Elicitor Activity of Curdlan and Its Potential Application in Protection of Hass Avocado Plants against Phytophthora cinnamomi Rands
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Pathogen
2.3. Elicitor Preparation and Application
2.4. Observation of Lesions Caused by Phytophthora Cinnamomi on Hass Avocado Plants under Greenhouse Conditions
2.5. Sample Preparation
2.6. Measurements of Active Defense Response-Related Enzymes, Antioxidant Enzymes Activities and Total Phenolic Content in Avocado Fruit
2.7. Protein Assay
2.8. Total Phenolic Content (TPC)
2.9. DPPH Free Radical Scavenging Activity
2.10. Determination of Chlorophyll and Carotenoid Content
2.11. Statistical Analysis
3. Results
3.1. Observation of Lesions Caused by Phytophthora Cinnamomi on Hass Avocado Plants under Greenhouse Conditions
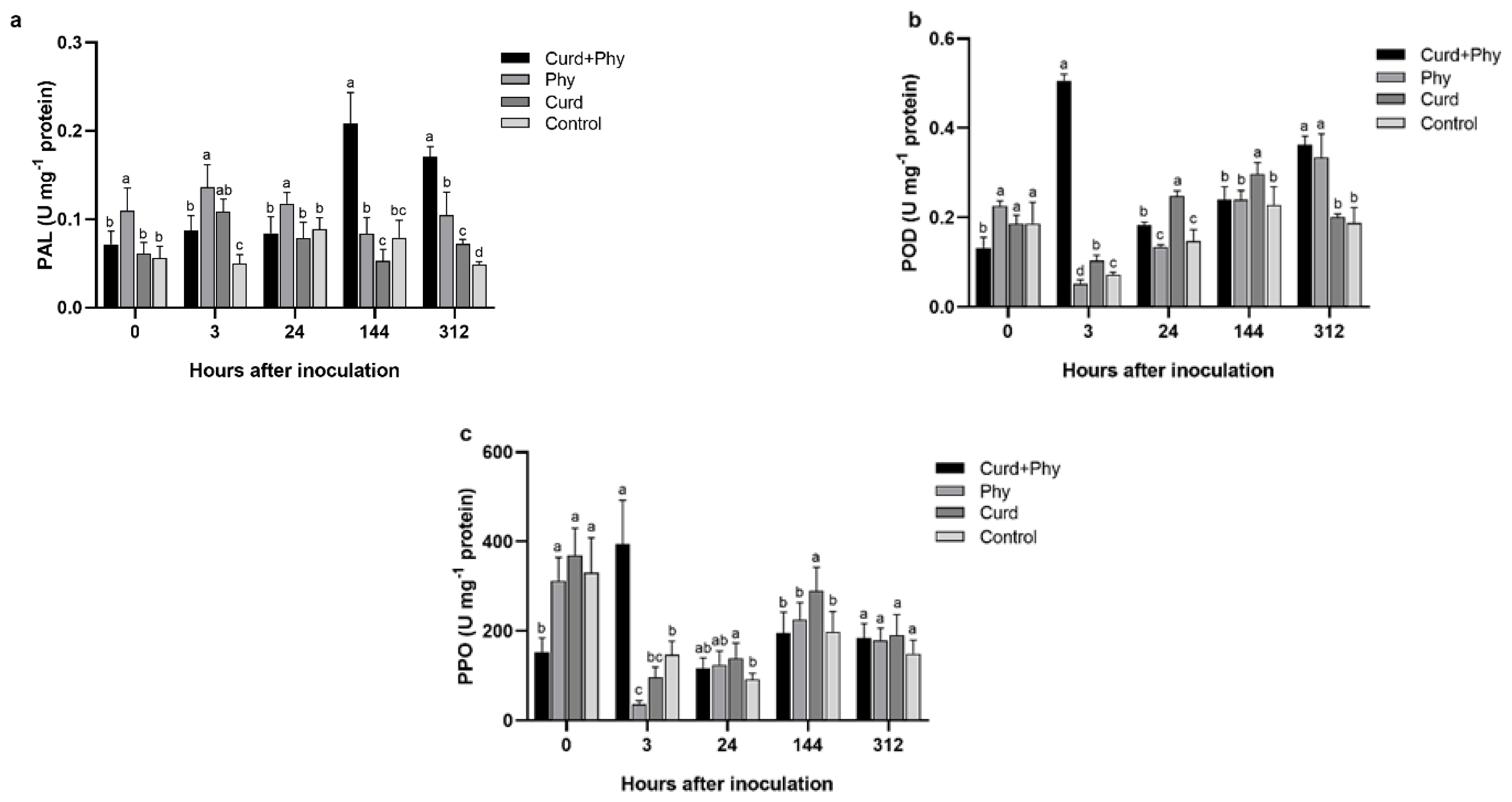
3.2. Effect of Curdlan on the Activities of Disease-Resistant Enzymes
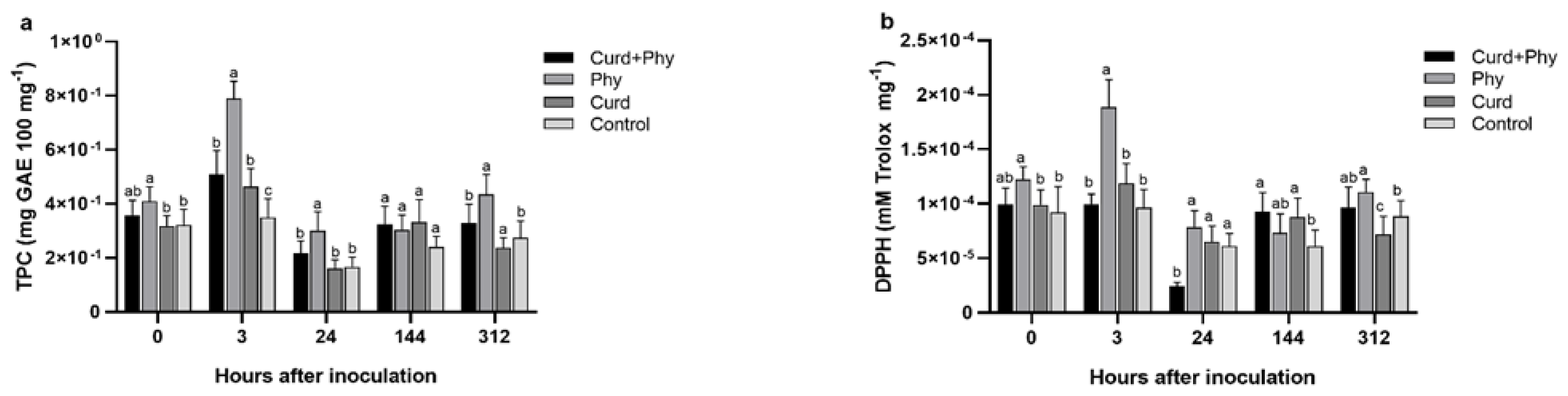
3.3. Effects of Curdlan Treatment on Total Phenol Content and Antioxidant Activity
3.4. Effect of Curdlan on Chlorophyll and Carotenoid Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Figueroa, J.G.; Borrás-Linares, I.; Lozano-Sánchez, J.; Segura-Carretero, A. Comprehensive characterization of phenolic and other polar compounds in the seed and seed coat of avocado by HPLC-DAD-ESI-QTOF-MS. Food Res. Int. 2018, 105, 752–763. [Google Scholar] [CrossRef] [PubMed]
- Migliore, G.; Farina, V.; Guccione, G.D.; Schifani, G. Quality Determinants of Avocado Fruit Consumption in Italy. Implications for Small Farms. Calitatea 2018, 19, 148–153. [Google Scholar]
- Vallejo Pérez, M.R.; Téliz Ortiz, D.; De La Torre Almaraz, R.; López Martinez, J.O.L.; Nieto Ángel, D. Avocado sunblotch viroid: Pest risk and potential impact in México. Crop Prot. 2017, 99, 118–127. [Google Scholar] [CrossRef]
- Kosińska, A.; Karamać, M.; Estrella, I.; Hernández, T.; Bartolomé, B.a.; Dykes, G.A. Phenolic compound profiles and antioxidant capacity of Persea americana Mill. peels and seeds of two varieties. J. Agric. Food Chem. 2012, 60, 4613–4619. [Google Scholar] [CrossRef] [PubMed]
- Ferreyra, R.; Sellés, G.; Saavedra, J.; Ortiz, J.; Zúñiga, C.; Troncoso, C.; Rivera, S.A.; González-Agüero, M.; Defilippi, B.G. Identification of pre-harvest factors that affect fatty acid profiles of avocado fruit (Persea americana Mill) cv. ‘Hass’ at harvest. S. Afr. J. Bot. 2016, 104, 15–20. [Google Scholar] [CrossRef]
- Ciro, D.; Rendon, K.; Navarro, R. Reconocimiento de la pudrición de raíces (Phytophthora cinnamomi) en aguacate (Persea americana) en Antioquia. Rev. Univ. Católica Oriente 2006, 22, 41–51. [Google Scholar]
- Tamayo Molano, P.J. Enfermedades del Aguacate. Rev. Politec. 2007, 3, 51–70. [Google Scholar]
