Root Morphological and Physiological Adaptations to Low Phosphate Enhance Phosphorus Efficiency at Melon (Cucumis melo L.) Seedling Stage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Experimental Design
2.1.1. Experiment 1: Low-Pi Stress Test
2.1.2. Experiment 2: Short-Term Phosphate Absorption Test
2.2. Measurement of Shoot Morphological Indicators
2.3. Measurement of Dry Weight and the Root: Shoot Ratio
2.4. Measurement of Root Morphology Traits
2.5. Measurement of Nutrient Element Content
2.6. Measurement of Acid Phosphatase Activity and Root Vitality
2.7. Gene Expression Analysis
2.8. Calculation of Pi Uptake Rate and P Utilization Efficiency
2.9. Statistical Analyses
3. Results
3.1. Growth of Melon Seedlings under Low-Pi Stress
3.2. Nutrient Homeostasis in Melon Seedlings under Low-Pi Stress
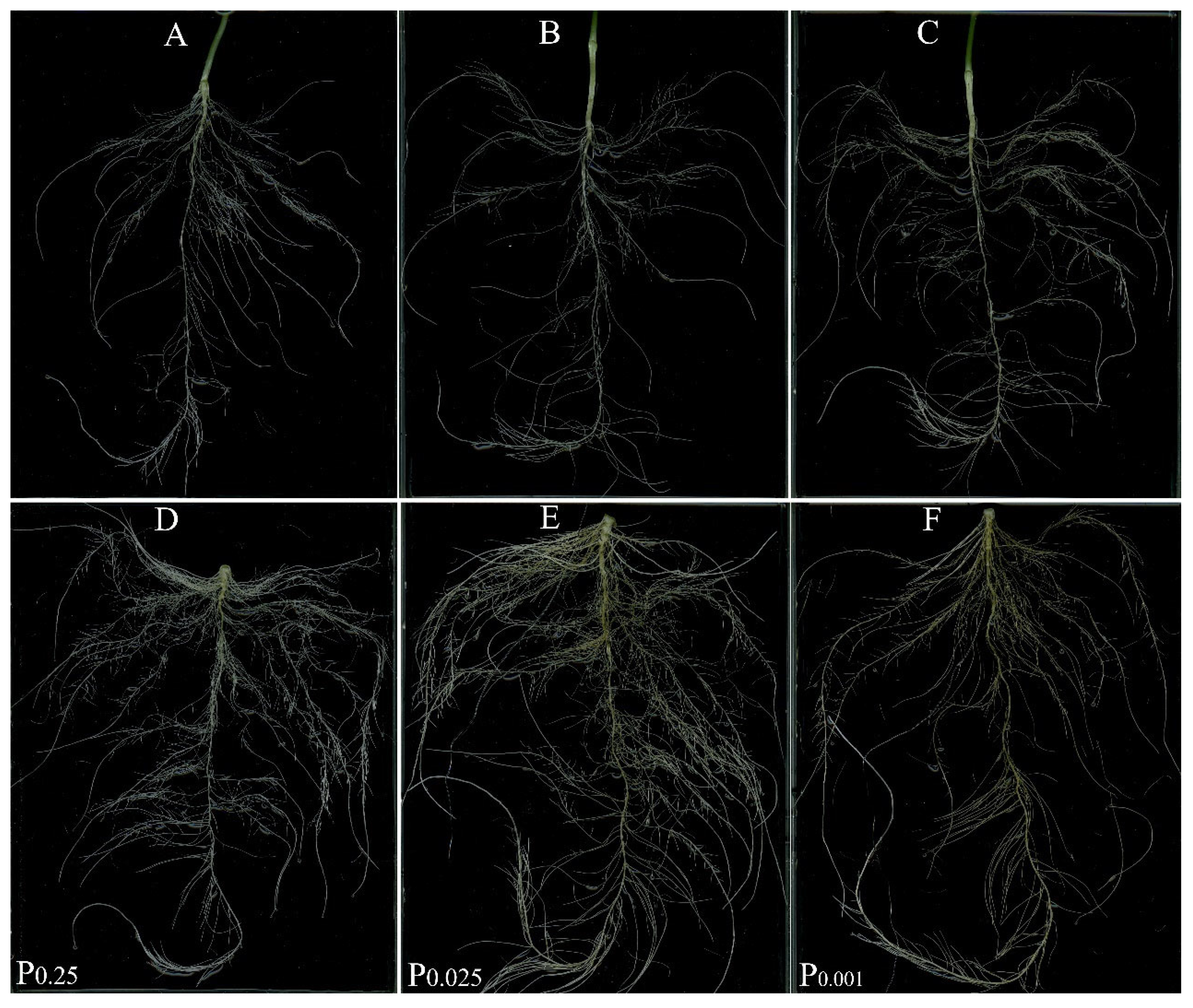
3.3. Root Morphology Changes under Low-Pi Stress
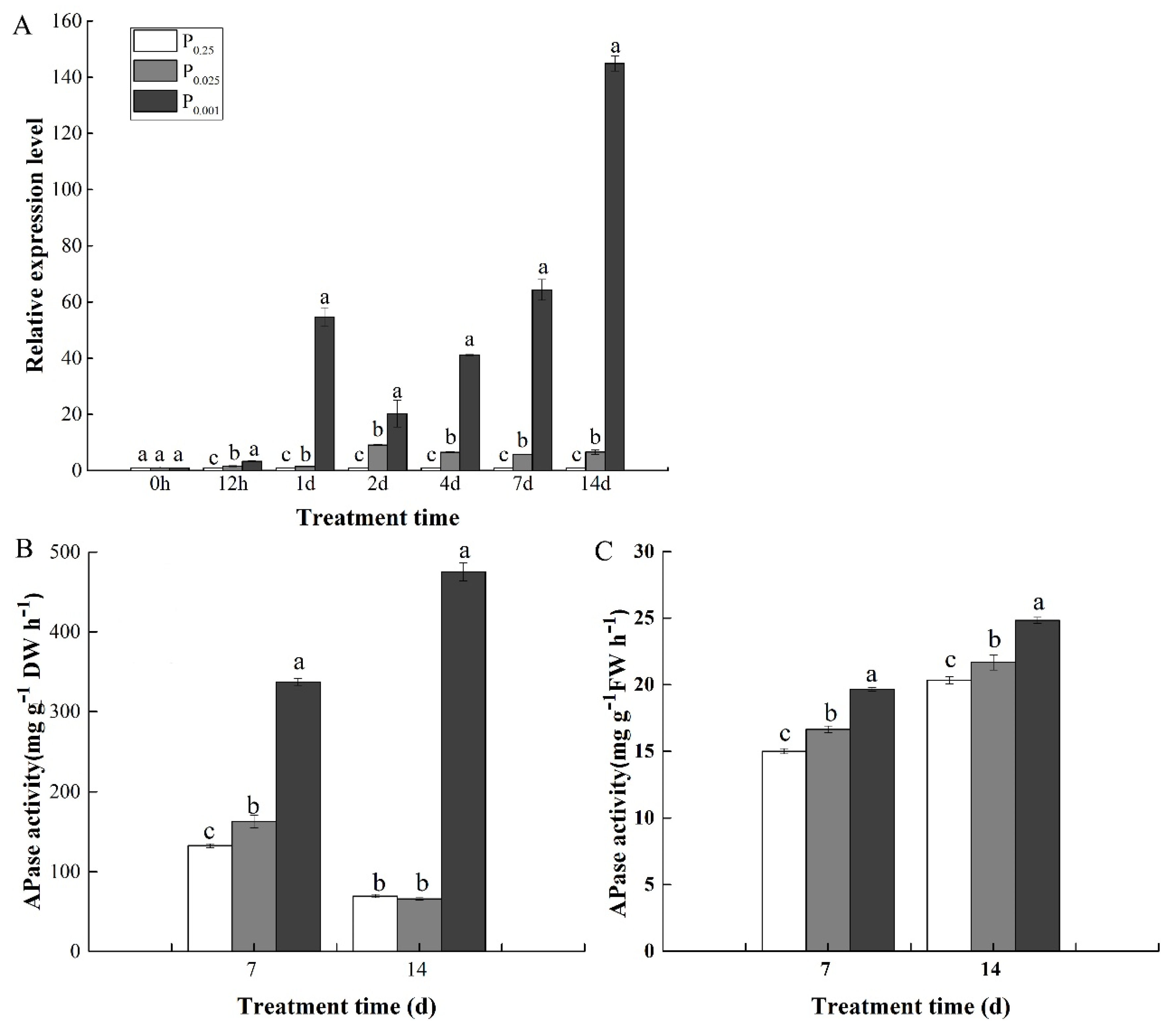
3.4. Gene Expression and Activity of Acid Phosphatase (APase) under Low-Pi Stress
3.5. Expression of High-Affinity Phosphate Transporter Genes under Low-Pi Stress
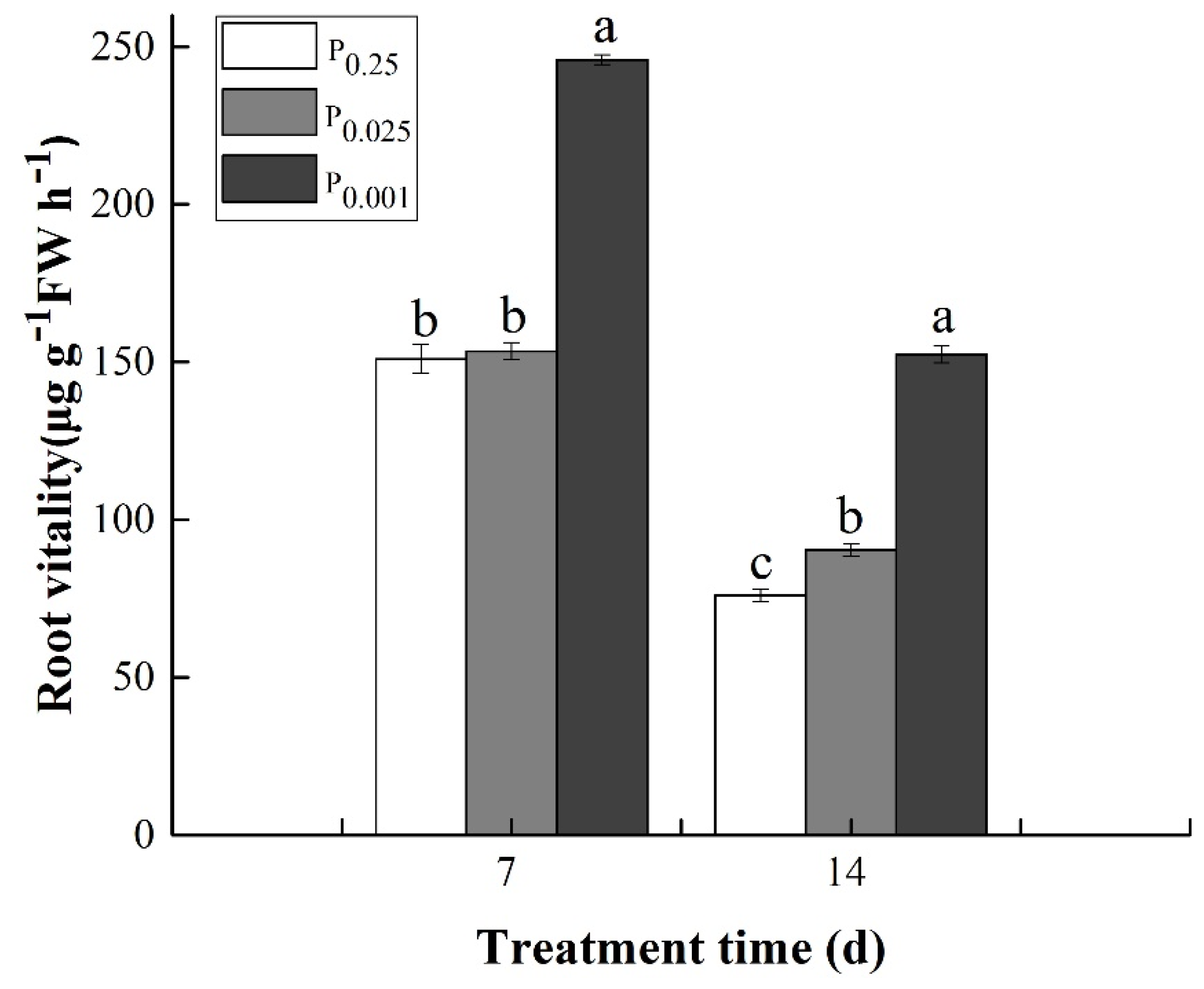
3.6. Root Vitality under Low-Pi Stress
3.7. PUR and PUE under Low-Pi Stress
4. Discussion
4.1. Effect of Low-Pi Stress on Nutrient Homeostasis in Melon Seedlings
4.2. The Longer Primary Root Was Induced by Low-Pi Stress in Melon Seedlings
4.3. Pi Absorption and Activation under Low-Pi Conditions
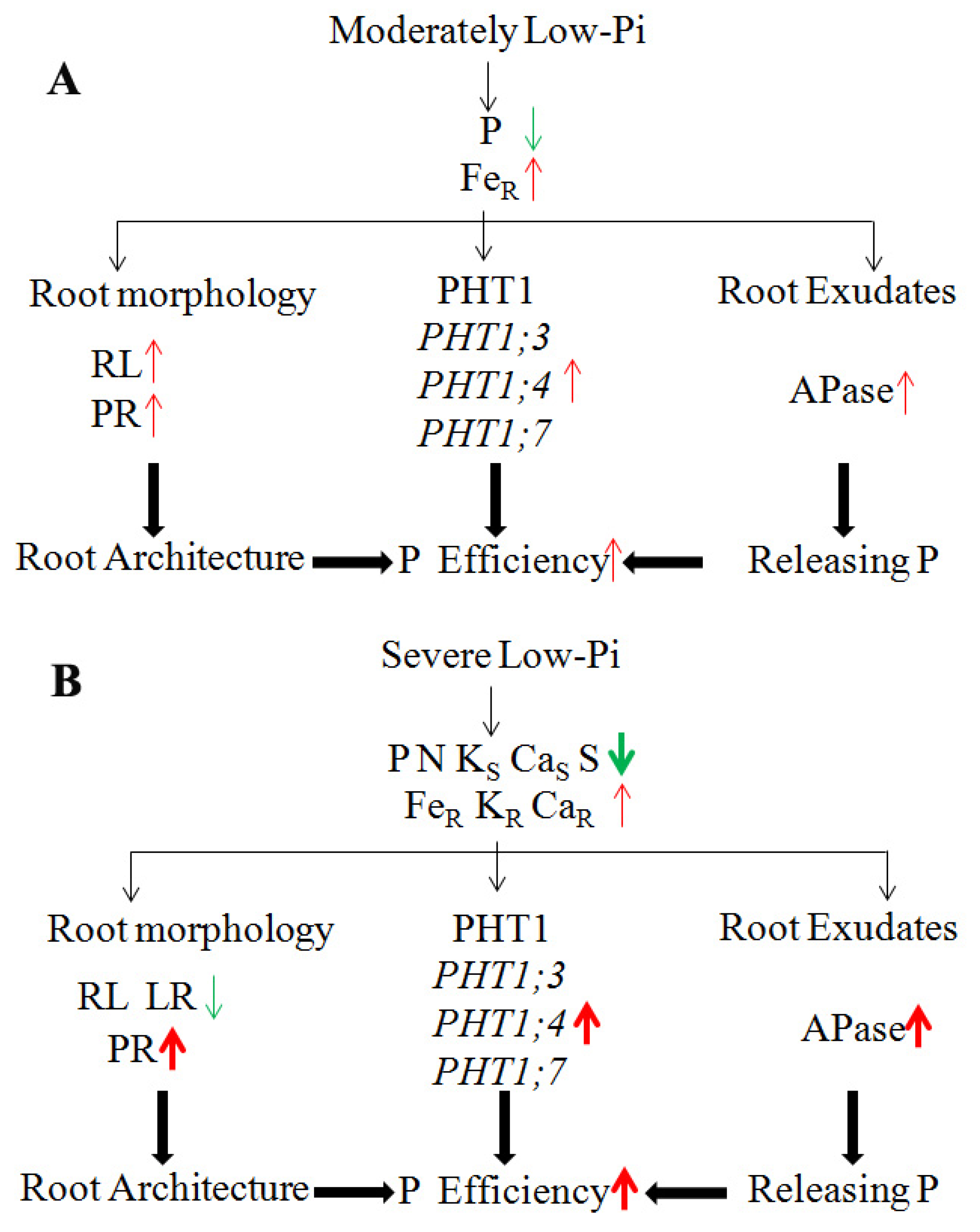
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Rausch, C.; Bucher, M. Molecular mechanisms of phosphate transport in plants. Planta 2002, 216, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Post, W.M.; Thornton, P.E.; Jain, A. The distribution of soil phosphorus for global biogeochemical modeling. Biogeosciences 2013, 10, 2525–2537. [Google Scholar] [CrossRef] [Green Version]
