A Fine Line between Phytotoxicity and Blue When Producing Hydrangea macrophylla in a Nursery at a Low Substrate pH
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bluing the Initial Year of Production: Year 1
2.1.1. Location, Plants and Substrate
2.1.2. Treatments and Other Fertilization
2.1.3. Substrate Pore-Water Chemical Properties
2.1.4. Flower Color Measurements
2.1.5. Plant Growth, Quality and Flower Index Measurements
2.1.6. Saturated Media Extract
2.2. Bluing a Crop Held Over: Year 2
2.2.1. Treatments and Other Fertilization
2.2.2. Substrate Pore-Water Chemical Properties
2.2.3. Flower Color Measurements
2.2.4. Plant Growth, Quality and Flower Index Measurements
2.3. Statistical Analysis
3. Results
3.1. Bluing the Initial Year of Production: Year 1 Results
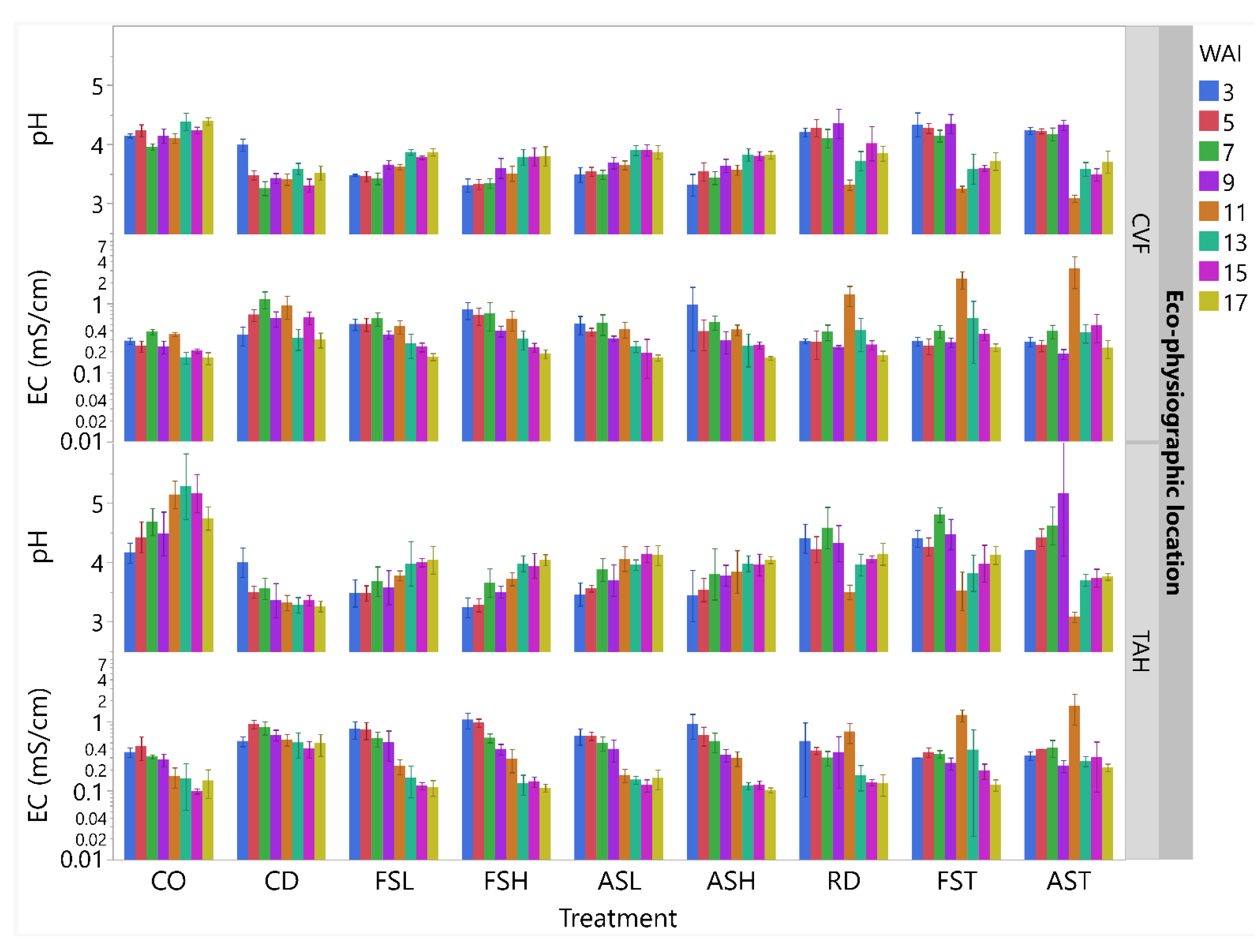
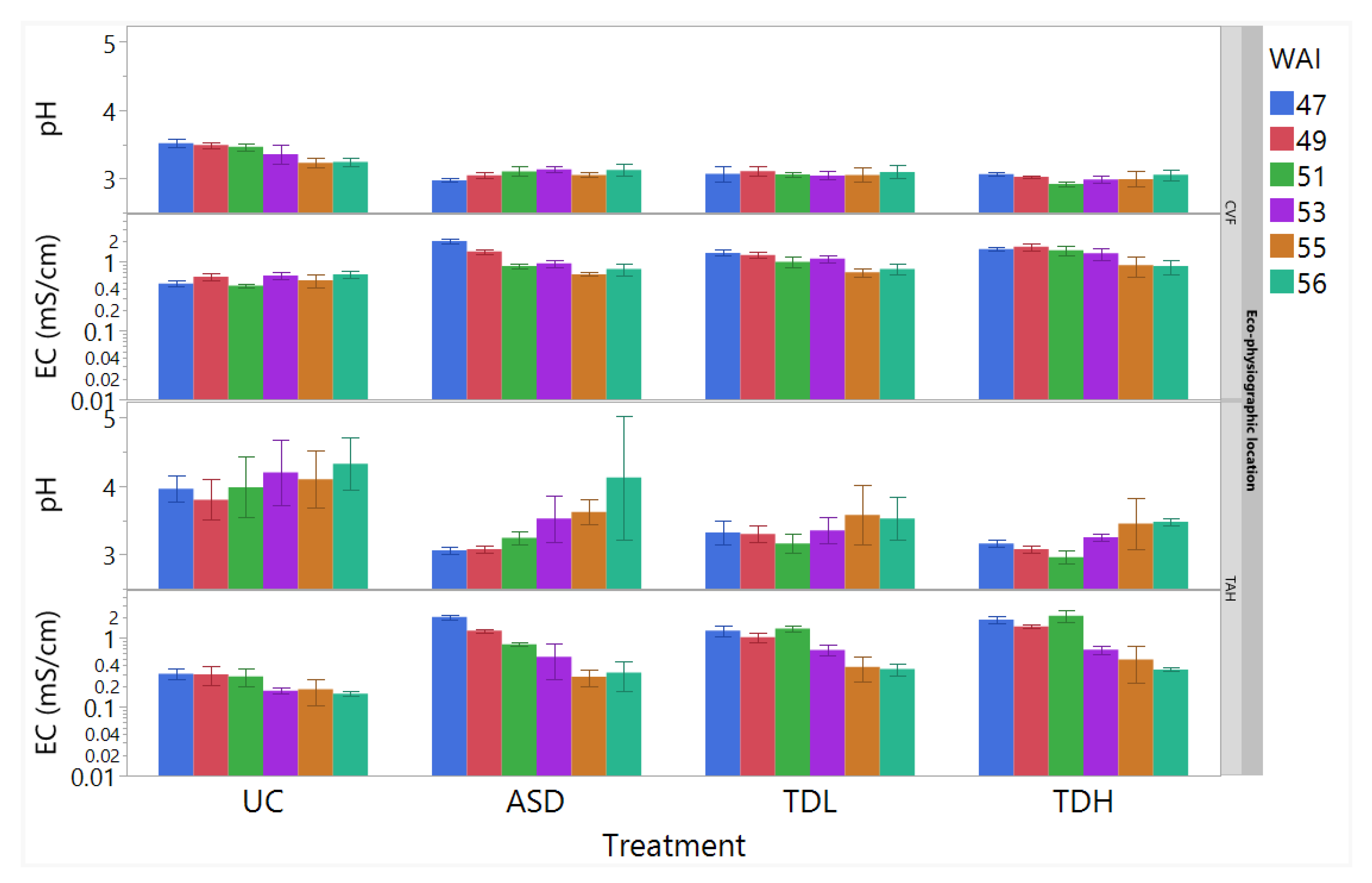
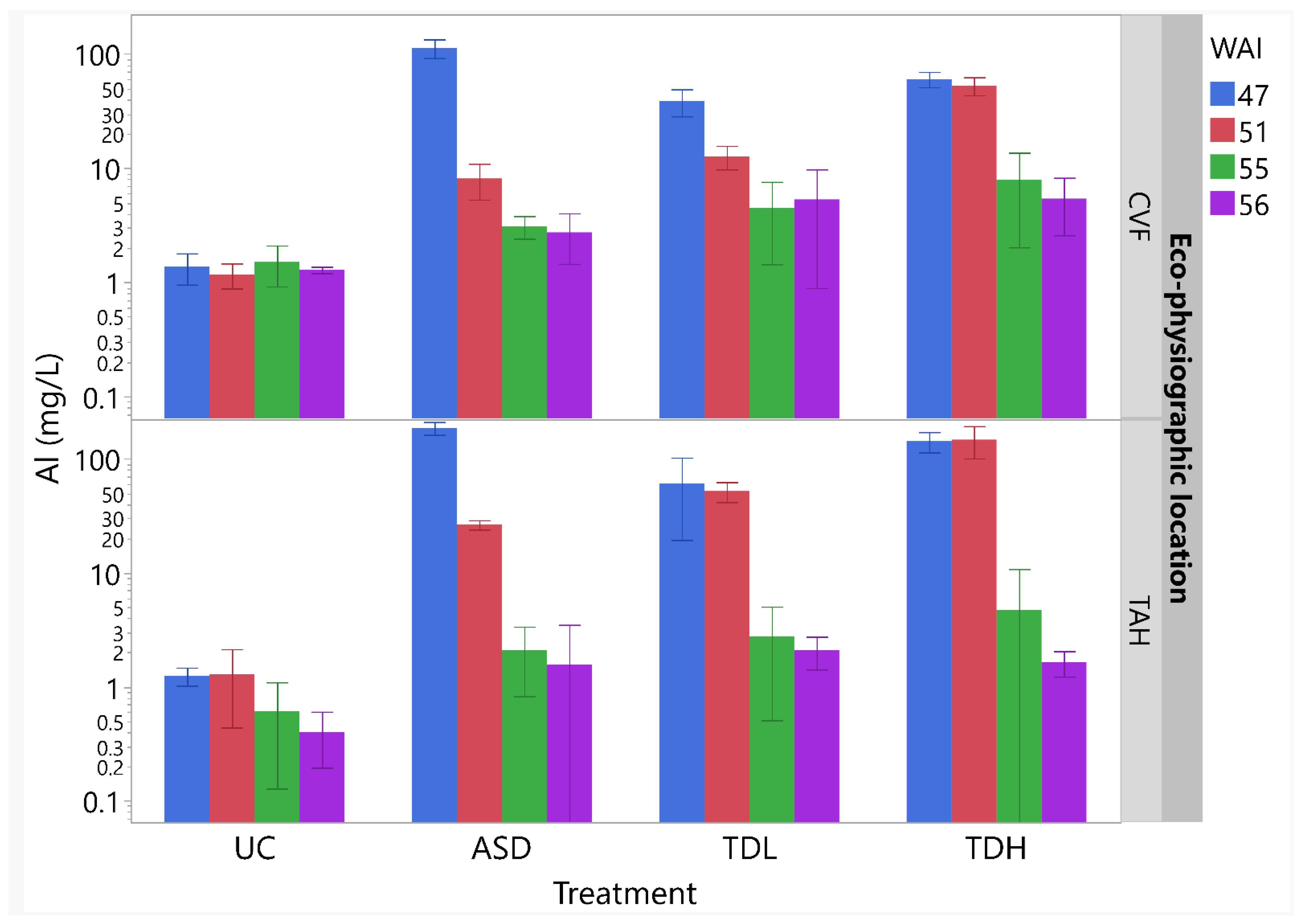
