Cell Division Controls Final Fruit Size in Three Apple (Malus x domestica) Cultivars
Abstract
1. Introduction
2. Materials and Methods
2.1. Pollination
2.2. Harvest
2.3. RNA Isolation and qPCR Analyses
2.4. Histology Slide Preparation and Cell Area Measurement
2.5. Phylogenetic Analysis
2.6. Statistical Analysis
3. Results
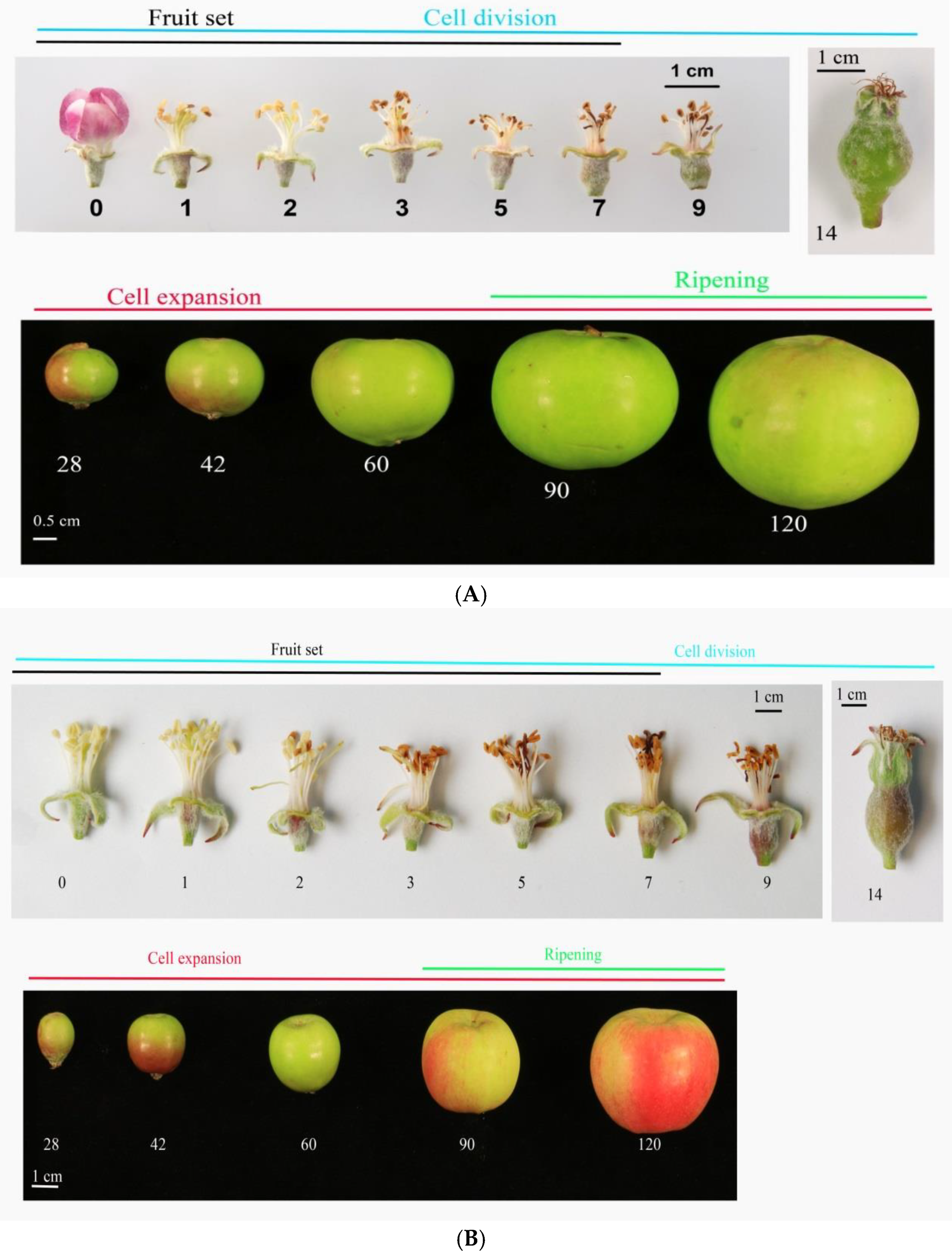
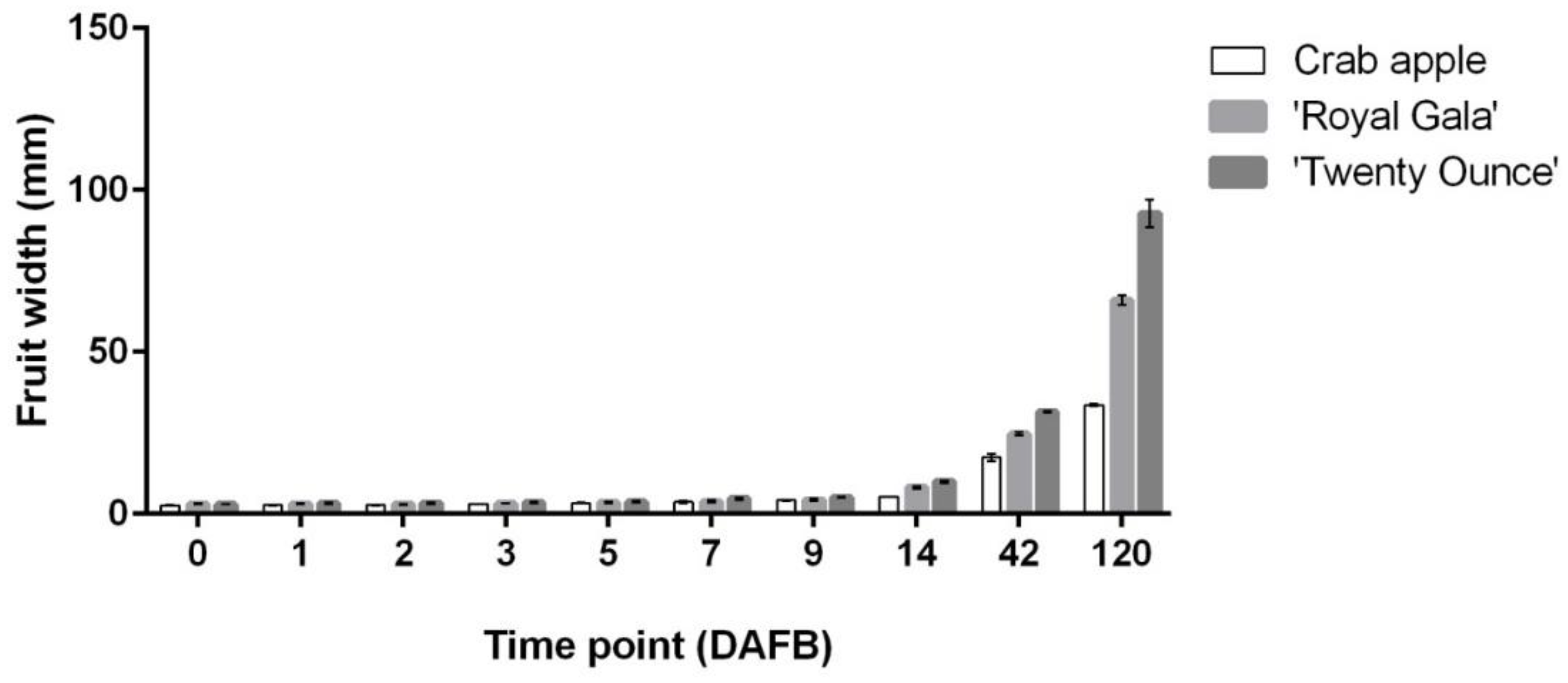
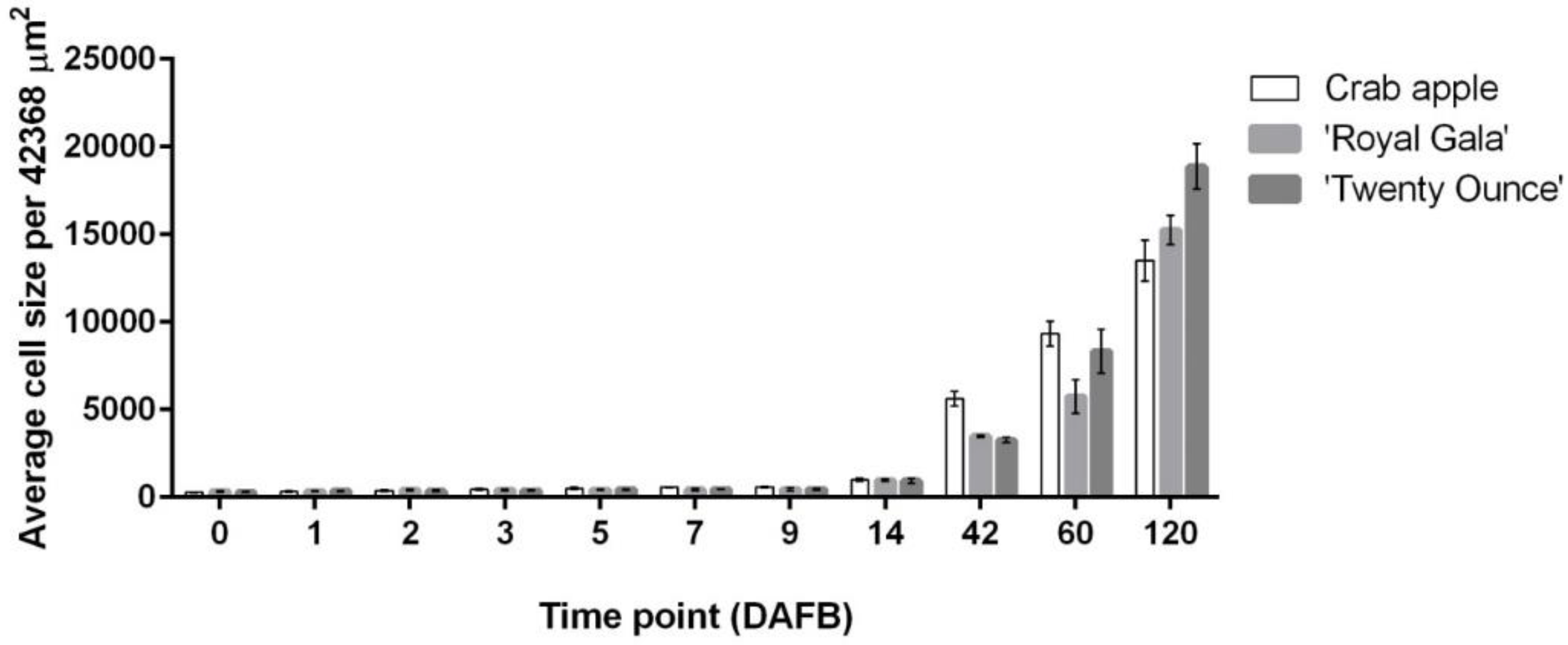
3.1. Cell Coverage Area at before Full Bloom and at Maturity
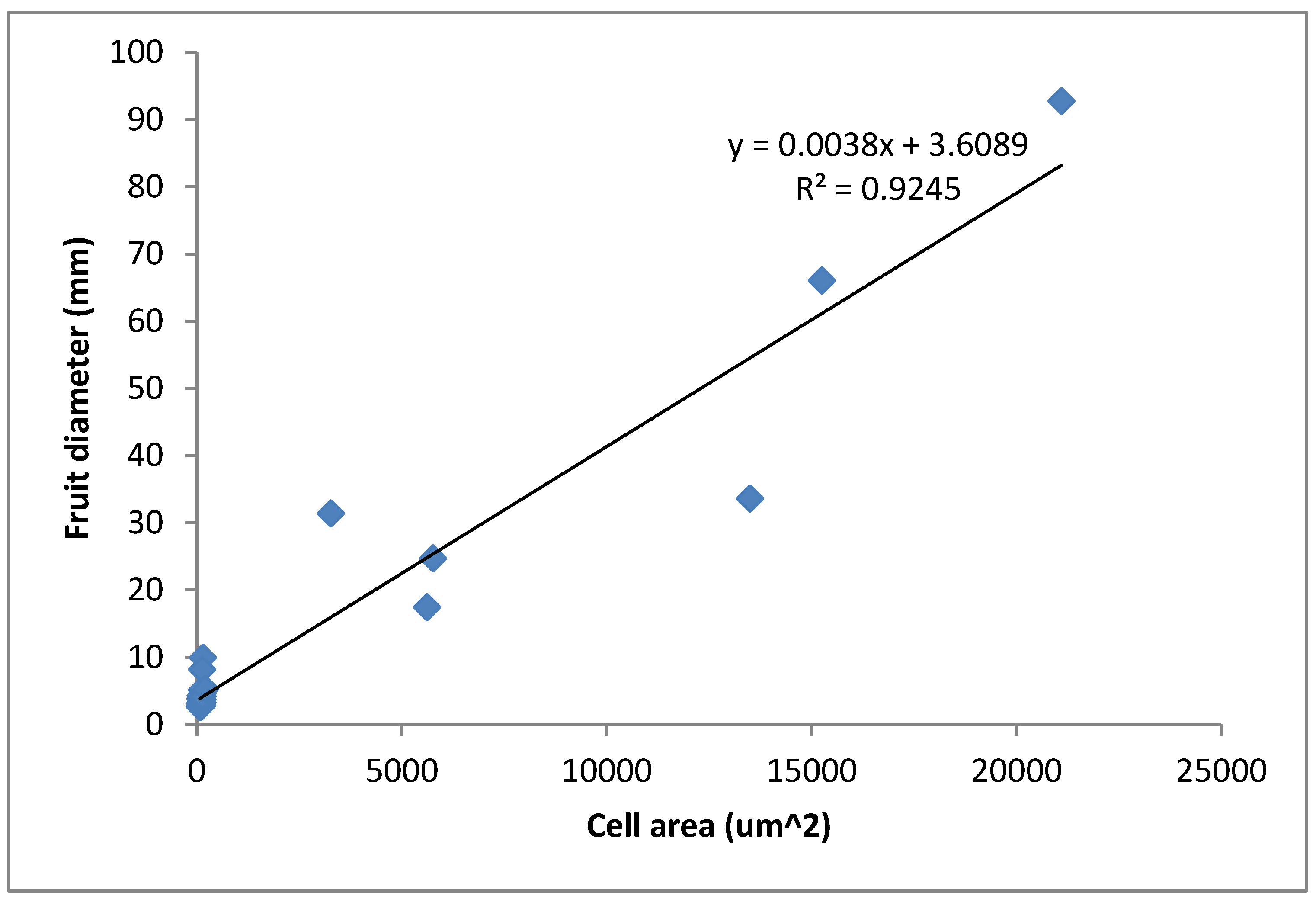
3.2. Relationship between Fruit Diameter, Cell Area, and Cell Number in Three Apple Cultivars
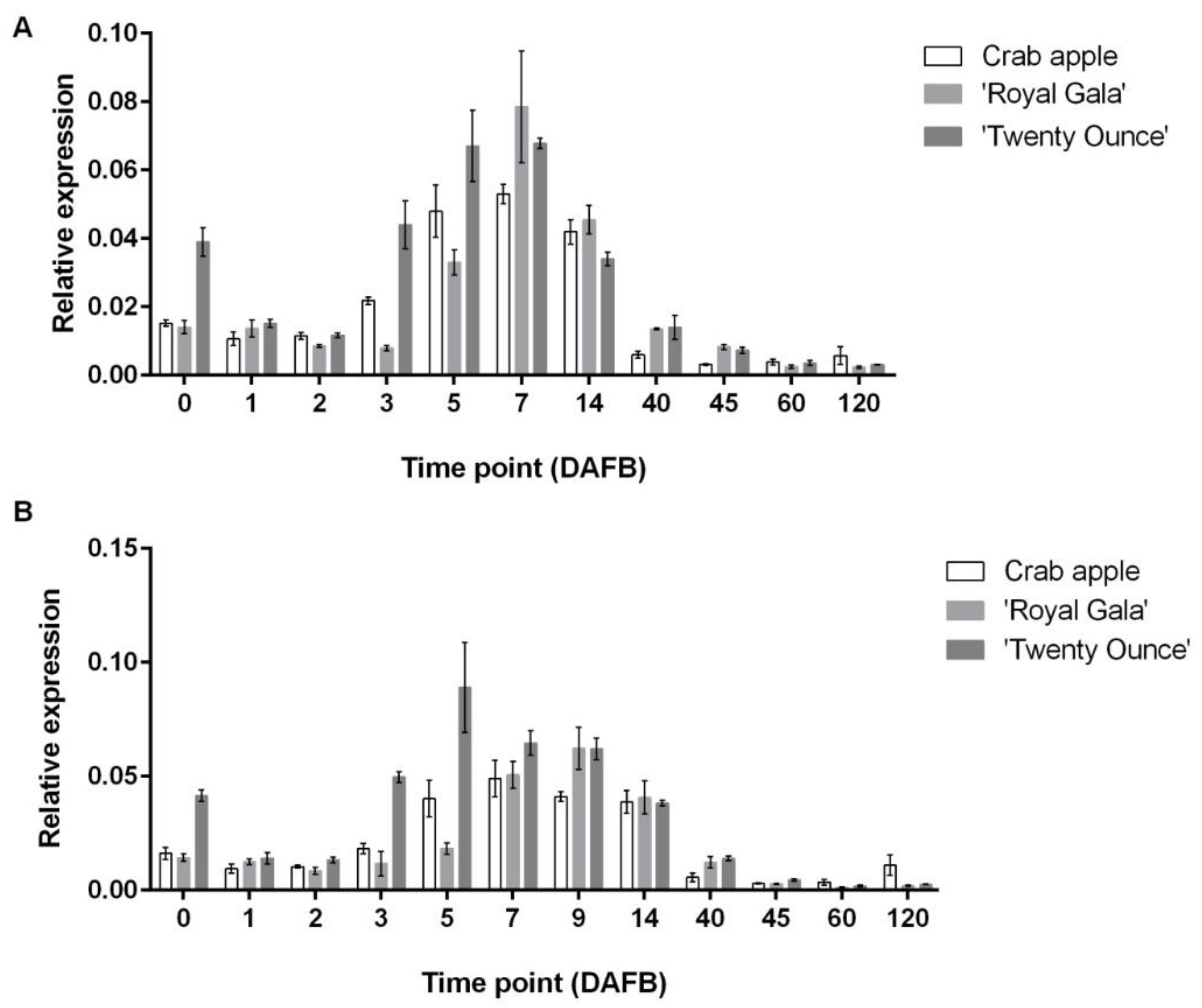
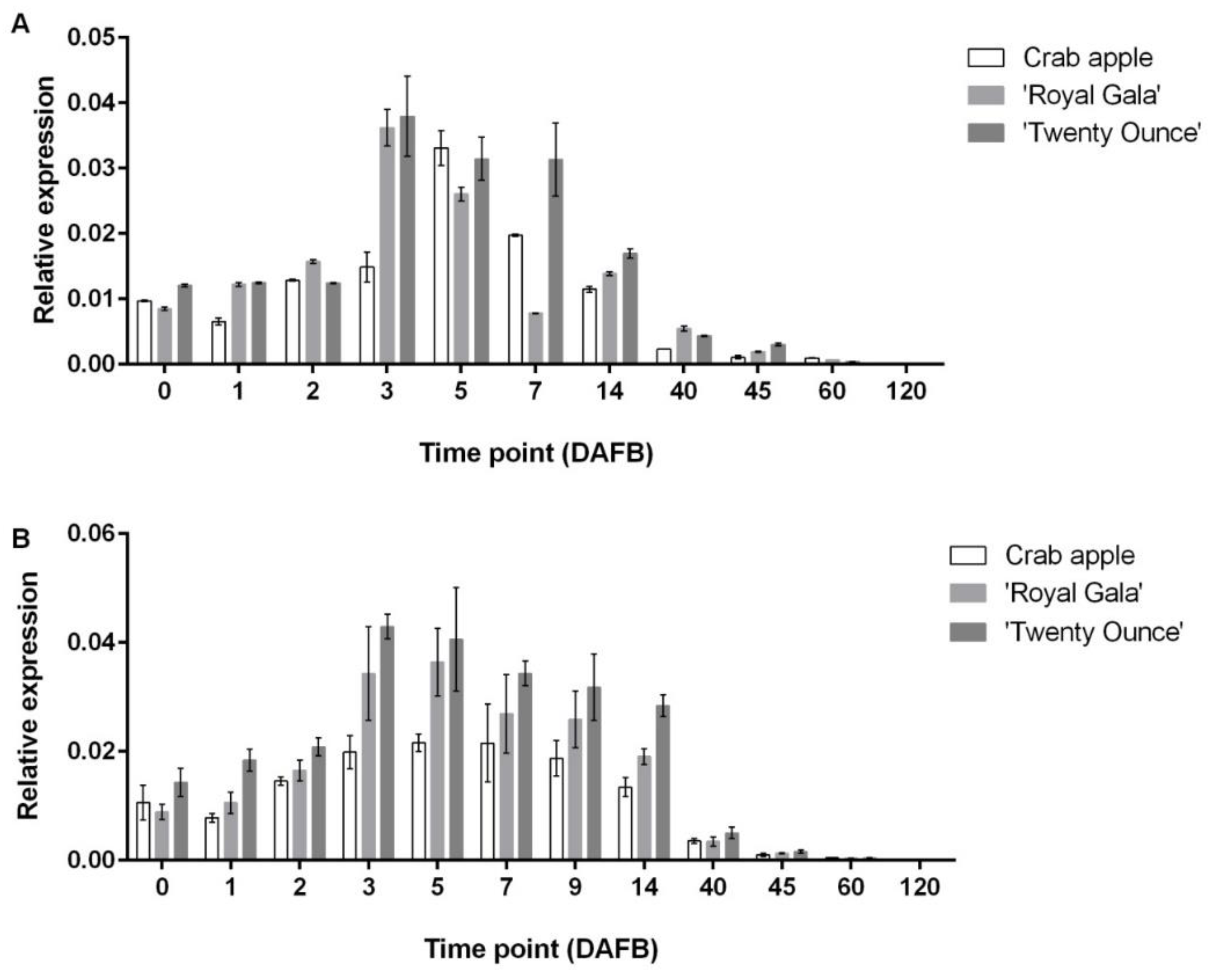
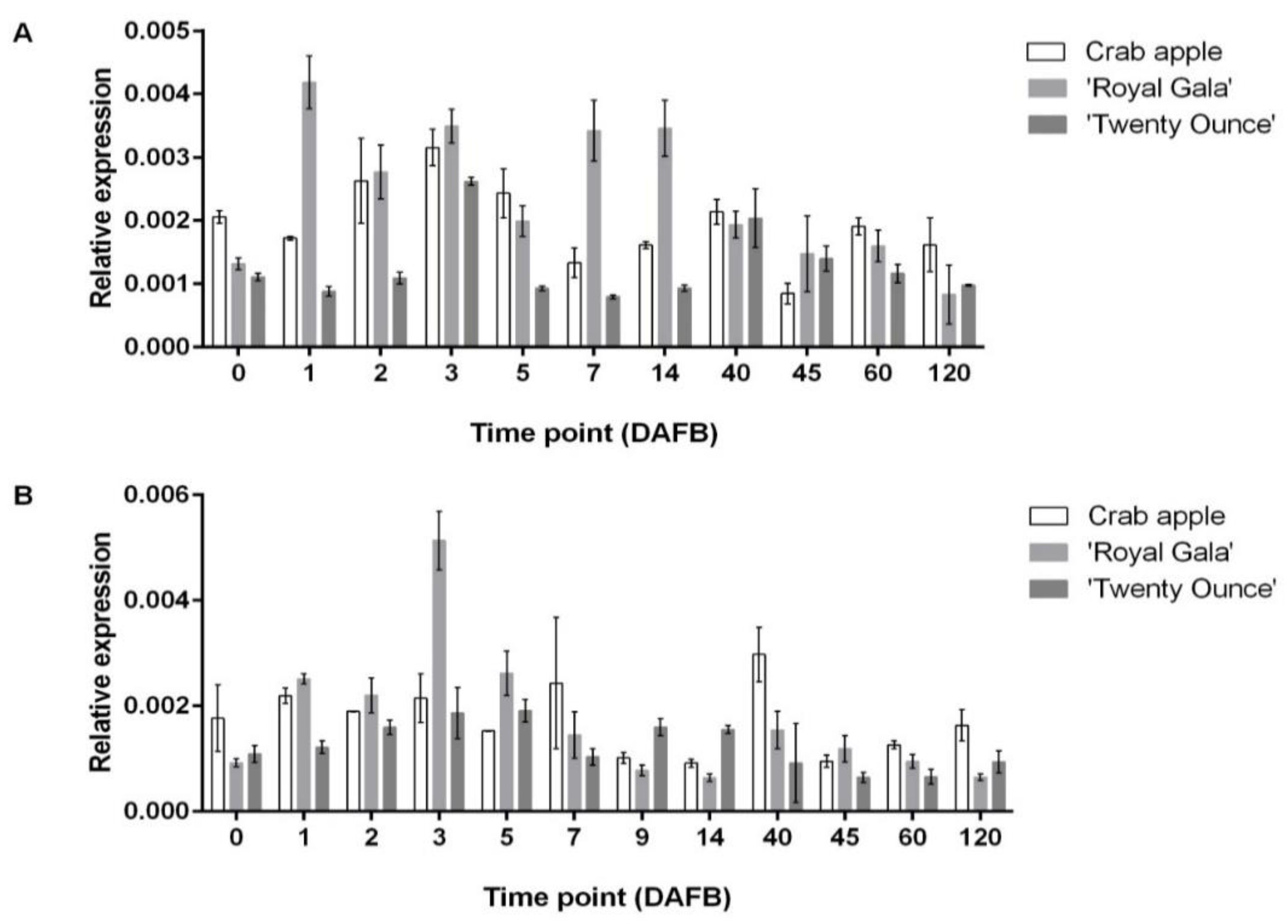
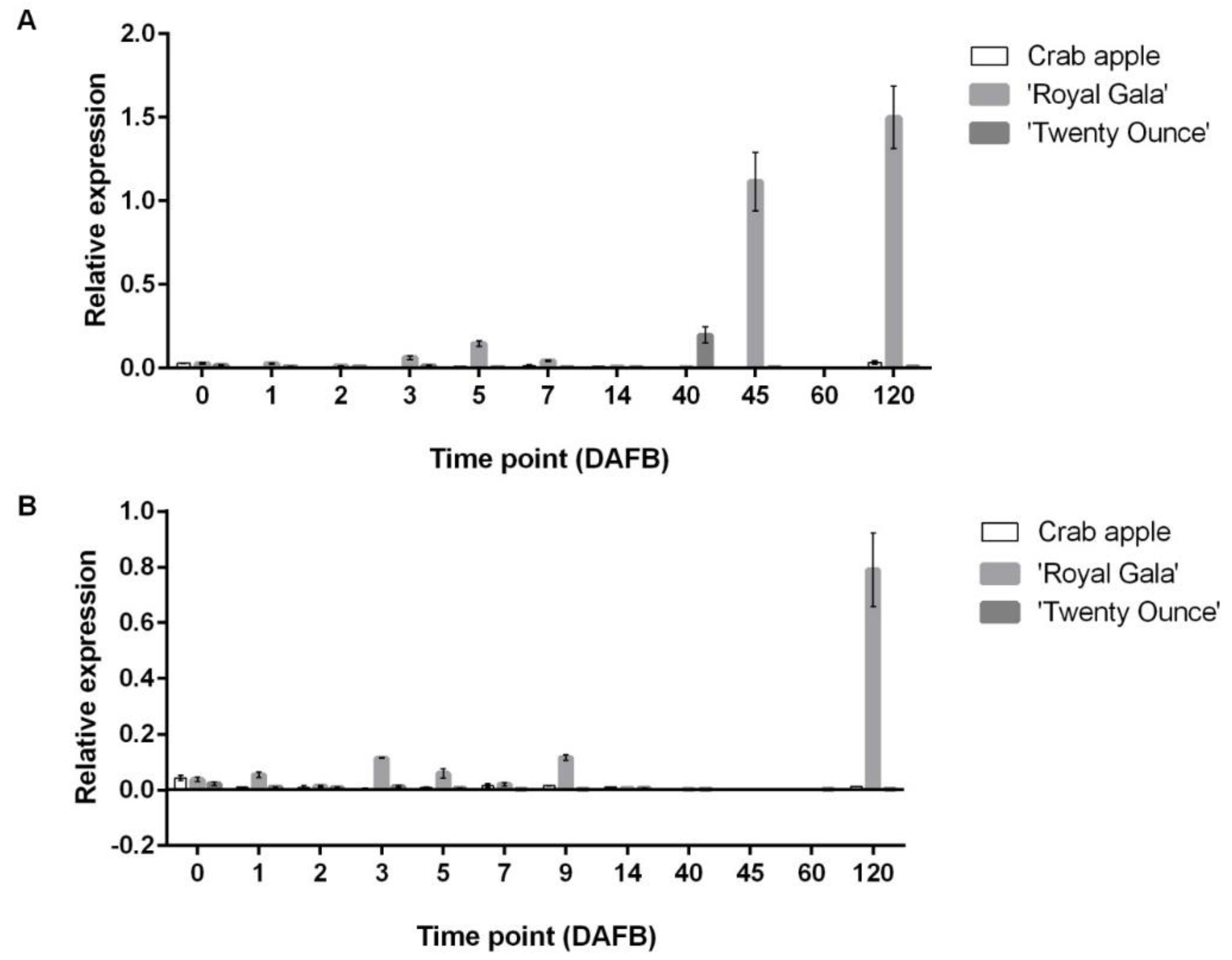
3.3. Comparison of Expression Patterns among Three Cultivars
3.4. Phylogenetic Analysis of CDK Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dash, M.; Malladi, A. The Aintegumenta genes, MdANT1 and MdANT2, are associated with the regulation of cell production during fruit growth in apple (Malus × domestica Borkh.). BMC Plant Biol. 2012, 12, 98. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Liu, K.; Yuan, G.; He, S.; Cong, P.; Zhang, C. Genome-wide identification and characterization of AINTEGUMENTA-LIKE (AIL) family genes in apple (Malus domestica Borkh.). Genomics 2022, 114, 110313. [Google Scholar] [CrossRef] [PubMed]
- Hirst, P.M. Advances in understanding flowering and pollination in apple trees. In Achieving Sustainable Cultivation of Apples; Burleigh Dodds Science Publishing: Cambridge, UK, 2017; Volume 31, pp. 109–126. [Google Scholar]