- MacDonald, G.K.; Bennett, E.M.; Potter, P.A.; Ramankutty, N. Agronomic phosphorus imbalances across the world’s croplands. Proc. Natl. Acad. Sci. USA 2011, 108, 3086–3091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alewell, C.; Ringeval, B.; Ballabio, C.; Robinson, D.A.; Panagos, P.; Borrelli, P. Global phosphorus shortage will be aggravated by soil erosion. Nat. Commun. 2020, 11, 4546. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Arredondo, D.L.; Leyva-González, M.A.; González-Morales, S.I.; López-Bucio, J.; Herrera-Estrella, L. Phosphate nutrition: Improving low-phosphate tolerance in crops. Annu. Rev. Plant Biol. 2014, 65, 95–123. [Google Scholar] [CrossRef]
- Andersson, H.; Bergström, L.; Djodjic, F.; Ulén, B.; Kirchmann, H. Topsoil and subsoil properties influence phosphorus leaching from four agricultural soils. J. Environ. Qual. 2013, 42, 455–463. [Google Scholar] [CrossRef] [Green Version]
- Lambers, H.; Ahmedi, I.; Berkowitz, O.; Dunne, C.; Finnegan, P.M.; Hardy, G.E.S.J.; Jost, R.; Laliberté, E.; Pearse, S.J.; Teste, F.P. Phosphorus nutrition of phosphorus-sensitive Australian native plants: Threats to plant communities in a global biodiversity hotspot. Conserv. Physiol. 2013, 1, cot010. [Google Scholar] [CrossRef] [Green Version]
- Cordell, D.; Drangert, J.; White, S. The story of phosphorus: Global food security and food for thought. Glob. Environ. Chang. 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Kopriva, S.; Chu, C. Are we ready to improve phosphorus homeostasis in rice? J. Exp. Bot. 2018, 69, 3515–3522. [Google Scholar] [CrossRef] [Green Version]