3.1.1. Substrate Pore-Water Chemical Properties
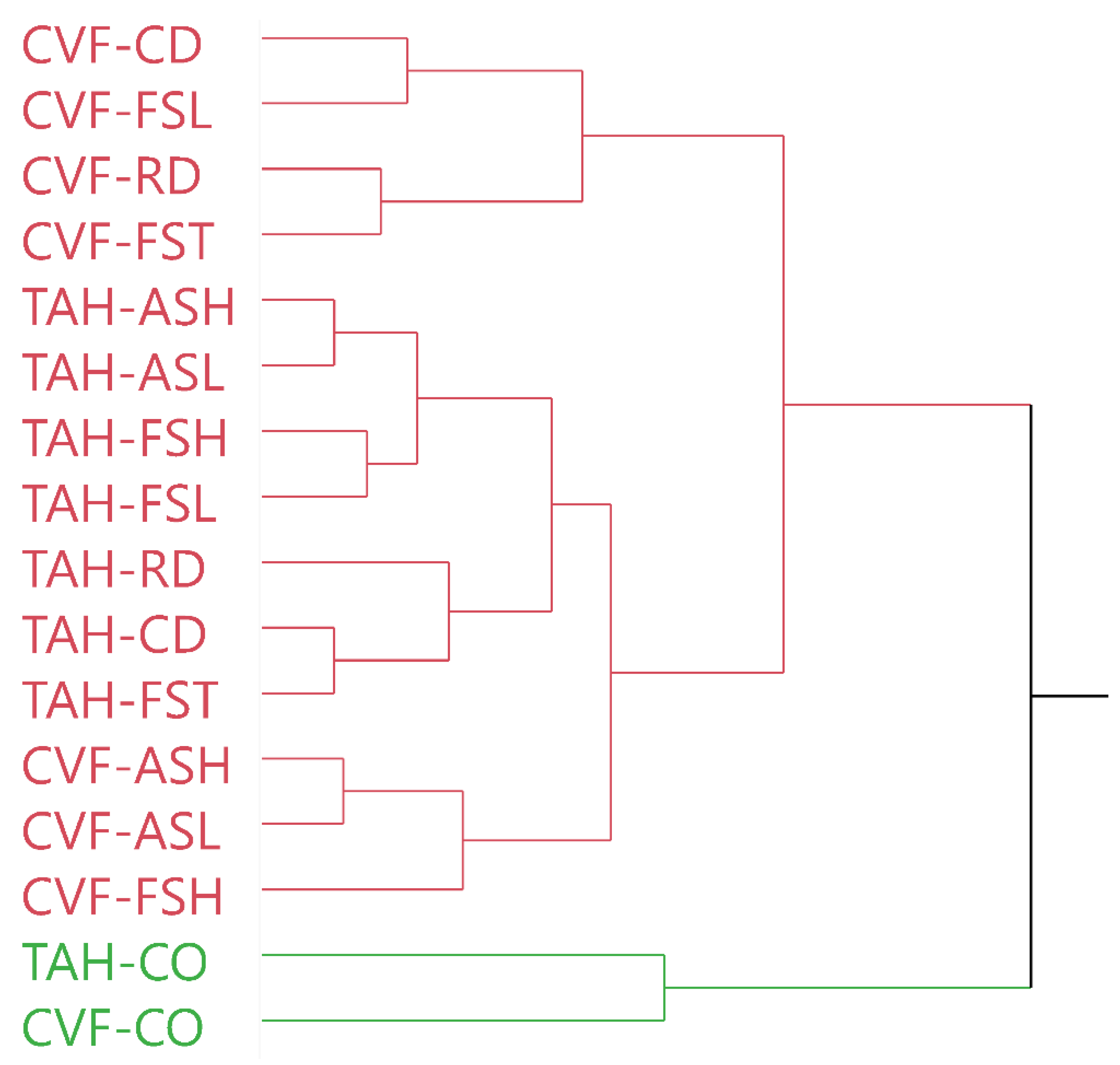
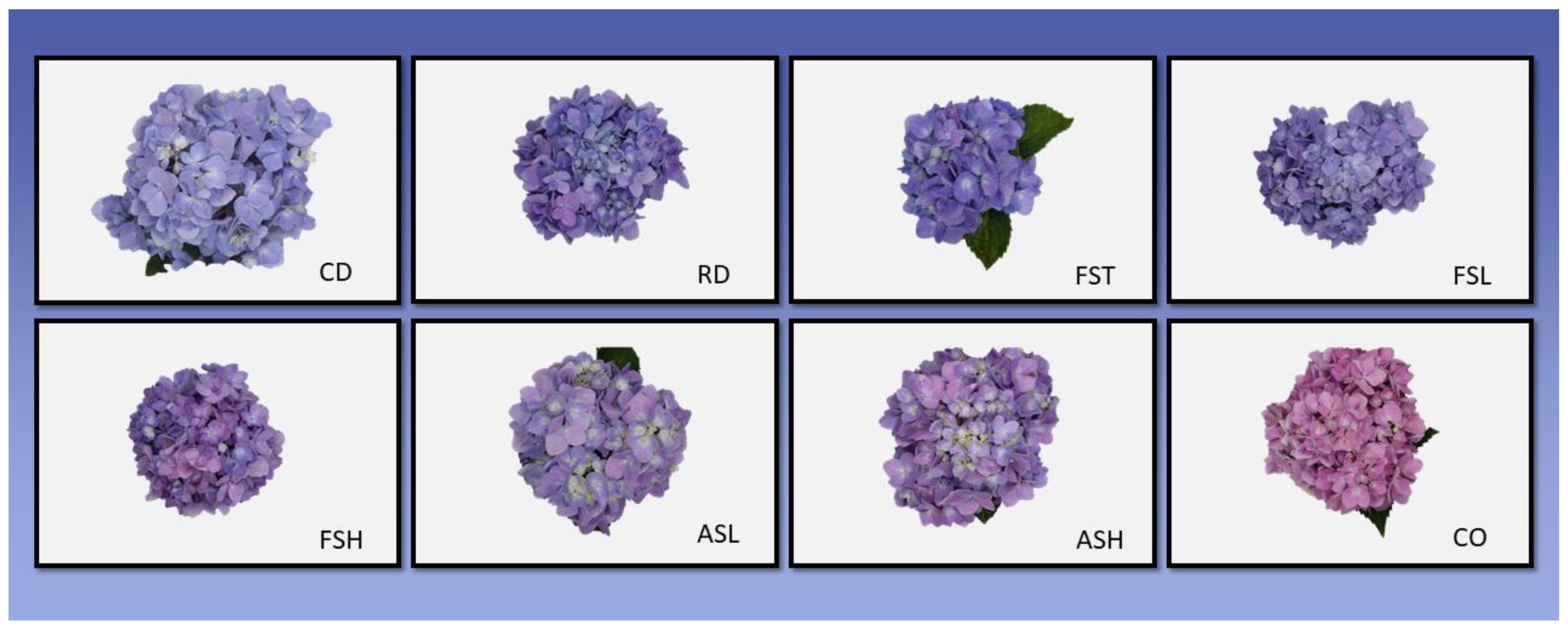
3.1.2. Flower Color Measurements
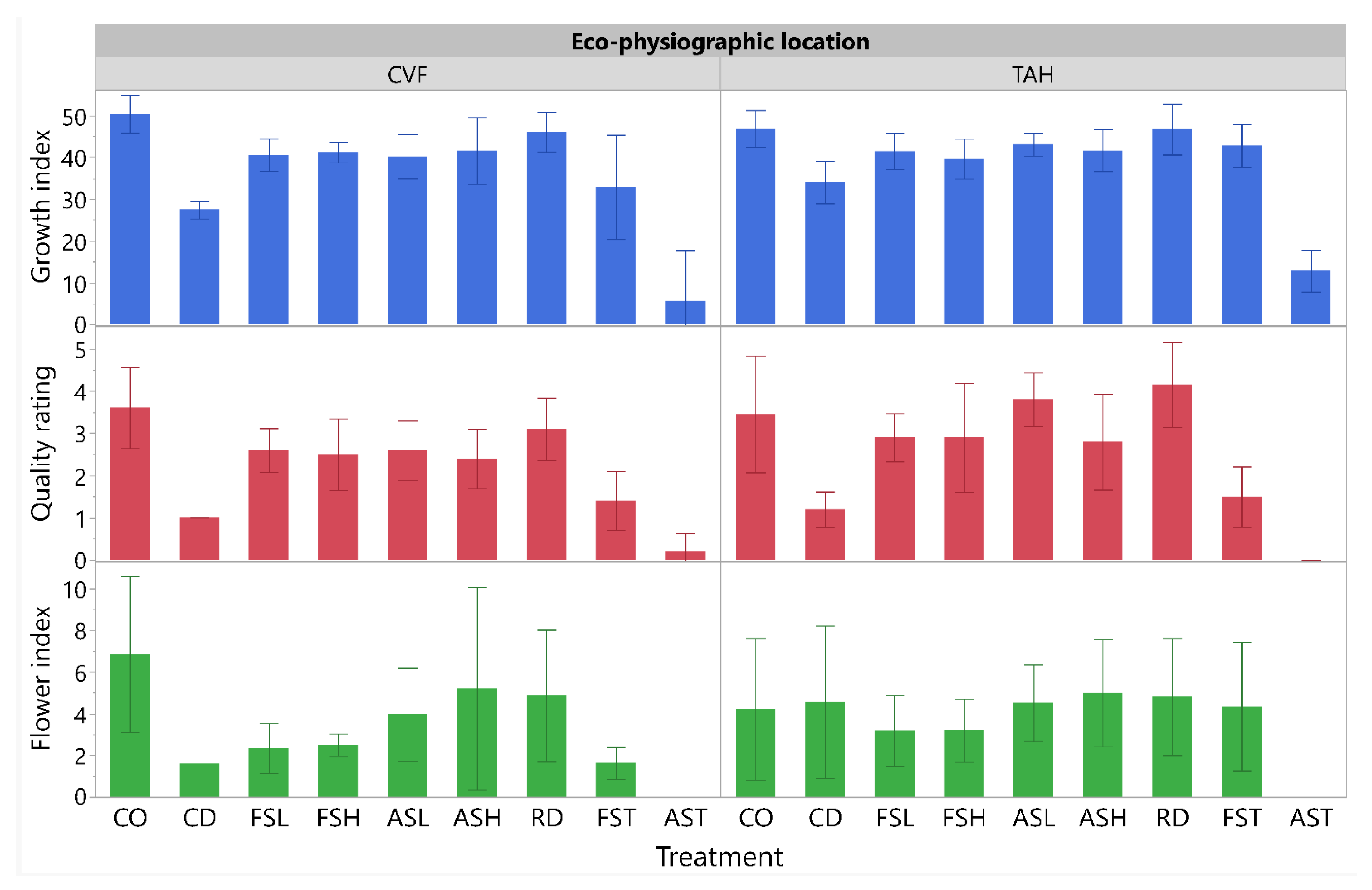
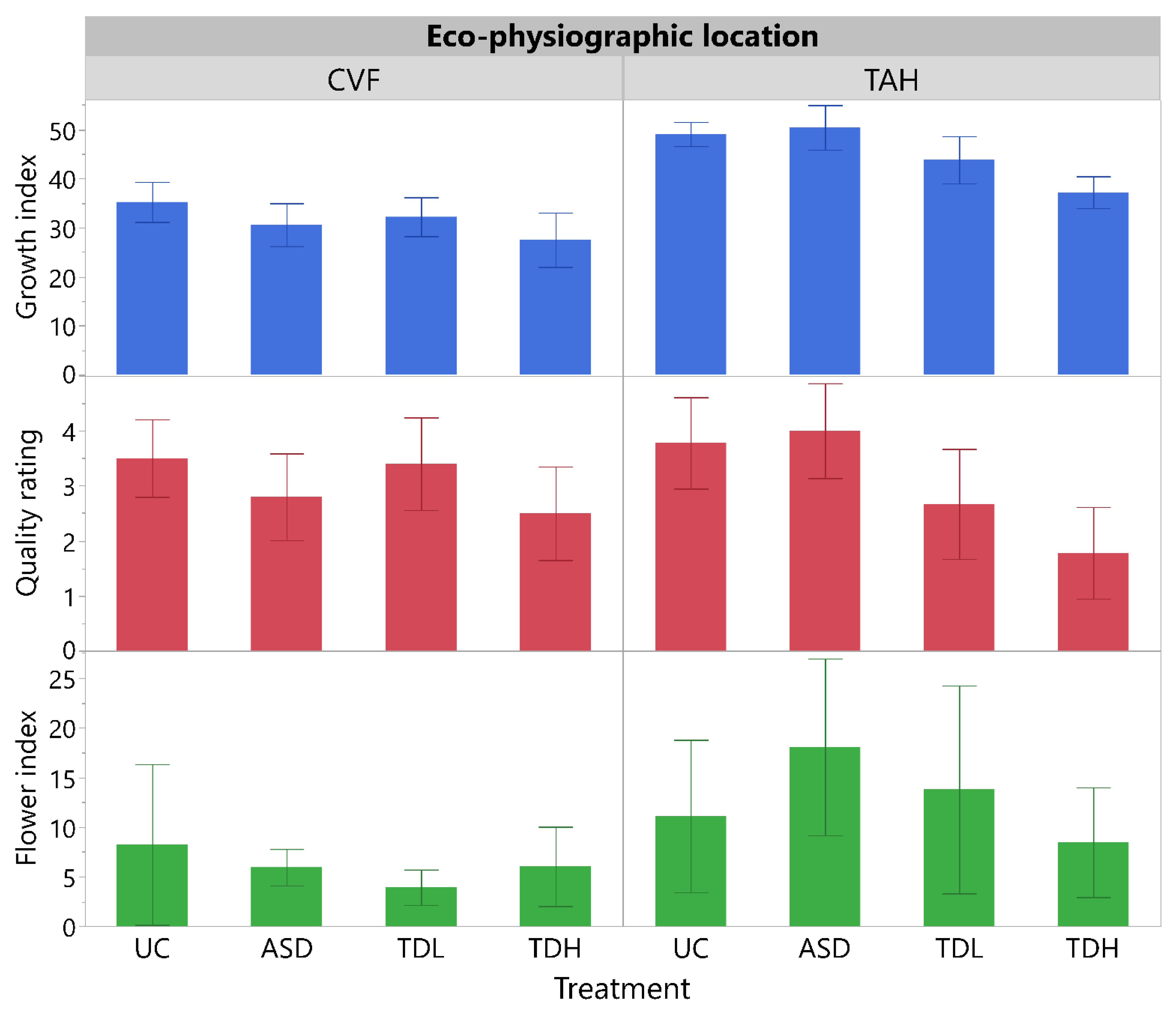
3.1.3. Plant Growth, Quality and Flower Index Measurements
3.1.4. SME Measurements
3.2. Bluing a Crop Held Over: Year 2 Results
3.2.1. Substrate Pore-Water Chemical Properties
3.2.2. Flower Color Measurements
3.2.3. Plant Growth, Quality and Flower Index Measurements
4. Discussion
4.1. Bluing the Initial Year of Production: Year 1 Discussion
4.2. Bluing a Crop Held Over: Year 2 Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allen, R.C. Influence of aluminum on the flower color of Hydrangea macrophylla DC. Contr. Boyce Thompson Inst. 1943, 13, 221–242. [Google Scholar]