- Bain, J.M.; Robertson, R.N. The physiology of growth in apple fruits I. Cell size, cell number, and fruit development. Aust. J. Biol. Sci. 1951, 4, 75–91. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Li, H.; Jiang, W.; Li, Q.; Xi, Y.; Wang, X.; Li, J. Phytochemical compositions, health-promoting properties and food applications of crabapples: A review. Food Chem. 2022, 23, 132789. [Google Scholar] [CrossRef]
- Gonzalez, L.; Torres, E.; Àvila, G.; Bonany, J.; Alegre, S.; Carbó, J.; Martín, B.; Recasens, I.; Asin, L. Evaluation of chemical fruit thinning efficiency using Brevis®(Metamitron) on apple trees (‘Gala’) under Spanish conditions. Sci. Hortic. 2020, 261, 109003. [Google Scholar] [CrossRef]
- Jonkers, H. Biennial bearing in apple and pear: A literature survey. Sci. Hortic. 1979, 11, 303–317. [Google Scholar] [CrossRef]
- Janssen, B.J.; Thodey, K.; Schaffer, R.J.; Alba, R.; Balakrishnan, L.; Bishop, R.; Bowen, J.H.; Crowhurst, R.N.; Gleave, A.P.; Ledger, S.; et al. Global gene expression analysis of apple fruit development from the floral bud to ripe fruit. BMC Plant Biol. 2008, 8, 16. [Google Scholar] [CrossRef]
- Forshey, C.G. Fruit numbers, fruit saize, and yield relationships in’McIntosh’apples. J. Am. Soc. Hort. Sci. 1977, 102, 399–402. [Google Scholar]
- McAtee, P.; Karim, S.; Schaffer, R.; David, K. A dynamic interplay between phytohormones is required for fruit development, maturation, and ripening. Front. Plant Scie. 2013, 4, 79. [Google Scholar] [CrossRef]
- Karim, S.K.; Read, N.; David, K.M.; Allan, A.C.; Schaffer, R.J. Characterisation of induced Malus x domestica ‘Royal Gala’cell differentiation by using different hormones in cell cultures. J. Hortic. Sci. Biotechnol. 2022, 2022, 1–5. [Google Scholar] [CrossRef]