- Pérez-Jiménez, R.M. Significant avocado diseases caused by fungi and oomycetes. Eur. J. Plant Sci. Biotechnol. 2008, 2, 1–24. [Google Scholar]
- Ramírez Gil, J.G.; Castañeda Sánchez, D.A.; Morales Osorio, J.G. Alternativas microbiológicas para el manejo de Phytophthora cinnamomi Rands., en Persea americana Mill. bajo condiciones de casa-malla. Cultiv. Trop. 2014, 35, 19–27. [Google Scholar]
- Kamoun, S.; Furzer, O.; Jones, J.D.G.; Judelson, H.S.; Ali, G.S.; Dalio, R.J.D.; Roy, S.G.; Schena, L.; Zambounis, A.; Panabières, F.; et al. The Top 10 oomycete pathogens in molecular plant pathology. Mol. Plant Pathol. 2015, 16, 413–434. [Google Scholar] [CrossRef]
- Hardham, A.R.; Blackman, L.M. Phytophthora cinnamomi. Mol. Plant Pathol. 2018, 19, 260–285. [Google Scholar] [CrossRef] [Green Version]
- Romero-Correa, M.T.; Villa-Gómez, R.; Castro-Mercado, E.; García-Pineda, E. The avocado defense compound phenol-2,4-bis (1,1-dimethylethyl) is induced by arachidonic acid and acts via the inhibition of hydrogen peroxide production by pathogens. Physiol. Mol. Plant Pathol. 2014, 87, 32–41. [Google Scholar] [CrossRef]
- El Modafar, C.; Elgadda, M.; El Boutachfaiti, R.; Abouraicha, E.; Zehhar, N.; Petit, E.; El Alaoui-Talibi, Z.; Courtois, B.; Courtois, J. Induction of natural defence accompanied by salicylic acid-dependant systemic acquired resistance in tomato seedlings in response to bioelicitors isolated from green algae. Sci. Hortic. 2012, 138, 55–63. [Google Scholar] [CrossRef]
- Cheplick, S.; Sarkar, D.; Bhowmik, P.C.; Shetty, K. Improved resilience and metabolic response of transplanted blackberry plugs using chitosan oligosaccharide elicitor treatment. Can. J. Plant Sci. 2017, 98, 717–731. [Google Scholar] [CrossRef] [Green Version]
- Shetty, N.P.; Jensen, J.D.; Knudsen, A.; Finnie, C.; Geshi, N.; Blennow, A.; Collinge, D.B.; Jørgensen, H.J.L. Effects of β-1,3-glucan from Septoria tritici on structural defence responses in wheat. J. Exp. Bot. 2009, 60, 4287–4300. [Google Scholar] [CrossRef] [Green Version]
- Davidsson, P.; Broberg, M.; Kariola, T.; Sipari, N.; Pirhonen, M.; Palva, E.T. Short oligogalacturonides induce pathogen resistance-associated gene expression in Arabidopsis thaliana. BMC Plant Biol. 2017, 17, 19. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef] [Green Version]
- Jukanti, A. Polyphenol Oxidases (PPOs) in Plants; Springer: Singapore, 2017. [Google Scholar] [CrossRef]
- Sobhy, I.S.; Erb, M.; Lou, Y.; Turlings, T.C.J. The prospect of applying chemical elicitors and plant strengtheners to enhance the biological control of crop pests. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20120283. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Davis, L.C.; Verpoorte, R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol. Adv. 2005, 23, 283–333. [Google Scholar] [CrossRef]