- Asen, S.; Siegelman, H.W. Effect of aluminum on absorption spectra of the anthocyanin and flavonols from sepals of Hydrangea macrophylla var. Merveille. Proc. Am. Soc. Hortic. Sci. 1957, 70, 478–481. [Google Scholar]
- Davis, T. State of the Crop: Hydrangeas. Nursery Management. 2012. Available online: https://www.nurserymag.com/article/nm0312-new-hydrangeas-market/ (accessed on 21 December 2021).
- Fulcher, A.; Owen, J.S., Jr.; LeBude, A.V. Hydrangea Production: Species-Specific Production Guide; University of Tennessee: Knoxville, TN, USA, 2016; p. 12. Available online: https://extension.tennessee.edu/publications/Documents/PB1840-B.pdf (accessed on 8 August 2021).
- Takeda, K.; Kariuda, M.; Itoi, H. Bluing of sepal colour of Hydrangea macrophylla. Phytochemistry 1985, 24, 2251–2254. [Google Scholar] [CrossRef]
- Schreiber, H.D.; Swink, A.M.; Godsey, T.D. The chemical mechanism for Al3+ complexing with delphinidin: A model for the bluing of hydrangea sepals. J. Inorg. Biochem. 2010, 104, 732–739. [Google Scholar] [CrossRef]
