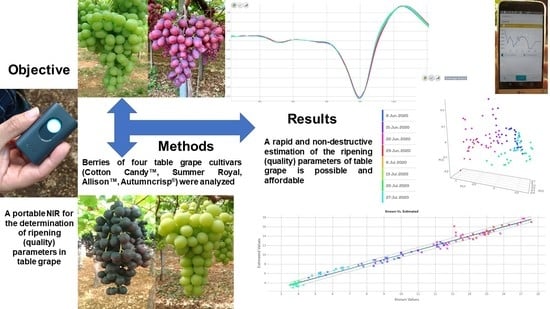
Ripeness Prediction in Table Grape Cultivars by Using a Portable NIR Device
Abstract
:1. Introduction
2. Materials and Methods
2.1. Table Grape Cultivars
2.2. SCiO™ Sensors
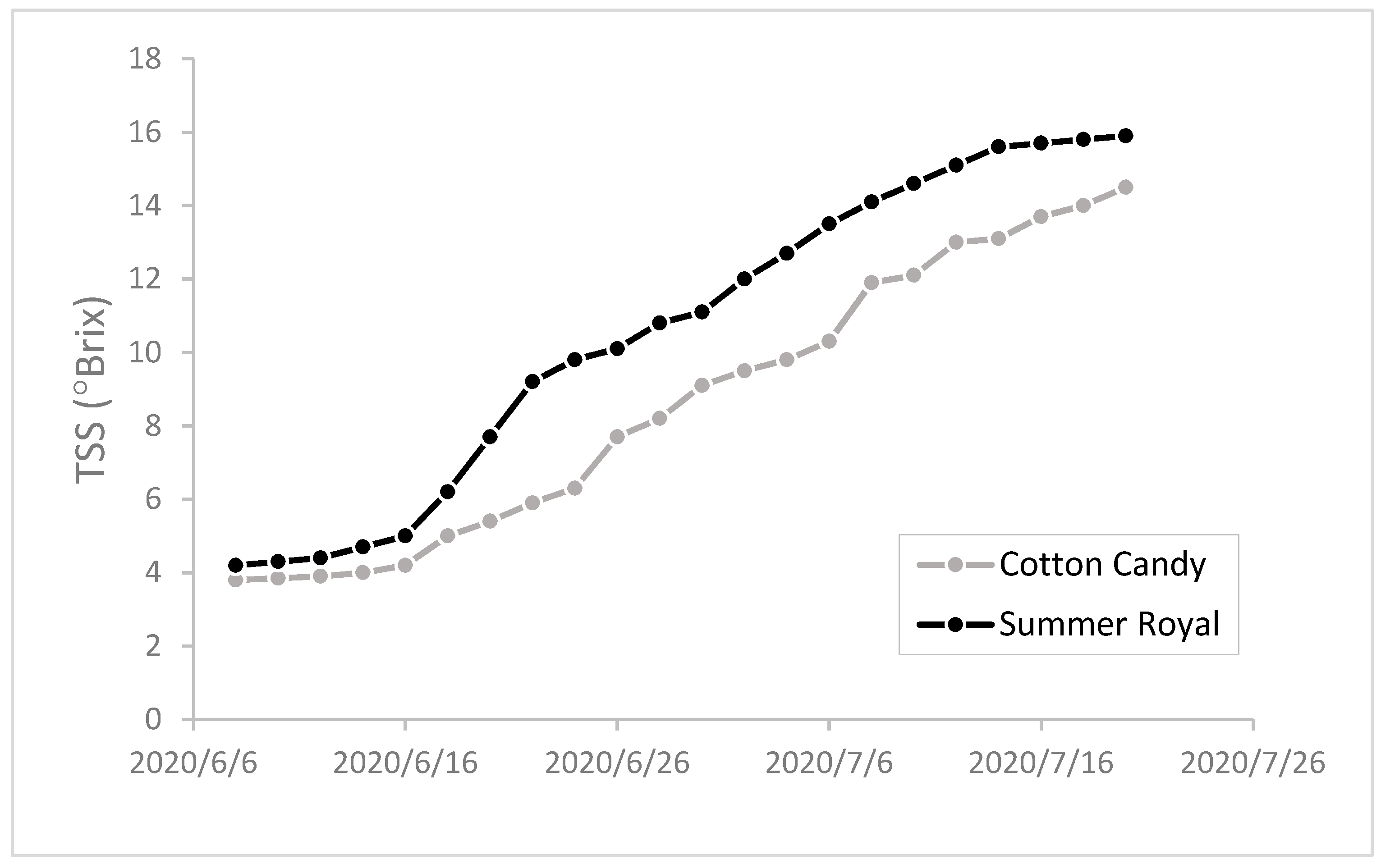
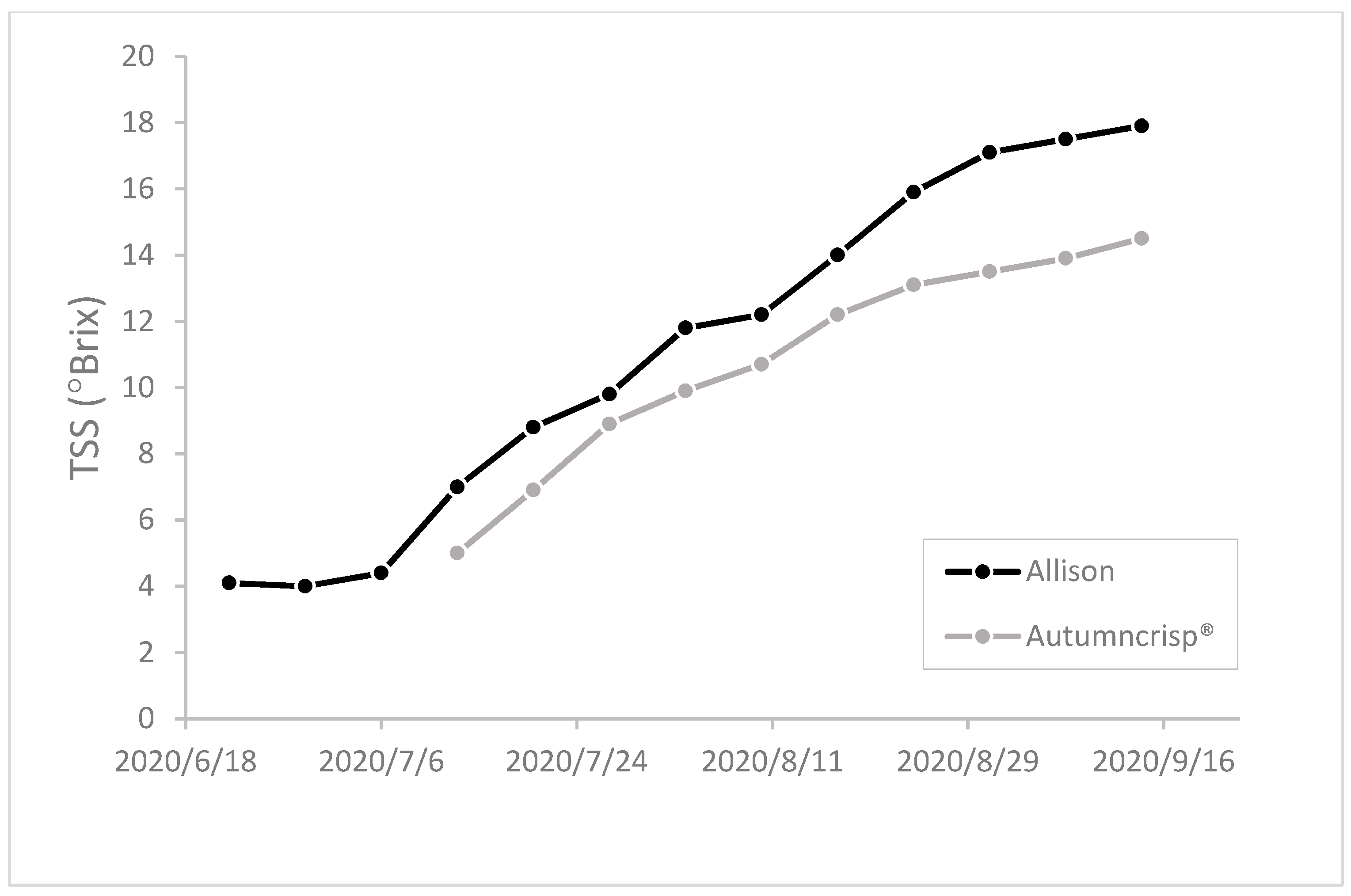
2.3. Berry Sampling and Analyses
2.4. Data Analysis
- Logarithmic transformation (LOGT), first derivative (FD), and SNV;
- Logarithmic transformation (LOGT), second derivative (SD), and SNV;
- First derivative (FD) and SNV;
- Second derivative (SD) and SNV.