- Maffei, M.E.; Arimura, G.-I.; Mithöfer, A. Natural elicitors, effectors and modulators of plant responses. Nat. Prod. Rep. 2012, 29, 1288–1303. [Google Scholar] [CrossRef]
- Li, J.; Zhu, L.; Lu, G.; Zhan, X.-B.; Lin, C.-C.; Zheng, Z.-Y. Curdlan β-1,3-glucooligosaccharides induce the defense responses against Phytophthora infestans infection of potato (Solanum tuberosum L. cv. McCain G1) leaf cells. PLoS ONE 2014, 9, e97197. [Google Scholar] [CrossRef]
- Fu, Y.; Yin, H.; Wang, W.; Wang, M.; Zhang, H.; Zhao, X.; Du, Y. β-1,3-Glucan with different degree of polymerization induced different defense responses in tobacco. Carbohydr. Polym. 2011, 86, 774–782. [Google Scholar] [CrossRef]
- Namdeo, A. Plant cell elicitation for production of secondary metabolites: A review. Pharmacogn. Rev. 2007, 1, 69–79. [Google Scholar]
- Dinis, L.-T.; Peixoto, F.; Zhang, C.; Martins, L.; Costa, R.; Gomes-Laranjo, J. Physiological and biochemical changes in resistant and sensitive chestnut (Castanea) plantlets after inoculation with Phytophthora cinnamomi. Physiol. Mol. Plant Pathol. 2011, 75, 146–156. [Google Scholar] [CrossRef]
- Jorge, T.F.; Rodrigues, J.A.; Caldana, C.; Schmidt, R.; van Dongen, J.T.; Thomas-Oates, J.; António, C. Mass spectrometry-based plant metabolomics: Metabolite responses to abiotic stress. Mass Spectrom. Rev. 2016, 35, 620–649. [Google Scholar] [CrossRef] [PubMed]
- Sellamuthu, P.S.; Sivakumar, D.; Soundy, P.; Korsten, L. Essential oil vapours suppress the development of anthracnose and enhance defence related and antioxidant enzyme activities in avocado fruit. Postharvest Biol. Technol. 2013, 81, 66–72. [Google Scholar] [CrossRef] [Green Version]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Demiral, T.; Türkan, İ. Comparative lipid peroxidation, antioxidant defense systems and proline content in roots of two rice cultivars differing in salt tolerance. Environ. Exp. Bot. 2005, 53, 247–257. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Smith, I.K.; Vierheller, T.L.; Thorne, C.A. Assay of glutathione reductase in crude tissue homogenates using 5,5′-dithiobis(2-nitrobenzoic acid). Anal. Biochem. 1988, 175, 408–413. [Google Scholar] [CrossRef]