- Takeda, K.; Kubota, R.; Yagioka, C. Copigments in the blueing of sepal color of Hydrangea macrophylla. Phytochemistry 1985, 24, 1207–1209. [Google Scholar] [CrossRef]
- Schreiber, H.D.; Jones, A.H.; Lariviere, C.M.; Mayhew, K.M.; Cain, J.B. Role of aluminum in red-to-blue color changes in Hydrangea macrophylla sepals. Biometals 2011, 24, 1005–1015. [Google Scholar] [CrossRef]
- Kodama, M.; Tanabe, Y.; Nakayama, M. Analyses of coloration-related components in Hydrangea sepals causing color variability according to soil conditions. Hortic. J. 2016, 85, 372–379. [Google Scholar] [CrossRef]
- Handreck, K.A. Production of blue hydrangea flowers without aluminum drenches. Commun. Soil Sci. Plant Anal. 2008, 28, 1191–1198. [Google Scholar] [CrossRef]
- Midcap, J.T.; Bilderback, T.E. Evaluating Hydrangea Production with Improved Substrates; Center Applied Nursery Research: Athens, GA, USA, 2002; p. 5. [Google Scholar]
- Opena, G.B.; Williams, K.A. Use of precharged zeolite to provide aluminum during blue hydrangea production. J. Plant Nutr. 2007, 26, 1825–1840. [Google Scholar] [CrossRef]
- Owen, J.S., Jr. Evaluation of Clay Amendments for Douglas Fir Bark; Agriculture Research Foundation: Corvallis, OR, USA, 2007; p. 8. [Google Scholar]
- Stoven, H.M.; Owen, J. Into the blue. Digger, April 2010; pp. 26–30. [Google Scholar]
- Stoven, H.M.; Owen, J.S., Jr. Comparison of substrate amendments for the adjustment of Hydrangea (Hydrangea macrophylla (THUNB.) SER ‘Bailmer’, Endless Summer) flower color. In Proceedings of the Southern Nursery Association Research Conference; Robbins, J.A., Ed.; 2008; pp. 32–35. Available online: https://www.sna.org/Resources/Documents/08resprocsec01.pdf (accessed on 3 March 2022).
