 1.53 bc | 3.08 bc | 11.32 c |
| FeNPs at 100 mg/L | 58.70 ef | 15.05 ef | 15.05 de | 89.61 ef | 3.86 fg | 0.97 ef | 1.71 ef | 6.54 gh |
| FeNPs at 150 mg/L | 63.86 de | 16.52 def | 16.19 de | 96.57 e | 4.84 ef | 1.00 ef | 2.07 cdef | 7.91 efg |
| ZnNPs at 150 mg/L | 59.51 ef | 15.97 def | 15.60 de | 90.27 ef | 4.82 ef | 0.99 ef | 1.76 def | 7.57 fg |
| ZnNPs at 200 mg/L | 65.10 de | 16.65 de | 17.90 cde | 99.65 e | 5.00 def | 1.07 def | 2.48 cde | 8.57 ef |
| SNPs at 20 mg/L | 92.04 b | 27.52 b | 27.17 ab | 146.72 b | 6.81 bc | 1.43 bcd | 2.85 bc | 11.29 c |
| SNPs at 40 mg/L | 97.33 b | 28.07 b | 28.80 a | 154.20 b | 7.79 ab | 1.70 b | 3.58 ab | 13.07 b |
| SNPs at 60 mg/L | 111.70 a | 34.267 a | 30.18 a | 176.15 a | 9.01 a | 2.19 a | 4.37 a | 15.57 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Shawa, G.M.R.; Alharbi, K.; AlKahtani, M.; AlHusnain, L.; Attia, K.A.; Abdelaal, K. Improving the Quality and Production of Philodendron Plants Using Nanoparticles and Humic Acid. Horticulturae 2022, 8, 678. https://doi.org/10.3390/horticulturae8080678
El-Shawa GMR, Alharbi K, AlKahtani M, AlHusnain L, Attia KA, Abdelaal K. Improving the Quality and Production of Philodendron Plants Using Nanoparticles and Humic Acid. Horticulturae. 2022; 8(8):678. https://doi.org/10.3390/horticulturae8080678
Chicago/Turabian StyleEl-Shawa, Ghada M. R., Khadiga Alharbi, Muneera AlKahtani, Latifa AlHusnain, Kotb A. Attia, and Khaled Abdelaal. 2022. "Improving the Quality and Production of Philodendron Plants Using Nanoparticles and Humic Acid" Horticulturae 8, no. 8: 678. https://doi.org/10.3390/horticulturae8080678
APA StyleEl-Shawa, G. M. R., Alharbi, K., AlKahtani, M., AlHusnain, L., Attia, K. A., & Abdelaal, K. (2022). Improving the Quality and Production of Philodendron Plants Using Nanoparticles and Humic Acid. Horticulturae, 8(8), 678. https://doi.org/10.3390/horticulturae8080678