- Xie, J.-H.; Chai, T.-T.; Xu, R.; Liu, D.; Yang, Y.-X.; Deng, Z.-C.; Jin, H.; He, H. Induction of defense-related enzymes in patchouli inoculated with virulent Ralstonia solanacearum. Electron. J. Biotechnol. 2017, 27, 63–69. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Makkar, H.P. Quantification of Tannins in Tree and Shrub Foliage: A Laboratory Manual; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Alcântara, M.A.; de Lima Brito Polari, I.; de Albuquerque Meireles, B.R.L.; de Lima, A.E.A.; da Silva Junior, J.C.; de Andrade Vieira, É.; dos Santos, N.A.; de Magalhães Cordeiro, A.M.T. Effect of the solvent composition on the profile of phenolic compounds extracted from chia seeds. Food Chem. 2019, 275, 489–496. [Google Scholar] [CrossRef]
- Metzner, H.; Rau, H.; Senger, H. Untersuchungen zur Synchronisierbarkeit einzelner Pigmentmangel-Mutanten von Chlorella. Planta 1965, 65, 186–194. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. hlorophylls and carotenoids: Pigments of photosynthetic biomembranes. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1987; Volume 148, pp. 350–382. [Google Scholar]
- Saed-Moucheshi, A.; Shekoofa, A.; Pessarakli, M. Reactive oxygen species (ROS) generation and detoxifying in plants. J. Plant Nutr. 2014, 37, 1573–1585. [Google Scholar] [CrossRef]
- Ge, Y.; Bi, Y.; Guest, D.I. Defence responses in leaves of resistant and susceptible melon (Cucumis melo L.) cultivars infected with Colletotrichum lagenarium. Physiol. Mol. Plant Pathol. 2013, 81, 13–21. [Google Scholar] [CrossRef]
- Siddique, Z.; Akhtar, K.P.; Hameed, A.; Sarwar, N.; Imran Ul, H.; Khan, S.A. Biochemical alterations in leaves of resistant and susceptible cotton genotypes infected systemically by cotton leaf curl Burewala virus. J. Plant Interact. 2014, 9, 702–711. [Google Scholar] [CrossRef] [Green Version]
- Kundu, S.; Chakraborty, D.; Pal, A. Proteomic analysis of salicylic acid induced resistance to Mungbean Yellow Mosaic India Virus in Vigna mungo. J. Proteom. 2011, 74, 337–349. [Google Scholar] [CrossRef]
- Bisswanger, H. Enzyme assays. Perspect. Sci. 2014, 1, 41–55. [Google Scholar] [CrossRef] [Green Version]
- Hammerschmidt, R. Phenols and plant–pathogen interactions: The saga continues. Physiol. Mol. Plant Pathol. 2005, 66, 77–78. [Google Scholar] [CrossRef]
- Witzell, J.; Martín, J.A. Phenolic metabolites in the resistance of northern forest trees to pathogens—Past experiences and future prospects. Can. J. For. Res. 2008, 38, 2711–2727. [Google Scholar] [CrossRef]
- Zhang, S.; Tang, W.; Jiang, L.; Hou, Y.; Yang, F.; Chen, W.; Li, X. Elicitor activity of algino-oligosaccharide and its potential application in protection of rice plant (Oryza saliva L.) against Magnaporthe grisea. Biotechnol. Biotechnol. Equip. 2015, 29, 646–652. [Google Scholar] [CrossRef] [Green Version]
- Taheri, P.; Tarighi, S. Riboflavin induces resistance in rice against Rhizoctonia solani via jasmonate-mediated priming of phenylpropanoid pathway. J. Plant Physiol. 2010, 167, 201–208. [Google Scholar] [CrossRef]