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shyam Narayan, J.H.A.; Matsuoka, T. Non-Destructive Techniques for Quality Evaluation of Intact Fruits and Vegetables. Food Sci. Technol. Res. 2000, 6, 248–251. [Google Scholar] [CrossRef] [Green Version]
- Dufour, É. Principles of infrared spectroscopy. In Infrared Spectroscopy for Food Quality Analysis and Control; Sun, D.W., Ed.; Academic Press: Cambridge, MA, USA, 2009; pp. 1–27. [Google Scholar]
- Wu, D.; He, Y.; Feng, S. Short-wave near-infrared spectroscopy analysis of major compounds in milk powder and wavelength assignment. Anal. Chim. Acta 2008, 610, 232–242. [Google Scholar] [CrossRef]
- Reeves, J.B. Effects of water on the spectra of model compounds in the short- wavelength near infrared spectral region (14,000–9091 cm−1 or 714–1100 nm). J. Near Infrared Spectrosc. 1994, 2, 199–212. [Google Scholar] [CrossRef]
- Li, M.; Qian, Z.; Shi, B.; Medlicott, J.; East, A. Evaluating the performance of a consumer scale SCiOTM molecular sensor to predict quality of horticultural products. Postharvest Biol. Technol. 2018, 145, 183–192. [Google Scholar] [CrossRef]
- Feng, C.; Wang, H.; Lu, N.; Tu, X.M. Log transformation: Application and interpretation in biomedical research. Stat. Med. 2013, 32, 230–239. [Google Scholar] [CrossRef]
- Shyam Narayan, J.H.A. Near infrared spectroscopy. In Nondestructive Evaluation of Food Quality: Theory and Practice; Jha, S.N., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 141–212. [Google Scholar]
- Moghimi, A.; Aghkhani, M.H.; Sazgarnia, A.; Sarmad, M. Vis/NIR spectroscopy and chemometrics for the prediction of soluble solids content and acidity (pH) of kiwifruit. Biosyst. Eng. 2010, 106, 295–302. [Google Scholar] [CrossRef]
- Ignat, T.; Lurie, S.; Nyasordzi, J.; Ostrovsky, V.; Egozi, H.; Hoffman, A.; Friedman, H.; Weksler, A.; Schmilovitch, Z. Forecast of Apple Internal Quality Indices at Harvest and During Storage by VIS-NIR Spectroscopy. Food Bioprocess. Tech. 2014, 7, 2951–2961. [Google Scholar] [CrossRef]
- Li, M.; Pullanagari, R.R.; Pranamornkith, T.; Yule, I.J.; East, A.R. Quantitative prediction of post storage ‘Hayward’ kiwifruit attributes using at harvest Vis-NIR spectroscopy. J. Food Eng. 2017, 202, 46–55. [Google Scholar] [CrossRef]
- Larraín, M.; Guesalaga, A.R.; Agosin, E. A multipurpose portable instrument for determining ripeness in wine grapes using NIR spectroscopy. IEEE Trans. Instrum. Meas. 2008, 57, 294–302. [Google Scholar] [CrossRef]
- Nturambirwe, J.F.I.; Nieuwoudt, H.H.; Perold, W.J.; Opara, U.L. Non-destructive measurement of internal quality of apple fruit by a contactless NIR spectrometer with genetic algorithm model optimization. Sci. Afr. 2019, 3, e00051. [Google Scholar] [CrossRef]
- Zhang, Y.; Nock, J.F.; Al Shoffe, Y.; Watkins, C.B. Non-destructive prediction of soluble solids and dry matter contents in eight apple cultivars using near-infrared spectroscopy. Postharvest Biol. Technol. 2019, 151, 111–118. [Google Scholar] [CrossRef]
- Pourdarbani, R.; Sabzi, S.; Kalantari, D.; Karimzadeh, R.; Ilbeygi, E.; Arribas, J.I. Automatic non-destructive video estimation of maturation levels in Fuji apple (Malus Malus pumila) fruit in orchard based on colour (Vis) and spectral (NIR) data. Biosyst. Eng. 2020, 195, 136–151. [Google Scholar] [CrossRef]
- Pourdarbani, R.; Sabzi, S.; Kalantari, D.; Arribas, J.I. Non-destructive visible and short-wave near-infrared spectroscopic data estimation of various physicochemical properties of Fuji apple (Malus pumila) fruits at different maturation stages. Chemom. Intell. Lab. Syst. 2020, 206, 104147. [Google Scholar] [CrossRef]