- Blom, T.J.; Piott, B.D. Florists’ hydrangea blueing with aluminum sulfate applications during forcing. HortScience 1992, 27, 1084–1087. [Google Scholar] [CrossRef]
- Edziak, P.J. The Effects of Slow-Release Aluminum Sulfate on Bloom Color of Hydrangea Macrophylla; California Polytechnic State University: San Luis Obispo, CA, USA, 2015; p. 37. Available online: https://digitalcommons.calpoly.edu/hcssp/33/ (accessed on 18 March 2022).
- Landis, H.; Hicks, K.; Henry, J.; McCall, I.; Whipker, B. Determining early season aluminum tissue values in hydrangea leaves to predict blue sepal color. HortScience 2018, 53, 205. [Google Scholar]
- Landis, H.G. Aluminum Effect on Hydrangea Plant Tissue Nutrient Concentrations and Sepal Coloration; Master of Science; North Carolina State University: Raleigh, NC, USA, 2019; p. 73. Available online: https://repository.lib.ncsu.edu/handle/1840.20/36346 (accessed on 3 March 2022).
- Midcap, J.T. Flower color control on remontant flowering Hydrangea macrophylla cultivars; Center Applied Nursery Research: Athens, GA, USA, 2002; p. 3. [Google Scholar]
- Bailey, D.A. Hydrangea production. In Hydrangea Production; Timber Press: Portland, OR, USA, 1989; p. 91. [Google Scholar]
- Yeager, T.; Bilderback, T.E.; Boyer, C.; Chappell, M.; Fain, G.; Fare, D.; Gilliam, C.; Jackson, B.; Lea-Cox, J.; LeBude, A.V.; et al. Best Management Practices: Guide for Producing Nursery Crops; Southern Nursery Association, Inc.: Acworth, GA, USA, 2013. [Google Scholar]
- Wright, R.D. The pour-through method: A quick and easy way to determine a medium’s nutrient availability. Am. Nurserym. 1984, 160. [Google Scholar]
- Collins, T.J. ImageJ for microscopy. Biotechniques 2007, 43 (Suppl. 1), 25–30. [Google Scholar] [CrossRef]
- Rhoades, J.D. Salinity: Electrical conductivity and total dissolved solids. In Methods of Soil Analysis: Part 3 Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N.P., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; Soil Science Society of America, Inc.: Madison, WI, USA; American Society of Agronomy, Inc.: Madison, WI, USA, 1996; pp. 417–435. [Google Scholar] [CrossRef]
- Bryson, G.M.; Mills, H.A. Plant Analysis Handbook IV: A Guide to Sampling, Preparation, Analysis, Interpretation and Use of Results of Agonomic and Horticultural Crop Plant Tissue, 4th ed.; Micro-Macro Publishing: Athens, GA, USA, 2014. [Google Scholar] [CrossRef]
- Ma, J.F.; Hiradate, S.; Nomoto, K.; Iwashita, T.; Matsumoto, H. Internal detoxification mechanism of Al in hydrangea: Identification of Al form in the leaves. Plant Physiol. 1997, 113, 1033–1039. [Google Scholar] [CrossRef]
- Ma, J.F. Role of organic acids in detoxification of aluminum in higher plants. Plant Cell Physiol. 2000, 41, 383–390. [Google Scholar] [CrossRef]
- Schreiber, H.D. Curious chemistry guides hydrangea colors. Am. Sci. 2014, 102, 444–451. [Google Scholar] [CrossRef]
- Clark, R.B. Effect of aluminum on growth and mineral elements of Al-tolerant and Al-intolerant corn. Plant Soil 1977, 47, 653–662. [Google Scholar] [CrossRef]
- Silva, S.; Pinto-Carnide, O.; Martins-Lopes, P.; Matos, M.; Guedes-Pinto, H.; Santos, C. Differential aluminium changes on nutrient accumulation and root differentiation in an Al sensitive vs. tolerant wheat. Environ. Exp. Bot. 2010, 68, 91–98. [Google Scholar] [CrossRef]
- Anonymous. Endless Summer: The Original Culture Sheet; Bailey Nurseries, Inc.: St. Paul, MN, USA, 2021; p. 2. [Google Scholar]
- Naumann, A.; Kunz, U.; Lehmann, H.; Stelzer, R.; Horst, W.J. Effect of aluminium on root morphology of Hydrangea macrophylla. In Plant Nutrition. Developments in Plant and Soil Sciences; Horst, W.J., Schenk, M.K., Burkert, A., Claassen, N., Flessa, H., Frommer, W.B., Goldbach, H., Olfs, H.W., Romheld, V., Eds.; Springer: Dordrecht, The Netherlands, 2001. [Google Scholar] [CrossRef]
- Tharpe, E.; Pietsch, G.; Fulcher, A. BloomStruck® Hydrangea: Controlling Flower Color. In TURF and Ornamental Field Day; University of Tennessee: Knoxville, TN, USA, 2017. [Google Scholar]
- Wallace, A.; Wieland, P.A.T. Mineral-nutrition associated with flower color in hydrangea. J. Plant Nutr. 1980, 2, 217–220. [Google Scholar] [CrossRef]