- van de Wiel, C.C.M.; van der Linden, C.G.; Scholten, O.E. Improving phosphorus use efficiency in agriculture: Opportunities for breeding. Euphytica 2016, 207, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Scheible, W.R.; Rojas Triana, M. Sensing, signalling, and control of phosphate starvation in plants: Molecular players and applications. Annu. Plant Rev. 2018, 48, 25–64. [Google Scholar] [CrossRef]
- Vengavasi, K.; Pandey, R.; Abraham, G.; Yadav, R.K. Comparative analysis of soybean root proteome reveals molecular basis of differential carboxylate efflux under low phosphorus stress. Genes 2017, 8, 341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chevalier, F.; Pata, M.; Nacry, P.; Doumas, P.; Rossignol, M. Effects of phosphate availability on the root system architecture: Large-scale analysis of the natural variation between Arabidopsis accessions. Plant Cell Environ. 2003, 26, 1839–1850. [Google Scholar] [CrossRef] [Green Version]
- Neumann, G.; Massonneau, A.; Martinoia, E.; Römheld, V. Physiological adaptations to phosphorus deficiency during proteoid root development in white lupin. Planta 1999, 208, 373–382. [Google Scholar] [CrossRef]
- Shimizu, A.; Yanagihara, S.; Kawasak, S.; Ikehashi, H. Phosphorus deficiency-induced root elongation and its QTL in rice (Oryza sativa L.). Theor. Appl. Genet. 2004, 109, 1361–1368. [Google Scholar] [CrossRef]
- Hoffland, E.; Wei, C.; Wissuwa, M. Organic anion exudation by lowland rice (Oryza sativa L.) at zinc and phosphorus deficiency. Plant Soil 2006, 283, 155–162. [Google Scholar] [CrossRef]
- Atemkeng, M.F.; Remans, R.; Michiels, J.; Tagne, A.; Ngonkeu, E.L.M. Inoculation with rhizobium etli enhances organic acid exudation in common bean (Phaseolus vulgaris L.) subjected to phosphorus deficiency. Afr. J. Agric. Res. 2011, 6, 2235–2242. [Google Scholar] [CrossRef]