- Kawano, S.; Fujiwara, T.; Iwamoto, M. Nondestructive Determination of Sugar Content in Satsuma Mandarin using Near Infrared (NIR) Transmittance. J. Jpn. Soc. Hortic. Sci. 1993, 62, 465–470. [Google Scholar] [CrossRef]
- McGlone, V.A.; Fraser, D.G.; Jordan, R.B.; Künnemeyer, R. Internal quality assessment of mandarin fruit by vis/NIR spectroscopy. J. Near Infrared Spectrosc. 2003, 11, 323–332. [Google Scholar] [CrossRef]
- Kawano, S.; Watanabe, H.; Iwamoto, M. Determination of Sugar Content in Intact Peaches by Near Infrared Spectroscopy with Fiber Optics in Interactance Mode. J. Jpn. Soc. Hortic. Sci. 1992, 61, 445–451. [Google Scholar] [CrossRef] [Green Version]
- Saranwong, S.; Sornsrivichai, J.; Kawano, S. Improvement of PLS calibration for Brix value and dry matter of mango using information from MLR calibration. J. Near Infrared Spectrosc. 2001, 9, 287–295. [Google Scholar] [CrossRef]
- Shyam Narayan, J.H.A.; Matsuoka, T. Non-destructive determination of acid-brix ratio of tomato juice using near infrared spectroscopy. Int. J. Food Sci. Technol. 2004, 39, 425–430. [Google Scholar] [CrossRef]
- Kemps, B.; Leon, L.; Best, S.; De Baerdemaeker, J.; De Ketelaere, B. Assessment of the quality parameters in grapes using VIS/NIR spectroscopy. Biosyst. Eng. 2010, 105, 507–513. [Google Scholar] [CrossRef]
- Verena, W.; Huck, C.W. Evaluation of the performance of three hand-held near- infrared spectrometer through investigation of total antioxidant capacity in gluten- free grains. Talanta 2018, 189, 233–240. [Google Scholar] [CrossRef]
- Porep, J.U.; Kammerer, D.R.; Carle, R. On-line application of near infrared (NIR) spectroscopy in food production. Trends Food Sci. Technod. 2015, 46, 211–230. [Google Scholar] [CrossRef]
- Nicolaï, B.M.; Beullens, K.; Bobelyn, E.; Peirs, A.; Theron, K.I.; Lammertyn, J. Nondestructive measurement of fruit and vegetable quality by means of NIR spectroscopy: A review. Postharvest Biol. Technol. 2007, 46, 99–118. [Google Scholar] [CrossRef]
- McGlone, V.A.; Jordan, R.B.; Martinsen, P.J. Vis/NIR estimation at harvest of pre- and post-storage quality indices for “Royal Gala” apple. Postharvest Biol. Technol. 2002, 25, 135–144. [Google Scholar] [CrossRef]
- Nicolaï, B.M.; Theron, K.I.; Lammertyn, J. Kernel PLS regression on wavelet transformed NIR spectra for prediction of sugar content of apple. Chemometr. Intell. Lab. Syst. 2006, 85, 243–252. [Google Scholar] [CrossRef]
- Sun, T.; Huang, K.; Xu, H.; Ying, Y. Research advances in nondestructive determination of internal quality in watermelon/melon: A review. J. Food Eng. 2010, 100, 569–577. [Google Scholar] [CrossRef]
- Haughey, S.A.; Galvin-King, P.; Malechaux, A.; Elliott, C.T. The use of handheld near-infrared reflectance spectroscopy (NIRS) for the proximate analysis of poultry feed and to detect melamine adulteration of soya bean meal. Anal. Methods 2015, 7, 181–186. [Google Scholar] [CrossRef]
- Kosmowsky, F.; Worku, T. Evaluation of a miniaturized NIR spectrometer for cultivar identification: The case of barley, chickpea and sorghum in Ethiopia. PLoS ONE 2018, 13, e0193620. [Google Scholar] [CrossRef] [PubMed]
- McVey, C.; Gordon, U.; Haughey, S.A.; Elliott, C.T. Assessment of the Analytical Performance of Three Near-Infrared Spectroscopy Instruments (Benchtop, Handheld and Portable) through the Investigation of Coriander Seed Authenticity. Foods 2021, 10, 956. [Google Scholar] [CrossRef]
- Kaur, H.; Künnemeyer, R.; McGlone, A. Comparison of hand-held near infrared spectrophotometers for fruit dry matter assessment. J. Near Infrared Spectrosc. 2017, 25, 267–277. [Google Scholar] [CrossRef]
- FAOSTAT (2021). Available online: https://www.fao.org/faostat/en/#compare (accessed on 14 May 2022).