- Sudisha, J.; Kumar, A.; Amruthesh, K.N.; Niranjana, S.R.; Shetty, H.S. Elicitation of resistance and defense related enzymes by raw cow milk and amino acids in pearl millet against downy mildew disease caused by Sclerospora graminicola. Crop Prot. 2011, 30, 794–801. [Google Scholar] [CrossRef]
- Rawal, H.; Singh, N.; Sharma, T. Conservation, divergence, and genome-wide distribution of PAL and POX A gene families in plants. Int. J. Genom. 2013, 2013, 678969. [Google Scholar] [CrossRef] [Green Version]
- Taheri, P.; Irannejad, A.; Goldani, M.; Tarighi, S. Oxidative burst and enzymatic antioxidant systems in rice plants during interaction with Alternaria alternata. Eur. J. Plant Pathol. 2014, 140, 829–839. [Google Scholar] [CrossRef]
- Thakker, J.N.; Patel, S.; Dhandhukia, P.C. Induction of defense-related enzymes in banana plants: Effect of live and dead pathogenic strain of Fusarium oxysporum f. sp. cubense. ISRN Biotechnol. 2013, 2013, 601303. [Google Scholar] [CrossRef] [Green Version]
- Koç, E.; Üstün, A.S. Influence of Phytophthora capsici L. inoculation on disease severity, necrosis length, peroxidase and catalase activity, and phenolic content of resistant and susceptible pepper (Capsicum annuum L.) plants. Turk. J. Biol. 2012, 36, 357–371. [Google Scholar] [CrossRef]
- Ngadze, E.; Icishahayo, D.; Coutinho, T.A.; van der Waals, J.E. Role of Polyphenol Oxidase, Peroxidase, Phenylalanine Ammonia Lyase, Chlorogenic Acid, and Total Soluble Phenols in Resistance of Potatoes to Soft Rot. Plant Dis. 2012, 96, 186–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Wang, D.; Gao, X.; Yue, Z.; Zhou, H. Exogenous caffeic acid and epicatechin enhance resistance against Botrytis cinerea through activation of the phenylpropanoid pathway in apples. Sci. Hortic. 2020, 268, 109348. [Google Scholar] [CrossRef]
- Silva, R.; Pascholati, S.; Bedendo, I. Induced resistance in tomato plants to Clavibacter michiganensis subsp. Michiganensis by Lentinula edodes and Agaricus subrufescens (syn. Agaricus brasiliensis). J. Plant Pathol. 2013, 95, 285–297. [Google Scholar] [CrossRef]
- Xu, H.; Li, J.; Wang, S.; Xu, L.; Cheng, R.; Zhang, J. Oligosaccharide elicitor prepared from Salecan triggers the defense responses of Arabidopsis thaliana Col0 against Botrytis cinerea infection. World J. Microbiol. Biotechnol. 2017, 33, 165. [Google Scholar] [CrossRef]
- Tománková, K.; Luhová, L.; Petřivalský, M.; Peč, P.; Lebeda, A. Biochemical aspects of reactive oxygen species formation in the interaction between Lycopersicon spp. and Oidium neolycopersici. Physiol. Mol. Plant Pathol. 2006, 68, 22–32. [Google Scholar] [CrossRef]
- Delpino-Rius, A.; Eras, J.; Vilaró, F.; Cubero, M.Á.; Balcells, M.; Canela-Garayoa, R. Characterisation of phenolic compounds in processed fibres from the juice industry. Food Chem. 2015, 172, 575–584. [Google Scholar] [CrossRef]