- Wu, L.; Chen, J.; Lin, H.; van Mantgem, P.; Harivandi, M.A.; Harding, J.A. Effects of regenerant wastewater irrigation on growth and ion uptake of landscape plants. J. Environ. Hortic. 1995, 13, 92–96. [Google Scholar] [CrossRef]
- Bañón, S.; Ochoa, J.; Bañón, D.; Ortuño, M.F.; Sánchez-Blanco, M.J. Controlling salt flushing using a salinity index obtained by soil dielectric sensors improves the physiological status and quality of potted hydrangea plant. Sci. Hortic. 2019, 247, 335–343. [Google Scholar] [CrossRef]
- Osaki, M.; Watanabe, T.; Tadano, T. Beneficial effect of aluminum on growth of plants adapted to low pH soils. Soil Sci. Plant Nutr. 1997, 43, 551–563. [Google Scholar] [CrossRef]
- Penn, C.; Camberato, J. A Critical Review on Soil Chemical Processes that Control How Soil pH Affects Phosphorus Availability to Plants. Agriculture 2019, 9, 120. [Google Scholar] [CrossRef]
- Naumann, A.; Horst, W.J. Effect of aluminium supply on aluminium uptake, translocation and blueing of Hydrangea macrophylla (Thunb.) Ser. cultivars in a peatclay substrate. J. Hortic. Sci. Biotechnol. 2003, 78, 463–469. [Google Scholar] [CrossRef]
- Schreiber, H.D.; Wade, S.E.; Mayhew, K.M.; Cobb, J.A. Characterization of Hydrangea macrophylla cultivars by the anthocyanin content in their sepals. J. Environ. Hortic. 2011, 29, 131–136. [Google Scholar] [CrossRef]
- Huxley, A.J.; Griffiths, M.; Levy, M. The New Royal Horticultural Society Dictionary of Gardening; Macmillan: London, UK, 1999. [Google Scholar]
- Park, W.-P.; Hyun, H.-N.; Koo, B.-J. Silicon fractionation of soluble silicon in volcanic ash soils that may affect groundwater silicon content on Jeju Island, Korea. Water 2020, 12, 2686. [Google Scholar] [CrossRef]
- Park, W.-P.; Koo, B.-J. Silicon and aluminum mobility in soils of Jeju Island, Korea. Appl. Environ. Soil Sci. 2020, 2020, 1–12. [Google Scholar] [CrossRef]
- Hodson, M.J.; Evans, D.E. Aluminium-silicon interactions in higher plants: An update. J. Exp. Bot. 2020, 71, 6719–6729. [Google Scholar] [CrossRef]
- Frantz, J.; Locke, J.C.; Sturtz, D.S.; Leisner, S. Silicon in ornamental crops: Detection, delivery, and function. In Silicio na Agricultura: Anais do V Simposio Brasileiro Sobre Silicio Agricultura; Rodriguez, F., Ed.; Universidade Federal de Vicosa: Vicosa, Brazil, 2010; pp. 111–134. [Google Scholar]
- Okada, M.; Okawa, K. The quantity of aluminium and phosphorus in Hydrangea macrophylla plants and its influence on sepal colour. J. Jpn. Soc. Hortic. Sci. 1974, 42, 361–370. [Google Scholar] [CrossRef]
| Application Method | Treatment/Product | Product Appl. Rate | Frequency of Appl. | Aluminum (Al) Added | Abbreviation | Location |
|---|---|---|---|---|---|---|
| Year 1 (2018) planted liner; base substrate or “control” (CO) contained no Al sulfate (0.00 kg Al·m−3) | ||||||
| Pre-plant incorporated | 90-day controlled release Al | 4.15 kg·m−3 | 1 appl. | 0.62 kg·m−3 | FSL | CVF, TAH |
| (provide Al throughout production) | 5.93 kg·m−3 | 0.89 kg·m−3 | FSH | |||
| Ground Al sulfate | 5.93 kg·m−3 | 1 appl. | 1.03 kg·m−3 | ASL | CVF, TAH | |
| 8.90 kg·m−3 | 1.56 kg·m−3 | ASH | ||||
| Topdressed | 90-day controlled release Al | 9.00 g·cont.−1 | 1 appl. | 1.35 g·cont.−1 | FST | TAH |
| (provide Al at time of flowering, rescue) | 15.00 g·cont.−1 | 2.25 g·cont.−1 | CVF | |||
| Ground Al sulfate | 26.17 g·cont.−1 | 1 appl. | 4.58 g·cont.−1 | AST | TAH | |
| 32.66 g·cont.−1 | 5.72 g·cont.−1 | CVF | ||||
| Drench | Dissolved Al sulfate | |||||
| (continuously every 2 wks. | 250 mL of 17.97 g·L−1 | 4.49 g·cont.−1 | 7 appl. | 5.50 g·cont.−1 | CD | CVF, TAH |
| One time rescue appl.) | 500 mL of 23.97 g·L−1 | 11.99 g·cont.−1 | 1 appl. | 2.10 g·cont.−1 | RD | |
| Year 2 (2019) 1-year old plant; base substrate or “control” (UC) contained 5.93 kg·m−3 aluminum sulfate (1.03 kg Al·m−3) | ||||||
| Topdressed | 90-day controlled release Al | 9.00 g·cont.−1 | 1 appl. | 1.35 g·cont.−1 | TDL | CVF, TAH |
| (provide Al at time of flowering, rescue) | 15.00 g·cont.−1 | 2.25 g·cont.−1 | TDH | |||
| Drench | Dissolved Al sulfate | 8.98 g·cont.−1 | 1.57 g·cont.−1 | CVF | ||
| (one time rescue appl.) | 500 mL of 17.97 g·L−1 | 1 appl. | ASD | |||
| 500 mL of 23.97 g·L−1 | 11.99 g·cont.−1 | 2.10 g·cont.−1 | TAH | |||
| Treatment | CVF 2 | TAH |
|---|---|---|