- Malladi, A.; Johnson, L.K. Expression profiling of cell cycle genes reveals key facilitators of cell production during carpel development, fruit set, and fruit growth in apple (Malus × domestica Borkh.). J. Exp. Bot. 2011, 62, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, J.J. Fruit ripening mutants yield insights into ripening control. Curr. Opin. Plant Biol. 2007, 10, 283–289. [Google Scholar] [CrossRef]
- Rugkong, A.; McQuinn, R.; Giovannoni, J.J.; Rose, J.K.; Watkins, C.B. Expression of ripening-related genes in cold-stored tomato fruit. Postharvest Biol. Technol. 2011, 61, 1–4. [Google Scholar] [CrossRef]
- Malladi, A. Molecular physiology of fruit growth in apple. Hortic. Rev. 2020, 47, 1–42. [Google Scholar]
- Kitsios, G.; Doonan, J.H. Cyclin dependent protein kinases and stress responses in plants. Plant Signal. Behav. 2011, 6, 204–209. [Google Scholar] [CrossRef]
- Endo, M.; Nakayama, S.; Umeda-Hara, C.; Ohtsuki, N.; Saika, H.; Umeda, M.; Toki, S. CDKB2 is involved in mitosis and DNA damage response in rice. Plant J. 2012, 69, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Klucher, K.M.; Chow, H.; Reiser, L.; Fischer, R.L. The AINTEGUMENTA gene of Arabidopsis required for ovule and female gametophyte development is related to the floral homeotic gene APETALA2. Plant Cell. 1996, 8, 137–153. [Google Scholar]
- Mizukami, Y.; Fischer, R.L. Plant organ size control: AINTEGUMENTA regulates growth and cell numbers during organogenesis. Proc. Natl. Acad. Sci. USA 2000, 97, 942–947. [Google Scholar] [CrossRef]
- Devoghalaere, F.; Doucen, T.; Guitton, B.; Keeling, J.; Payne, W.; Ling, T.J.; Ross, J.J.; Hallett, I.C.; Gunaseelan, K.; Dayatilake, G.A.; et al. A genomics approach to understanding the role of auxin in apple (Malus x domestica) fruit size control. BMC Plant Biol. 2012, 12, 7. [Google Scholar] [CrossRef]
- Jiao, Q.Q.; Cui, M.; Chen, X.S.; Zhang, Z.Y.; Wang, N.; Jiang, Y.M.; Mao, Z.Q.; Shu, J.; Shen, X.; Jiang, S.H.; et al. MdCKS1 Expression Involved in Fruit Size in Apple Cultivar ‘Fuji’and ‘Ralls’. Russ. J. Plant Physiol. 2021, 68, 1059–1068. [Google Scholar] [CrossRef]