- Bonser, A.M.; Lynch, J.; Snapp, S. Effect of phosphorus deficiency on growth angle of basal roots in Phaseolus vulgaris. New Phytol. 1996, 132, 281–288. [Google Scholar] [CrossRef]
- Tang, H.; Chen, X.; Gao, Y.; Hong, L.; Chen, Y. Alteration in root morphological and physiological traits of two maize cultivars in response to phosphorus deficiency. Rhizosphere 2020, 14, 100201. [Google Scholar] [CrossRef]
- Zhang, Z.; Liao, H.; Lucas, W.J. Molecular mechanisms underlying phosphate sensing, signaling, and adaptation in plants. J. Integr. Plant Biol. 2014, 56, 192–220. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, H.; Tao, P.; Chen, H. Comparative proteomic analyses provide new insights into low phosphorus stress responses in maize leaves. PLoS ONE 2014, 9, e98215. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Jain, A.; Ai, H.; Liu, X.; Wang, X.; Hu, Z.; Sun, Y.; Hu, S.; Shen, X.; Lan, X.; et al. OsPDR2 mediates the regulation on the development response and maintenance of Pi homeostasis in rice. Plant Physiol. Biochem. 2020, 149, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chiou, T.; Lin, S. Signaling network in sensing phosphate availability in plants. Annu. Rev. Plant. Biol. 2011, 62, 185–206. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.M.; Wang, Z.Q.; Wang, J.Y.; Li, P.F.; Jin, J.F.; Chen, W.W.; Fan, W.; Kochian, L.V.; Zheng, S.J.; Yang, J.L. Low phosphate represses histone deacetylase complex1 to regulate root system architecture remodeling in Arabidopsis. New Phytol. 2020, 225, 1732–1745. [Google Scholar] [CrossRef]
- Available online: https://www.fao.org/faostat/zh/#data/QC (accessed on 18 November 2021).