- Zhang, X.; Li, K.; Liu, S.; Xing, R.; Yu, H.; Chen, X.; Li, P. Size effects of chitooligomers on the growth and photosynthetic characteristics of wheat seedlings. Carbohydr. Polym. 2016, 138, 27–33. [Google Scholar] [CrossRef]
- Gomathi, R.; Rakkiyapan, P. Comparative lipid peroxidation, leaf membrane thermostability, and antioxidant system in four sugarcane genotypes differing in salt tolerance. Int. J. Plant Physiol. Biochem. 2011, 3, 67–74. [Google Scholar] [CrossRef]
- Muley, A.B.; Shingote, P.R.; Patil, A.P.; Dalvi, S.G.; Suprasanna, P. Gamma radiation degradation of chitosan for application in growth promotion and induction of stress tolerance in potato (Solanum tuberosum L.). Carbohydr. Polym. 2019, 210, 289–301. [Google Scholar] [CrossRef]
- Zong, H.; Liu, S.; Xing, R.; Chen, X.; Li, P. Protective effect of chitosan on photosynthesis and antioxidative defense system in edible rape (Brassica rapa L.) in the presence of cadmium. Ecotoxicol. Environ. Saf. 2017, 138, 271–278. [Google Scholar] [CrossRef]
- Zou, P.; Tian, X.; Dong, B.; Zhang, C. Size effects of chitooligomers with certain degrees of polymerization on the chilling tolerance of wheat seedlings. Carbohydr. Polym. 2017, 160, 194–202. [Google Scholar] [CrossRef]
- Abhayashree, M.S.; Murali, M.; Thriveni, M.C.; Sindhu, G.M.; Amruthesh, K.N. Crude oligosaccharides mediated resistance and histo-chemical changes in Capsicum annuum against anthracnose disease caused by Colletotrichum capsici. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2017, 151, 221–233. [Google Scholar] [CrossRef]
- Basavaraju, P.; Shetty, N.P.; Shetty, H.S.; de Neergaard, E.; Jørgensen, H.J. Infection induced defence responses in sorghum, with special emphasis on accumulation of reactive oxygen species and cell wall modifications. Arch. Phytopathol. Plant Prot. 2010, 43, 1295–1317. [Google Scholar] [CrossRef]
- García-Pineda, E.; Benezer-Benezer, M.; Gutiérrez-Segundo, A.; Rangel-Sánchez, G.; Arreola-Cortés, A.; Castro-Mercado, E. Regulation of defence responses in avocado roots infected with Phytophthora cinnamomi (Rands). Plant Soil 2010, 331, 45–56. [Google Scholar] [CrossRef]
- Vleeshouwers, V.G.A.A.; van Dooijeweert, W.; Govers, F.; Kamoun, S.; Colon, L.T. The hypersensitive response is associated with host and nonhost resistance to Phytophthora infestans. Planta 2000, 210, 853–864. [Google Scholar] [CrossRef]