- Cho, H.T.; Kende, H. Expansins and internodal growth of deepwater rice. Plant Physiol. 1997, 113, 1145–1151. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, B.; Shen, F.; Wang, X.; Zheng, W.Y.; Xiao, C.; Deng, Y.; Wang, T.; Yu Huang, Z.; Zhou, Q.; Wang, Y.; et al. Role of MdERF3 and MdERF118 natural variations in apple flesh firmness/crispness retainability and development of QTL-based genomics-assisted prediction. Plant Biotechnol. J. 2021, 19, 1022–1037. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, D.J. Loosening of plant cell walls by expansins. Nature 2000, 407, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.T.; Cosgrove, D.J. Altered expression of expansin modulates leaf growth and pedicel abscission in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2000, 97, 9783–9788. [Google Scholar] [CrossRef] [PubMed]
- Percy, A.E.; Melton, L.D.; Jameson, P.E. Expansion during early apple fruit development induced by auxin and N-(2-chloro-4-pyridyl)-N′-phenylurea: Effect on cell wall hemicellulose. Plant Growth Regul. 1998, 26, 1–6. [Google Scholar] [CrossRef]
- Harada, T.; Kurahashi, W.; Yanai, M.; Wakasa, Y.; Satoh, T. Involvement of cell proliferation and cell enlargement in increasing the fruit size of Malus species. Sci. Hortic. 2005, 105, 447–456. [Google Scholar] [CrossRef]
- Wakasa, Y.; Hatsuyama, Y.; Takahashi, A.; Sato, T.; Niizeki, M.; Harada, T. Divergent expression of six expansin genes during apple fruit ontogeny. Eur. J. Hortic. Sci. 2003, 68, 253–259. [Google Scholar]
- Dash, M.; Johnson, L.K.; Malladi, A. Reduction of fruit load affects early fruit growth in apple by enhancing carbohydrate availability, altering the expression of cell production-related genes, and increasing cell production. J. Am. Soc. Hortic. Sci. 2013, 138, 253–262. [Google Scholar] [CrossRef]