- Fita, A.; Nuez, F.; Picó, B. Diversity in root architecture and response to P deficiency in seedlings of Cucumis melo L. Euphytica 2011, 181, 323–339. [Google Scholar] [CrossRef]
- Fita, A.; Bowen, H.C.; Hayden, R.M.; Nuez, F.; Pico, B.; Hammond, J.P. Diversity in expression of phosphorus responsive genes in Cucumis melo L. PLoS ONE 2012, 7, e35387. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Tang, X.; Vance, C.P.; White, P.J.; Zhang, F.; Shen, J. Interactions between light intensity and phosphorus nutrition affect the phosphate-mining capacity of white lupin (Lupinus albus L.). J. Exp. Bot. 2014, 65, 2995–3003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunter, M.; Leong, G.; Mitchell, J.; Dieters, M.; Fujinuma, R. Constant water table sub-irrigation of pots allows derivation of root weights (without physical recovery) and repeated measures of in situ growth and water use efficiencies. Plant Soil 2018, 425, 1–19. [Google Scholar] [CrossRef]
- Ogner, G.; Wickstrøm, T.; Remedios, G.; Gjelsvik, S.; Hensel, G.R.; Jacobsen, J.E.; Olsen, M.; Skretting, E.; Sørlie, B. The Chemical Analysis Program of the Norwegian Forest Research Institute 2000; Internal Report; Norwegian Forest Research Institute: Ås, Norway, 1999. [Google Scholar]
- Gilbert, G.A.; Knight, J.D.; Vance, C.P.; Allan, D.L. Acid phosphatase activity in phosphorus-deficient white lupin roots. Plant Cell Environ. 1999, 22, 801–810. [Google Scholar] [CrossRef]
- McMichael, B.L.; Burke, J.J. Metabolic activity of cotton roots in response to temperature. Environ. Exp. Bot. 1994, 34, 201–206. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, S.; Li, P.; Yin, Y.; Niu, Q.; Yan, J.; Huang, D. Plant buffering against the high-light stress-induced accumulation of CsGA2ox8 transcripts via alternative splicing to finely tune gibberellin levels and maintain hypocotyl elongation. Hortic. Res. 2021, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, B.; Zhao, L.; Gao, P.; Ma, H.; Luan, S. Expression analysis of Fusarium wilt resistance gene in melon by real-time quantitative PCR. Pak. J. Bot. 2014, 46, 713–717. [Google Scholar]
- Weng, J.; Rehman, A.; Li, P.; Chang, L.; Zhang, Y.; Niu, Q. Physiological and transcriptomic analysis reveals the responses and difference to high temperature and humidity stress in two melon genotypes. Int. J. Mol. Sci. 2022, 23, 734. [Google Scholar] [CrossRef]
- Colpaert, J.V.; Van Tichelen, K.K.; Van Assche, J.A.; Van Laere, A. Short-term phosphorus uptake rates in mycorrhizal and non-mycorrhizal roots of intact Pinus sylvestris seedlings. New Phytol. 1999, 143, 589–597. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C. Phosphorus-use efficiency by corn genotypes. J. Plant Nutr. 1997, 20, 1267–1277. [Google Scholar] [CrossRef]
- Li, P.; Weng, J.; Zhang, Q.; Yu, L.; Yao, Q.; Chang, L.; Niu, Q. Physiological and biochemical responses of Cucumis melo L. chloroplasts to low-phosphate stress. Front. Plant Sci. 2018, 9, 1525. [Google Scholar] [CrossRef] [Green Version]
- Broadley, M.R.; Bowen, H.C.; Cotterill, H.L.; Hammond, J.P.; Meacham, M.C.; Mead, A.; White, P.J. Phylogenetic variation in the shoot mineral concentration of angiosperms. J. Exp. Bot. 2004, 55, 321–336. [Google Scholar] [CrossRef] [Green Version]
- Pieters, A.J.; Paul, M.J.; Lawlor, D.W. Low sink demand limits photosynthesis under Pi deficiency. J. Exp. Bot. 2001, 52, 1083–1091. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.; Chen, W.W.; Wang, Y.Y.; Huang, Z.R.; Ye, X.; Chen, L.S.; Yang, L.T. Effects of phosphorus deficiency on the absorption of mineral nutrients, photosynthetic system performance and antioxidant metabolism in Citrus grandis. PLoS ONE 2021, 16, e0246944. [Google Scholar] [CrossRef]