- Islam, M.T.; Rookes, J.E.; Cahill, D.M. Active defence by an Australian native host, Lomandra longifolia, provides resistance against Phytophthora cinnamomi. Funct. Plant Biol. 2017, 44, 386–399. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.; Wan, G.; Liang, Z. Accumulation of salicylic acid-induced phenolic compounds and raised activities of secondary metabolic and antioxidative enzymes in Salvia miltiorrhiza cell culture. J. Biotechnol. 2010, 148, 99–104. [Google Scholar] [CrossRef]
- Künstler, A.; Bacsó, R.; Gullner, G.; Hafez, Y.M.; Király, L. Staying alive–is cell death dispensable for plant disease resistance during the hypersensitive response? Physiol. Mol. Plant Pathol. 2016, 93, 75–84. [Google Scholar] [CrossRef]
- Eshraghi, L.; Anderson, J.; Aryamanesh, N.; Shearer, B.; McComb, J.; Hardy, G.E.S.; O’Brien, P.A. Phosphite primed defence responses and enhanced expression of defence genes in Arabidopsis thaliana infected with Phytophthora cinnamomi. Plant Pathol. 2011, 60, 1086–1095. [Google Scholar] [CrossRef]
- Trouvelot, S.; Héloir, M.-C.; Poinssot, B.; Gauthier, A.; Paris, F.; Guillier, C.; Combier, M.; Trdá, L.; Daire, X.; Adrian, M. Carbohydrates in plant immunity and plant protection: Roles and potential application as foliar sprays. Front. Plant Sci. 2014, 5, 592. [Google Scholar] [CrossRef] [Green Version]



| Treatment | Average Length of the Lesion (cm) | ||
|---|---|---|---|
| 3 dai | 6 dai | 9 dai | |
| Curd + Phy | 2.32 ± 0.32 a | 3.39 ± 0.34 a | 3.90 ± 0.24 a |
| Phy | 3.56 ± 0.24 b | 4.53 ± 0.28 b | 5.69 ± 0.31 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guarnizo, N.; Álvarez, A.; Oliveros, D.; Barbosa, O.; Joli, J.E.; Bermúdez-Cardona, M.B.; Murillo-Arango, W. Elicitor Activity of Curdlan and Its Potential Application in Protection of Hass Avocado Plants against Phytophthora cinnamomi Rands. Horticulturae 2022, 8, 646. https://doi.org/10.3390/horticulturae8070646
Guarnizo N, Álvarez A, Oliveros D, Barbosa O, Joli JE, Bermúdez-Cardona MB, Murillo-Arango W. Elicitor Activity of Curdlan and Its Potential Application in Protection of Hass Avocado Plants against Phytophthora cinnamomi Rands. Horticulturae. 2022; 8(7):646. https://doi.org/10.3390/horticulturae8070646
Chicago/Turabian StyleGuarnizo, Nathalie, Andree Álvarez, Diego Oliveros, Oveimar Barbosa, Jordi Eras Joli, María Bianney Bermúdez-Cardona, and Walter Murillo-Arango. 2022. "Elicitor Activity of Curdlan and Its Potential Application in Protection of Hass Avocado Plants against Phytophthora cinnamomi Rands" Horticulturae 8, no. 7: 646. https://doi.org/10.3390/horticulturae8070646
APA StyleGuarnizo, N., Álvarez, A., Oliveros, D., Barbosa, O., Joli, J. E., Bermúdez-Cardona, M. B., & Murillo-Arango, W. (2022). Elicitor Activity of Curdlan and Its Potential Application in Protection of Hass Avocado Plants against Phytophthora cinnamomi Rands. Horticulturae, 8(7), 646. https://doi.org/10.3390/horticulturae8070646