- Chang, S.; Puryear, J.; Cairney, A. A simple and efficient method for isolating RNA from pine trees. Plant Mol. Biol. Rep. 1993, 11, 113–116. [Google Scholar] [CrossRef]
- Schaffer, R.J.; Friel, E.N.; Souleyre, E.J.; Bolitho, K.; Thodey, K.; Ledger, S.; Bowen, J.H.; Ma, J.H.; Nain, B.; Cohen, D.; et al. A genomics approach reveals that aroma production in apple is controlled by ethylene predominantly at the final step in each biosynthetic pathway. Plant Physiol. 2007, 144, 1899–1912. [Google Scholar] [CrossRef]
- Olmstead, J.W.; Iezzoni, A.F.; Whiting, M.D. Genotypic differences in sweet cherry fruit size are primarily a function of cell number. J. Am. Soc. Hortic. Sci. 2007, 132, 697–703. [Google Scholar] [CrossRef]
- Bohner, J.; Bangerth, F. Effects of fruit set sequence and defoliation on cell number, cell size and hormone levels of tomato fruits (Lycopersicon esculentum Mill.) within a truss. Plant Growth Regul. 1988, 7, 141–155. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Kolarič, J.; Pleško, I.M.; Tojnko, S.; Stopar, M. Apple fruitlet ethylene evolution and MdACO1, MdACS5A, and MdACS5B expression after application of naphthaleneacetic acid, 6-benzyladenine, ethephon, or shading. HortScience 2011, 46, 1381–1386. [Google Scholar] [CrossRef]
- Sass, J.E. Botanical Microtechnique, 3rd ed.; The Iowa State University Press: Ames, IA, USA, 1958. [Google Scholar]
- Vandepoele, K.; Raes, J.; Veylder, L.; de Rouzé, P.; Rombauts, S.; Inzé, D. Genome-wide analysis of core cell cycle genes in Arabidopsis. Plant Cell 2002, 14, 903–916. [Google Scholar] [CrossRef]