- Baxter, I.R.; Vitek, O.; Lahner, B.; Muthukumar, B.; Borghi, M.; Morrissey, J.; Guerinot, M.L.; Salt, D.E. The leaf ionome as a multivariable system to detect a plant’s physiological status. Proc. Natl. Acad. Sci. USA 2008, 105, 12081–12086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Y.; Wang, Z.; Ren, M.; Zhang, P.; Li, Z.; Chen, S.; Ge, C.; Wang, Y. Iron and callose homeostatic regulation in rice roots under low phosphorus. BMC Plant Biol. 2018, 18, 326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirsch, J.; Marin, E.; Floriani, M.; Chiarenza, S.; Richaud, P.; Nussaume, L.; Thibaud, M.C. Phosphate deficiency promotes modification of iron distribution in Arabidopsis plants. Biochimie 2006, 88, 1767–1771. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.J.; Hu, Y.; Zhu, Y.G.; Gao, R.T.; Zhao, Q.L. The mechanisms of iron plaque formation on the surface of rice roots induced by phosphorus starvation. Plant Nutr. Fertil. Sci. 2008, 14, 22–27. [Google Scholar]
- Jiang, F.Y.; Chen, X.; Luo, A.C. Iron plaque formation on wetland plants and its influence on phosphorus, calcium and metal uptake. Aquat. Ecol. 2009, 43, 879–890. [Google Scholar] [CrossRef]
- Yang, X.J.; Xu, Z.; Shen, H. Drying–submergence alternation enhanced crystalline ratio and varied surface properties of iron plaque on rice (Oryza sativa) roots. Environ. Sci. Pollut. Res. 2018, 25, 3571–3587. [Google Scholar] [CrossRef]
- Morris, E.C.; Griffiths, M.; Golebiowska, A.; Mairhofer, S.; Burr-Hersey, J.; Goh, T.; Wangenheim, D.; Atkinson, B.; Sturrock, C.; Lynch, J.; et al. Shaping 3D root system architecture. Curr. Biol. 2017, 27, R919–R930. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Bucio, J.; Hernandez-Abreu, E.; Sanchez-Calderon, L.; Nieto-Jacobo, M.F.; Simpson, J.; Herrera-Estrella, L. Phosphate availability alters architecture and causes changes in hormone sensitivity in the Arabidopsis root system. Plant Physiol. 2002, 129, 244–256. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.; Zhang, Z.H.; Chen, X.; Jia, Z.; Yi, J.; Su, Y. Responses of root morphology and architecture to phosphorus deficiency at seedling stage of tobacco growth. Aust. J. Crop Sci. 2013, 7, 1967–1972. [Google Scholar]
- Mollier, A.; Pellerin, S. Maize root system growth and development as influenced by phosphorus deficiency. J. Exp. Bot. 1999, 50, 487–497. [Google Scholar] [CrossRef]
- Camilo, S.; Odindo, A.O.; Kondwakwenda, A.; Sibiya, J. Root traits related with drought and phosphorus tolerance in common bean (Phaseolus vulgaris L.). Agronomy 2021, 11, 552. [Google Scholar] [CrossRef]
- Ha, S.; Tran, L. Understanding plant responses to phosphorus starvation for improvement of plant tolerance to phosphorus deficiency by biotechnological approaches. Crit. Rev. Biotechnol. 2014, 34, 16–30. [Google Scholar] [CrossRef]
- Kumar, S.; Chugh, C.; Seem, K.; Kumar, S.; Vinod, K.K.; Mohapatra, T. Characterization of contrasting rice (Oryza sativa L.) genotypes reveals the Pi-efficient schema for phosphate starvation tolerance. BMC Plant Biol. 2021, 21, 282. [Google Scholar] [CrossRef]
- Tadano, T.; Sakai, H. Secretion of acid phosphatase by the roots of several crop species under phosphorus-deficient conditions. Soil Sci. Plant Nutr. 1991, 37, 129–140. [Google Scholar] [CrossRef]
- Li, Y.; Gao, Y.; Tian, Q.; Shi, F.; Li, L.; Zhang, W. Stimulation of root acid phosphatase by phosphorus deficiency is regulated by ethylene in Medicago falcata. Environ. Exp. Bot. 2011, 71, 114–120. [Google Scholar] [CrossRef]
- Roche, J.; Turnbull, M.H.; Guo, Q.; Novak, O.; Spath, J.; Gieseg, S.P.; Jameson, P.E.; Love, J. Coordinated nitrogen and carbon remobilization for nitrate assimilation in leaf, sheath and root and associated cytokinin signals during early regrowth of Lolium perenne. Ann. Bot. 2017, 119, 1353–1364. [Google Scholar] [CrossRef]