| Non-Rescue Treatments | ||
| CO | 34.34 b3 | 34.04 b |
| Drench | ||
| CD | 40.48 a | 40.16 a |
| Incorporated | ||
| FSL | 39.45 a | 38.77 a |
| FSH | 38.65 a | 37.96 a |
| ASL | 38.54 a | 38.33 a |
| ASH | 38.33 a | 38.96 a |
| Rescue Treatments | ||
| RD | 38.17 ab | 40.09 a |
| FST | 38.65 a | 39.96 a |
| AST 4 | . | . |
| p < 0.0001 | p < 0.0019 | |
| Treatment | Al, Extractant (mg·L−1) | Al, Foliar Tissue (µg·g−1 Dry Weight) | ||
|---|---|---|---|---|
| CVF 1 | TAH | CVF | TAH | |
| Non-Rescue Treatments Control | ||||
| CO | 0.12 b 2 | 0.10 b | 105 d | 121 d |
| Drench | ||||
| CD | 1.82 a | 11.59 a | 1237 a | 1633 a |
| Incorporated | ||||
| FSL | 0.37 b | 0.26 b | 444 c | 577 c |
| FSH | 0.30 b | 0.23 b | 523 bc | 501 cd |
| ASL | 0.26 b | 0.31 b | 534 bc | 569 c |
| ASH | 0.29 b | 0.24 b | 567 bc | 492 cd |
| Rescue Treatments | ||||
| RD | 0.29 b | 0.29 b | 587 bc | 538 c |
| FST | 0.40 b | 0.26 b | 783 b | 1147 b |
| AST 3 | 0.39 b | 0.34 b | . | . |
| p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | |
| Treatment | N (%) | K (%) | P (%) | Mg (%) | Ca (%) | |
|---|---|---|---|---|---|---|
| Recommended rate [26] | 2.24–5.6% | 2.2–7.8% | 0.25–0.70% | 0.22–0.61% | 0.60–2.00% | |
| CVF 1 | CO | 2.21 | 1.30 | 0.17 bc 2 | 0.31 a | 0.91 a |
| CD | 2.40 | 1.29 | 0.12 d | 0.18 d | 0.50 c | |
| FSL | 2.39 | 1.38 | 0.23 a | 0.29 ab | 0.74 ab | |
| FSH | 2.66 | 1.32 | 0.23 a | 0.30 a | 0.77 a | |
| ASL | 1.69 | 1.38 | 0.20 ab | 0.25 abc | 0.68 abc | |
| ASH | 2.42 | 1.35 | 0.23 a | 0.28 abc | 0.75 ab | |
| RD | 2.61 | 1.31 | 0.14 cd | 0.23 bcd | 0.67 abc | |
| FST | 2.71 | 1.49 | 0.14 cd | 0.21 cd | 0.52 bc | |
| AST 3 | . | . | . | . | . | |
| p-value | 0.3914 | 0.2705 | <0.0001 | <0.0001 | <0.0001 | |
| TAH | CO | 1.59 c | 0.96 | 0.17 ab | 0.31 | 1.29 |
| CD | 2.28 a | 1.06 | 0.12 b | 0.26 | 1.08 | |
| FSL | 1.94 abc | 0.84 | 0.18 ab | 0.32 | 1.35 | |
| FSH | 1.83 abc | 0.86 | 0.18 ab | 0.32 | 1.26 | |
| ASL | 1.85 abc | 0.81 | 0.18 ab | 0.36 | 1.56 | |
| ASH | 2.11 abc | 0.96 | 0.22 a | 0.30 | 1.25 | |
| RD | 1.80 bc | 0.92 | 0.14 ab | 0.32 | 1.24 | |
| FST | 2.44 ab | 1.07 | 0.14 ab | 0.29 | 1.08 | |
| AST | . | . | . | . | . | |
| p-value | 0.0087 | 0.5181 | 0.0765 | 0.3150 | 0.1694 | |
| Treatment | CVF 1 | TAH |
|---|---|---|
| UC | 40.97 | 39.44 c 2 |
| ASD | 41.43 | 41.28 bc |
| TDL | 41.60 | 45.75 a |
| TDH | 42.29 | 44.42 ab |
| p-value | 0.6519 | 0.0006 |
| Treatment | N (%) | K (%) | P (%) | Mg (%) | Ca (%) | |
|---|---|---|---|---|---|---|
| Recommended rate [26] | 2.24–5.6% | 2.2–7.8% | 0.25–0.70% | 0.22–0.61% | 0.60–2.00% | |
| CVF 1 | UC | 2.93 b 2 | 1.49 b | 0.17 | 0.22 ab | 0.34 ab |
| ASD | 3.76 a | 1.80 ab | 0.17 | 0.26 a | 0.42 a | |
| TDL | 3.58 a | 1.64 b | 0.12 | 0.22 ab | 0.32 b | |
| TDH | 3.99 a | 2.05 a | 0.19 | 0.21 b | 0.31 b | |
| p-value | <0.0001 | 0.0013 | 0.0526 | 0.0317 | 0.0069 | |
| TAH | UC | 2.77 b | 0.87 b | 0.14 | 0.38 a | 0.78 a |
| ASD | 2.99 ab | 1.14 b | 0.12 | 0.37 a | 0.69 ab | |
| TDL | 3.54 ab | 1.58 a | 0.13 | 0.32 ab | 0.57 bc | |
| TDH | 3.68 a | 1.65 a | 0.14 | 0.25 b | 0.49 c | |
| p-value | 0.0287 | <0.0001 | 0.1533 | 0.0005 | <0.0001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pietsch, G.M.; Brindley, J.C.; Owen, J.S., Jr.; Fulcher, A. A Fine Line between Phytotoxicity and Blue When Producing Hydrangea macrophylla in a Nursery at a Low Substrate pH. Horticulturae 2022, 8, 690. https://doi.org/10.3390/horticulturae8080690
Pietsch GM, Brindley JC, Owen JS Jr., Fulcher A. A Fine Line between Phytotoxicity and Blue When Producing Hydrangea macrophylla in a Nursery at a Low Substrate pH. Horticulturae. 2022; 8(8):690. https://doi.org/10.3390/horticulturae8080690
Chicago/Turabian StylePietsch, Grace M., Julie C. Brindley, James S. Owen, Jr., and Amy Fulcher. 2022. "A Fine Line between Phytotoxicity and Blue When Producing Hydrangea macrophylla in a Nursery at a Low Substrate pH" Horticulturae 8, no. 8: 690. https://doi.org/10.3390/horticulturae8080690
APA StylePietsch, G. M., Brindley, J. C., Owen, J. S., Jr., & Fulcher, A. (2022). A Fine Line between Phytotoxicity and Blue When Producing Hydrangea macrophylla in a Nursery at a Low Substrate pH. Horticulturae, 8(8), 690. https://doi.org/10.3390/horticulturae8080690