- Huang, Y.-W.; Tsay, W.-S.; Chen, C.-C.; Lin, C.-W.; Huang, H.-J. Increased expression of the rice C-type cyclin-dependent protein kinase gene, Orysa;CDKC;1, in response to salt stress. Plant Physiol. Biochem. 2008, 46, 71–81. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, B.; Cao, Z.-Y.; Ling, T.-F.; Shen, W.-B. Heme oxygenase is involved in cobalt chloride-induced lateral root development in tomato. Biometals 2011, 24, 181–191. [Google Scholar] [CrossRef]
- Czerednik, A.; Busscher, M.; Bielen, B.M.; Wolters-Arts, M.; de Maagd, R.A.; Angenent, G.C. Regulation of tomato fruit pericarp development by an interplay between CDKB and CDKA1 cell cycle genes. J. Exp. Bot. 2012, 63, 2605–2617. [Google Scholar] [CrossRef]
- Gentric, N.; Genschik, P.; Noir, S. Connections between the cell cycle and the DNA damage response in plants. Int. J. Mol. Sci. 2021, 22, 9558. [Google Scholar] [CrossRef]
- McAtee, P.A.; Hallett, I.C.; Johnston, J.W.; Schaffer, R.J. A rapid method of fruit cell isolation for cell size and shape measurements. Plant Methods 2009, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Krizek, B.A. Ectopic expression of Aintegumenta in Arabidopsis plants results in increased growth of floral organs. Dev. Genet. 1999, 25, 224–236. [Google Scholar] [CrossRef]
- Ng, J.K.; Schröder, R.; Sutherland, P.W.; Hallett, I.C.; Hall, M.I.; Prakash, R.; Smith, B.G.; Melton, L.D.; Johnston, J.W. Cell wall structures leading to cultivar differences in softening rates develop early during apple (Malus x domestica) fruit growth. BMC Plant Biol. 2013, 13, 128. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.K.; Malladi, A.; NeSmith, D.S. Differences in cell number facilitate fruit size variation in rabbiteye blueberry genotypes. J. Am. Soc. Hortic. Sci. 2011, 136, 10–15. [Google Scholar] [CrossRef]
- Patricia Denne, M. Fruit development and some tree factors affecting it. N. Z. J. Bot. 1963, 1, 265–294. [Google Scholar] [CrossRef]