- Nussaume, L.; Kanno, S.; Javot, H.; Marin, E.; Nakanishi, T.M.; Thibaud, M. Phosphate import in plants: Focus on the PHT1 transporters. Front. Plant Sci. 2011, 2, 83. [Google Scholar] [CrossRef] [Green Version]
- Walder, F.; Brulé, D.; Koegel, S.; Wiemken, A.; Boller, T.; Courty, P.E. Plant phosphorus acquisition in a common mycorrhizal network: Regulation of phosphate transporter genes of the Pht1 family in sorghum and flax. New Phytol. 2015, 205, 1632–1645. [Google Scholar] [CrossRef]
- Remy, E.; Cabrito, T.R.; Batista, R.A.; Teixeira, M.C.; Sá-Correia, I.; Duque, P. The Pht1; 9 and Pht1; 8 transporters mediate inorganic phosphate acquisition by the Arabidopsis thaliana root during phosphorus starvation. New Phytol. 2012, 195, 356–371. [Google Scholar] [CrossRef]
- Ayadi, A.; David, P.; Arrighi, J.F.; Chiarenza, S.; Thibaud, M.C.; Nussaume, L.; Marin, E. Reducing the genetic redundancy of Arabidopsis PHOSPHATE TRANSPORTER1 transporters to study phosphate uptake and signaling. Plant Physiol. 2015, 167, 1511–1526. [Google Scholar] [CrossRef] [Green Version]
- Chang, M.X.; Gu, M.; Xia, Y.W.; Dai, X.L.; Dai, C.R.; Zhang, J.; Wang, S.C.; Qu, H.Y.; Yamaji, N.; Ma, J.F.; et al. OsPHT1; 3 mediates uptake, translocation, and remobilization of phosphate under extremely low phosphate regimes. Plant Physiol. 2019, 179, 656–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, A.; Chen, X.; Wang, H.; Liao, D.; Gu, M.; Qu, H.; Sun, S.; Xu, G. Genome-wide investigation and expression analysis suggest diverse roles and genetic redundancy of Pht1 family genes in response to Pi deficiency in tomato. BMC Plant. Biol. 2014, 14, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ming, F.; Mi, G.H.; Lu, Q.; Yin, S.; Zhang, S.S.; Guo, B.; Shen, D.L. Cloning and characterization of cDNA for the Oryza sativa phosphate transporter. Cell. Mol. Biol. Lett. 2005, 10, 401–411. [Google Scholar] [PubMed]
- Qin, L.; Guo, Y.; Chen, L.; Liang, R.; Gu, M.; Xu, G.; Zhao, J.; Walk, T.; Liao, H. Functional characterization of 14 Pht1 family genes in yeast and their expressions in response to nutrient starvation in soybean. PLoS ONE 2012, 7, e47726. [Google Scholar] [CrossRef]
- Shin, H.; Shin, H.S.; Dewbre, G.R.; Harrison, M.J. Phosphate transport in Arabidopsis: Pht1;1 and Pht1;4 play a major role in phosphate acquisition from both low- and high-phosphate environments. Plant J. 2004, 39, 629–642. [Google Scholar] [CrossRef]
- Teng, W.; Zhao, Y.; Zhao, X.; He, X.; Ma, W.; Deng, Y.; Chen, X.; Tong, Y. Genome-wide identification, characterization, and expression analysis of PHT1 phosphate transporters in wheat. Front. Plant Sci. 2017, 8, 543. [Google Scholar] [CrossRef] [Green Version]
- Schünmann, P.H.D.; Richardson, A.E.; Smith, F.W.; Delhaize, E. Characterization of promoter expression patterns derived from the Pht1 phosphate transporter genes of barley (Hordeum vulgare L.). J. Exp. Bot. 2004, 55, 855–865. [Google Scholar] [CrossRef] [Green Version]
- Baker, A.; Ceasar, S.A.; Palmer, A.J.; Paterson, J.B.; Qi, W.; Muench, S.P.; Baldwin, S.A. Replace, reuse, recycle: Improving the sustainable use of phosphorus by plants. J. Exp. Bot. 2015, 66, 3523–3540. [Google Scholar] [CrossRef] [Green Version]


| Target Gene ID | Gene Description | Sense Primer 5′→3′ | Anti-Sense Primer 5′→3′ |
|---|---|---|---|
| Actin | Reference gene | TCTATTCCAGCCATCTCTC | GACCCTCCAATCCAAAC |
| cmo:103488012 | Purple acid phosphatase | CGGAAGTCTATCAAGAAGGT | CATGGAATGGATAACGATCTG |
| cmo:103483597 | PHT1;3 | CAATAGATTCTCAGCACCTTC | GCCTCAACCTCTACTTGTAA |
| cmo:103483596 | PHT1;4 | GACATTAGAAGCCAACAGAA | GGACTCAGGAACCAACAA |
| MELO3C022994 | PHT1;7 | GCATTCATCGCCGCTGTCTT | GCAGTGTATCTCGCCGTCTC |