- Srivichien, S.; Terdwongworakul, A.; Teerachaichayut, S. Quantitative prediction of nitrate level in intact pineapple using Vis–NIRS. J. Food Eng. 2015, 150, 29–34. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Ouyang, A. Nondestructive deermination of pear internal quality indices by visible and near-infrared spectrometry. LWT 2008, 41, 1720–1725. [Google Scholar] [CrossRef]
- Donis-González, I.R.; Valero, C.; Momin, M.A.; Kaur, A.; Slaughter, D.C. Performance evaluation of two commercially available portable spectrometers to non-invasively determine table grape and peach quality attributes. Agronomy 2020, 10, 148. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Wang, Q.; Xu, L.; Tian, X.; Xia, Y.; Fan, S. Comparison and optimization of models for determination of sugar content in pear by portable Vis-NIR spectroscopy coupled with wavelength selection algorithm. Food Anal. Methods 2019, 12, 12–22. [Google Scholar] [CrossRef]
- Bobelyn, E.; Serban, A.S.; Nicu, M.; Lammertyn, J.; Nicolai, B.M.; Saeys, W. Postharvest quality of apple predicted by NIR-spectroscopy: Study of the effect of biological variability on spectra and model performance. Postharvest Biol. Technol. 2010, 55, 133–143. [Google Scholar] [CrossRef]
- Marques, E.J.N.; de Freitas, S.T.; Pimentel, M.F.; Pasquini, C. Rapid and non-destructive determination of quality parameters in the ‘Tommy Atkins’ mango using a novel handheld near infrared spectrometer. Food Chem. 2016, 197, 1207–1214. [Google Scholar] [CrossRef]
- Cayuela, A.; Weiland, C. Intact orange quality prediction with two portable NIR spectrometers. Postharvest Biol. Technol. 2010, 58, 113–120. [Google Scholar] [CrossRef] [Green Version]
- Sánchez, M.T.; De la Haba, M.J.; Guerrero, J.E.; Garrido-Varo, A.; Pérez-Marín, D. Testing of a local approach for the prediction of quality parameters in intact nectarines using a portable NIRS instrument. Postharvest Biol. Technol. 2011, 60, 130–135. [Google Scholar] [CrossRef]
- Bayer, A.; Bachmann, M.; Müller, A.; Kaufmann, H. A Comparison of Feature-Based MLR and PLS Regression Techniques for the Prediction of Three Soil Constituents in a Degraded South African Ecosystem. Appl. Environ. Soil Sci. 2012, 2012, 971252. [Google Scholar] [CrossRef] [Green Version]
- Chauchard, F.; Cogdill, R.; Roussel, S.; Roger, J.M.; Bellon-Maurel, V. Application of LS-SVM to non-linear phenomena in NIR spectroscopy: Development of a robust and portable sensor for acidity prediction in grapes. Chemometr. Intell. Lab. Syst. 2004, 71, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Barnes, R.J.; Dhanoa, M.S.; Lister, S.J. Standard normal variate transformation and de-trending of near-infrared diffuse reflectance spectra. Appl. Spectrosc. 1989, 43, 772–777. [Google Scholar] [CrossRef]
- Ruggiero, G.; Parlavecchia, M.; Dal Sasso, P. Typological charcterisation and territorial distribution of traditional rural buildings in the Apulian territory (Italy). J. Cult. Herit. 2019, 39, 278–287. [Google Scholar] [CrossRef]
- Viscarra Rossel, R.A. ParLeS: Software for chemometric analysis of spectroscopic data. Chemom. Intell. Lab. Syst. 2008, 90, 72–83. [Google Scholar] [CrossRef]
- Sandak, J.; Sandak, A.; Meder, R. Assessing trees, wood and derived products with near infrared spectroscopy: Hints and tips. J. Near Infrared Spectrosc. 2016, 24, 485–505. [Google Scholar] [CrossRef] [Green Version]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-regression: A basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Barca, E.; Porcu, E.; Bruno, D.; Passarella, G. An Automated Decision Support System for Aided Assessment of Variogram Models. Environ. Model. Softw. 2017, 87, 72–83. [Google Scholar] [CrossRef]