- Hugh Smith, W. The histological structure of the flesh of the apple in relation to growth and senescence. J. Pomol. Hortic. Sci. 1941, 18, 249–260. [Google Scholar] [CrossRef]
- Chen, P.; Li, Z.; Zhang, D.; Shen, W.; Xie, Y.; Zhang, J.; Jiang, L.; Li, X.; Shen, X.; Geng, D.; et al. Insights into the effect of human civilization on Malus evolution and domestication. Plant Biotechnol. J. 2021, 19, 2206–2220. [Google Scholar] [CrossRef]
- Bertin, N.; Génard, M.; Fishman, S. A model for an early stage of tomato fruit development: Cell multiplication and cessation of the cell proliferative activity. Ann. Bot. 2003, 92, 65–72. [Google Scholar] [CrossRef]
- Malladi, A.; Hirst, P.M. Increase in fruit size of a spontaneous mutant of ‘Gala’apple (Malus × domestica Borkh.) is facilitated by altered cell production and enhanced cell size. J. Exp. Bot. 2010, 61, 3003–3013. [Google Scholar] [CrossRef]
- Khan, A.; Gutierrez, B.; Chao, C.T.; Singh, J. Origin of the Domesticated Apples. In The Apple Genome; Springer: Cham, Switzerland, 2021; pp. 383–394. [Google Scholar]




| Primer | Sequence 5′-3′ |
|---|---|
| MdCDKB2:2 | TGCACAGGGATCTTAAGC |
| MdCDKB2:2 | ATACTTCTTGAGTGGCAC |
| MdANT2 | CCAAGGTGATCGAACCTAACATTGCAG |
| MdANT2 | TCCTCCAATGCCATTGAGAATGAGAGA |
| MdARF106 | GAGGGGAAGCCGTTTGAGGT |
| MdARF106 | GCCGTCCAAAACACCTTCAAT |
| MdEXP3 | GATGCAGGAGAAGAGGAGGC |
| MdEXP3 | ATTGCACATCTCCAGCACCA |
| MdACO1 | CAGTCGGATGGGACCAGAA |
| MdACO1 | GCTTGGAATTTCAGGCCAGA |
| MdPG1 | TGAACACTTTGCAGCACGAT |
| MdPG1 | GGCGGTTCAAGTGAAAAATG |
| MdActin | ACCATCTGCAACTCATCCGAACCT |
| MdActin | ACAATGCTAGGGAACACGGCTCTT |
| MdGAPDH | TGAGGGCAAGCTGAAGGGTATCTT |
| MdGAPDH | TCAAGTCAACCACACGGGTACTGT |
| Gene Name | Accession Number |
|---|---|
| Arath; CDKA;1 | AT3G48750.1 |