| Treatment Time (d) | Pi Treatment(mM) | Nutrient Element Contents (mg g−1 DW) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A Pr | A Ps | Nr | Ns | Kr | Ks | Car | Cas | Fer | Fes | Sr | Ss | ||
| 7 | 0.25 | 10.45 ± 0.23 a | 12.54 ± 0.29 a | 46.97 ± 0.42 a | 63.94 ± 0.15 a | 69.07 ± 2.58 a | 39.57 ± 2.29 a | 6.99 ± 0.34 a | 38.99 ± 0.25 b | 1.33 ± 0.02 c | 0.18 ± 0.02 b | 4.01 ± 0.07 a | 4.62 ± 0.45 b |
| 0.025 | 6.66 ± 0.37 b | 7.97 ± 0.24 b | 44.67 ± 0.30 a | 64.14 ± 0.04 a | 67.74 ± 1.53 a | 41.90 ± 1.44 a | 6.69 ± 0.42 a | 41.83 ± 1.21 a | 1.49 ± 0.14 b | 0.17 ± 0.03 b | 3.94 ± 0.09 a | 5.42 ± 0.16 a | |
| 0.001 | 4.02 ± 0.14 c | 3.89 ± 0.21 c | 45.54 ± 0.05 a | 60.67 ± 0.11 a | 66.91 ± 2.98 a | 38.82 ± 1.34 a | 6.51 ± 0.31 a | 31.77 ± 1.59 c | 1.78 ± 0.04 a | 0.24 ± 0.02 a | 3.40 ± 0.21 b | 4.78 ± 0.06 b | |
| 14 | 0.25 | 12.92 ± 0.21 a | 13.84 ± 0.31 a | 53.70 ± 1.29 a | 58.00 ± 2.58 a | 74.30 ± 2.02 b | 41.70 ± 1.39 b | 6.10 ± 0.16 b | 44.10 ± 1.91 a | 1.17 ± 0.03 b | 0.13 ± 0.04 b | 3.96 ± 0.15 b | 4.55 ± 0.33 a |
| 0.025 | 5.30 ± 0.37 b | 4.65 ± 0.15 b | 54.50 ± 1.48 a | 59.70 ± 2.45 a | 73.40 ± 2.03 b | 52.80 ± 1.23 a | 6.00 ± 0.24 b | 34.10 ± 1.25 b | 1.65 ± 005 a | 0.14 ± 0.05 b | 4.43 ± 0.20 a | 4.25 ± 0.45 a | |
| 0.001 | 2.62 ± 0.42 c | 2.03 ± 0.13 c | 47.20 ± 1.40 b | 43.20 ± 1.79 b | 79.40 ± 1.94 a | 29.70 ± 1.04 c | 6.90 ± 0.35 a | 27.40 ± 1.20 c | 1.66 ± 0.03 a | 0.27 ± 0.03 a | 3.04 ± 0.07 c | 3.77 ± 0.26 b | |
| Treatment Time (d) | Pi Treatment (mM) | TRL (cm plant−1) | SA (cm2 plant−1) | V (cm3 plant−1) | D (mm) | PRL (cm plant−1) | LRZL (cm plant−1) |
|---|---|---|---|---|---|---|---|
| 7 | 0.25 | 643.84 ± 42.66 b | 66.35 ± 3.45 b | 0.54 ± 0.02 b | 0.33 ± 0.01 ab | 34.83 ± 2.41 a | 30.60 ± 2.68 a |
| 0.025 | 689.27 ± 25.13 b | 68.91 ± 3.68 b | 0.55 ± 0.04 b | 0.32 ± 0.01 b | 33.54 ± 4.96 a | 27.38 ± 4.69 a | |
| 0.001 | 736.93 ± 27.18 a | 78.46 ± 2.31 a | 0.66 ± 0.04 a | 0.34 ± 0.01 a | 32.60 ± 1.76 a | 28.15 ± 1.56 a | |
| 14 | 0.25 | 1088.12 ± 48.09 b | 126.24 ± 14.00 ab | 1.17 ± 0.16 a | 0.36 ± 0.00 a | 39.45 ± 0.93c | 36.10 ± 2.08 b |
| 0.025 | 1288.80 ± 40.73 a | 145.50 ± 16.94 a | 1.30 ± 0.17 a | 0.36 ± 0.01 a | 45.34 ± 1.38 b | 39.19 ± 0.19 b | |
| 0.001 | 961.77 ± 28.14c | 101.03 ± 9.20 b | 0.85 ± 0.10 b | 0.33 ± 0.01 b | 51.10 ± 2.05 a | 44.67 ± 2.10 a |
| Treatment Time (d) | Pi Treatment (mM) | PUR (μg Pi cm−1 TRL) | PUE (g DW mg−1 P) |
|---|---|---|---|
| 7 | 0.25 | 0.0044 ± 0.0015 c | 0.0819 ± 0.0012 c |
| 0.025 | 0.0109 ± 0.0012 b | 0.1549 ± 0.0026 b | |
| 0.001 | 0.0180 ± 0.0020 a | 0.5727 ± 0.0018 a | |
| 14 | 0.25 | 0.0319 ± 0.0030 b | 0.0725 ± 0.0022 c |
| 0.025 | 0.0327 ± 0.0021 b | 0.2327 ± 0.0038 b | |
| 0.001 | 0.0399 ± 0.0026 a | 0.8678 ± 0.0048 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Weng, J.; Rehman, A.; Niu, Q. Root Morphological and Physiological Adaptations to Low Phosphate Enhance Phosphorus Efficiency at Melon (Cucumis melo L.) Seedling Stage. Horticulturae 2022, 8, 636. https://doi.org/10.3390/horticulturae8070636
Li P, Weng J, Rehman A, Niu Q. Root Morphological and Physiological Adaptations to Low Phosphate Enhance Phosphorus Efficiency at Melon (Cucumis melo L.) Seedling Stage. Horticulturae. 2022; 8(7):636. https://doi.org/10.3390/horticulturae8070636
Chicago/Turabian StyleLi, Pengli, Jinyang Weng, Asad Rehman, and Qingliang Niu. 2022. "Root Morphological and Physiological Adaptations to Low Phosphate Enhance Phosphorus Efficiency at Melon (Cucumis melo L.) Seedling Stage" Horticulturae 8, no. 7: 636. https://doi.org/10.3390/horticulturae8070636
APA StyleLi, P., Weng, J., Rehman, A., & Niu, Q. (2022). Root Morphological and Physiological Adaptations to Low Phosphate Enhance Phosphorus Efficiency at Melon (Cucumis melo L.) Seedling Stage. Horticulturae, 8(7), 636. https://doi.org/10.3390/horticulturae8070636