- Pillonel, L.; Dufour, E.; Schaller, E.; Bosset, J.O.; De Baerdemaeker, J.; Karoui, R. Prediction of colour of European Emmental cheeses by using near infrared spectroscopy: A feasibility study. Eur. Food Res. Technol. 2007, 226, 63–69. [Google Scholar] [CrossRef]
- Janik, L.J.; Forrestera, S.T.; Rawsonb, A. The prediction of soil chemical and physical properties from mid-infrared spectroscopy and combined partial least-squares regression and neural networks (PLS-NN) analysis. Chemom. Intell. Lab. Syst. 2009, 97, 179–188. [Google Scholar] [CrossRef]
- Camacho-Tamayo, J.H.; Rubiano, S.Y.; Maria del Pilar Hurtado, S. Near-infrared (NIR) diffuse reflectance spectroscopy for the prediction of carbon and nitrogen in an Oxisol. Agron. Colomb. 2014, 32, 86–94. [Google Scholar] [CrossRef]
- Saeys, W.; Mouazen, A.M.; Ramon, H. Potential for onsite and online analysis of pig manure using visible and near infrared reflectance spectroscopy. Biosyst. Eng. 2005, 91, 393–402. [Google Scholar] [CrossRef]
- Mouazen, A.M.; De Baerdemaeker, J.; Ramon, H. Effect of wavelength range on the measurement accuracy of some selected soil constituents using visual-near infrared spectroscopy. J. Near Infrared Spectrosc. 2006, 14, 189–199. [Google Scholar] [CrossRef]
- Colombo, C.; Palumbo, G.; Di Iorio, E.; Sellitto, V.M.; Comolli, R.; Stellacci, A.M.; Castrignanò, A. Soil organic carbon variation in alpine landscape (Northern Italy) as evaluated by diffuse reflectance spectroscopy. Soil Sci. Soc. Am. J. 2014, 78, 794–804. [Google Scholar] [CrossRef]
- Nicolaï, B.M.; Verlinden, B.E.; Desmet, M.; Saevels, S.; Saeys, W.; Theron, K.; Cubeddu, R.; Pifferi, A.; Torricelli, A. Time-resolved and continuous wave NIR reflectance spectroscopy to predict soluble solids content and firmness of pear. Postharvest Biol. Technol. 2008, 47, 68–74. [Google Scholar] [CrossRef]
- Travers, S.; Bertelsen, M.G.; Kucheryavskiy, S.V. Predicting apple (cv. Elshof) postharvest dry matter and soluble solids content with near infrared spectroscopy. J. Sci. Food Agric. 2014, 94, 955–962. [Google Scholar] [CrossRef]
- McGlone, V.A.; Kawano, S. Firmness, dry-matter and soluble-solids assessment of postharvest kiwifruit by NIR spectroscopy. Postharvest Biol. Technol. 1998, 13, 131–141. [Google Scholar] [CrossRef]
- Jarén, C.; Ortuño, J.C.; Arazuri, S.; Arana, J.I.; Salvadores, M.C. Sugar determination in grapes using NIR technology. Int. J. Infrared Millim. Waves 2001, 22, 1521–1530. [Google Scholar] [CrossRef]
- Urraca, R.; Sanz-Garcia, A.; Tardaguila, J.; Diago, M.P. Estimation of total soluble solids in grape berries using a hand-held NIR spectrometer under field conditions. J. Sci. Food Agric. 2016, 96, 3007–3016. [Google Scholar] [CrossRef]
- Gómez, A.H.; He, Y.; Pereira, A.G. Non-destructive measurement of acidity, soluble solids and firmness of Satsuma mandarin using Vis/NIR-spectroscopy techniques. J. Food Eng. 2006, 77, 313–319. [Google Scholar] [CrossRef]
- Maniwara, P.; Nakano, K.; Boonyakiat, D.; Ohashi, S.; Hiroi, M.; Tohyama, T. The use of visible and near infrared spectroscopy for evaluating passion fruit postharvest quality. J. Food Eng. 2014, 143, 33–43. [Google Scholar] [CrossRef]