| Arath;CDKB1;1 | AT3G54180.1 |
| Arath;CDKB1;2 | AT2G38620.2 |
| Arath;CDKB2;1 | AT1G76540.1 |
| Arath;CDKB2;2 | AT1G20930.1 |
| Arath;CDKC;1 | AT5G10270.1 |
| Arath;CDKC;2 | AT5G64960.1 |
| Arath;CDKD;1 | AT1G73690.1 |
| Arath;CDKD;2 | AT1G66750.1 |
| Arath;CDKD;3 | AT1G18040.1 |
| Arath;CDKE;1 | AT5G63610.1 |
| Arath;CDKF;1 | AT4G28980.2 |
| MdCDKA1 | MDP0000185491 |
| MdCDKB1;1 | MDP0000223519 |
| MdCDKB1;2 | MDP0000240040 |
| MdCDKB2;1 | MDP0000418062 |
| MdCDKB2;2 | MDP0000722904 |
| MdCDKC1 | MDP0000243737 |
| MdCDKC2 | MDP0000253325 |
| MdCDKD1 | MDP0000128357 |
| MdCDKE1 | MDP0000206441 |
| MdCDKE2 | MDP0000235270 |
| MdCDKF1;1 | MDP0000303768 |
| MdCDKG2 | MDP0000263387 |
| SlCDKA1 | Solyc08g066330.1.1 |
| SlCDKA2 | Solyc12g095860.1.1 |
| SlCDKB1;1 | Solyc10g074720.1.1 |
| SlCDKB2;1 | Solyc04g082840.2.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karim, S.K.A.; Allan, A.C.; Schaffer, R.J.; David, K.M. Cell Division Controls Final Fruit Size in Three Apple (Malus x domestica) Cultivars. Horticulturae 2022, 8, 657. https://doi.org/10.3390/horticulturae8070657
Karim SKA, Allan AC, Schaffer RJ, David KM. Cell Division Controls Final Fruit Size in Three Apple (Malus x domestica) Cultivars. Horticulturae. 2022; 8(7):657. https://doi.org/10.3390/horticulturae8070657
Chicago/Turabian StyleKarim, Siti Khadijah A., Andrew C. Allan, Robert J. Schaffer, and Karine M. David. 2022. "Cell Division Controls Final Fruit Size in Three Apple (Malus x domestica) Cultivars" Horticulturae 8, no. 7: 657. https://doi.org/10.3390/horticulturae8070657
APA StyleKarim, S. K. A., Allan, A. C., Schaffer, R. J., & David, K. M. (2022). Cell Division Controls Final Fruit Size in Three Apple (Malus x domestica) Cultivars. Horticulturae, 8(7), 657. https://doi.org/10.3390/horticulturae8070657