- Amuah, C.L.Y.; Teye, E.; Lamptey, F.P.; Nyandey, K.; Opoku-Ansah, J.; Osei-Wusu Adueming, P. Feasibility Study of the Use of Handheld NIR Spectrometer for Simultaneous Authentication and Quantification of Quality Parameters in Intact Pineapple Fruits. J. Spectrosc. 2019, 2019, 5975461. [Google Scholar] [CrossRef] [Green Version]
- Cen, H.; He, Y. Theory and application of near infrared reflectance spectroscopy in determination of food quality. Trends Food Sci. Technod. 2007, 18, 72–83. [Google Scholar] [CrossRef]
- Ma, T.; Li, X.; Inagaki, T.; Yang, H.; Tsuchikawa, S. Noncontact evaluation of soluble solids content in apples by near-infrared hyperspectral imaging. J. Food Eng. 2018, 224, 53–61. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, D.; Liu, L.; Wang, Z. How to predict the sugariness and hardness of melons: A near-infrared hyper-spectral imaging method. Food Chem. 2017, 218, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Liu, J.; Huang, K.; Kuang, J.; Liu, D. Evaluation of moisture content in processed apple chips using NIRS and wavelength selection techniques. Infrared Phys. Technol. 2019, 98, 305–310. [Google Scholar] [CrossRef]
- Kuhn, M.; Johnson, K. Applied Predictive Modelling; Springer: New York, NY, USA, 2013. [Google Scholar]
- Magwaza, L.S.; Tesfay, S.Z. A Review of Destructive and Non-destructive Methods for Determining Avocado Fruit Maturity. Food Bioprocess. Technol. 2015, 8, 1995–2011. [Google Scholar] [CrossRef]
- Goisser, S.; Fernandes, M.; Ulrichs, C.; Mempel, H. Non-destructive measurement method for a fast quality evaluation of fruit and vegetables by using food-scanner. DGG-Proceedings 2018, 8, 1–5. [Google Scholar] [CrossRef]
- Subedi, P.P.; Walsh, K.B. Assessment of avocado fruit dry matter content using portable near infrared spectroscopy: Method and instrumentation optimisation. Postharvest Biol. Technol. 2020, 161, 111078. [Google Scholar] [CrossRef]
- Kasim, N.F.M.; Mishra, P.; Schouten, R.E.; Woltering, E.J.; Boer, M.P. Assessing firmness in mango comparing broadband and miniature spectrophotometers. Infrared Phys. Technol. 2021, 115, 103733. [Google Scholar] [CrossRef]
- Goi, A.; Simoni, M.; Righi, F.; Visentin, G.; De Marchi, M. Application of a Handheld Near-Infrared Spectrometer to Predict Gelatinized Starch, Fiber Fractions, and Mineral Content of Ground and Intact Extruded Dry Dog Food. Animals 2020, 10, 1660. [Google Scholar] [CrossRef]
- Pérez-Marín, D.; Paz, P.; Guerrero, J.E.; Garrido-Varo, A.; Sánchez, M.T. Miniature handheld NIR sensor for the on-site non-destructive assessment of post-harvest quality and refrigerated storage behavior in plums. J. Food Eng. 2010, 99, 294–302. [Google Scholar] [CrossRef]
- Büning-Pfaue, H. Analysis of water in food by near infrared spectroscopy. J. Food Chem. 2003, 82, 107–115. [Google Scholar] [CrossRef]
- Ma, Y.B.; Babu, K.S.; Amamcharla, J.K. Prediction of total protein and intact casein in cheddar cheese using a low-cost handheld short-wave near-infrared spectrometer. LWT 2019, 109, 319–326. [Google Scholar] [CrossRef]
- Walsh, K.B.; McGlone, V.A.; Han, D.H. The uses of near infra-red spectroscopy in postharvest decision support: A review. Postharvest Biol. Technol. 2020, 163, 111139. [Google Scholar] [CrossRef]
- Zheng, W.; Bai, Y.; Luo, H.; Li, Y.; Yang, X.; Zhang, B. Self-adaptive models for predicting soluble solid content of blueberries with biological variability by using near-infrared spectroscopy and chemometrics. Postharvest Biol. Technol. 2020, 169, 111286. [Google Scholar] [CrossRef]
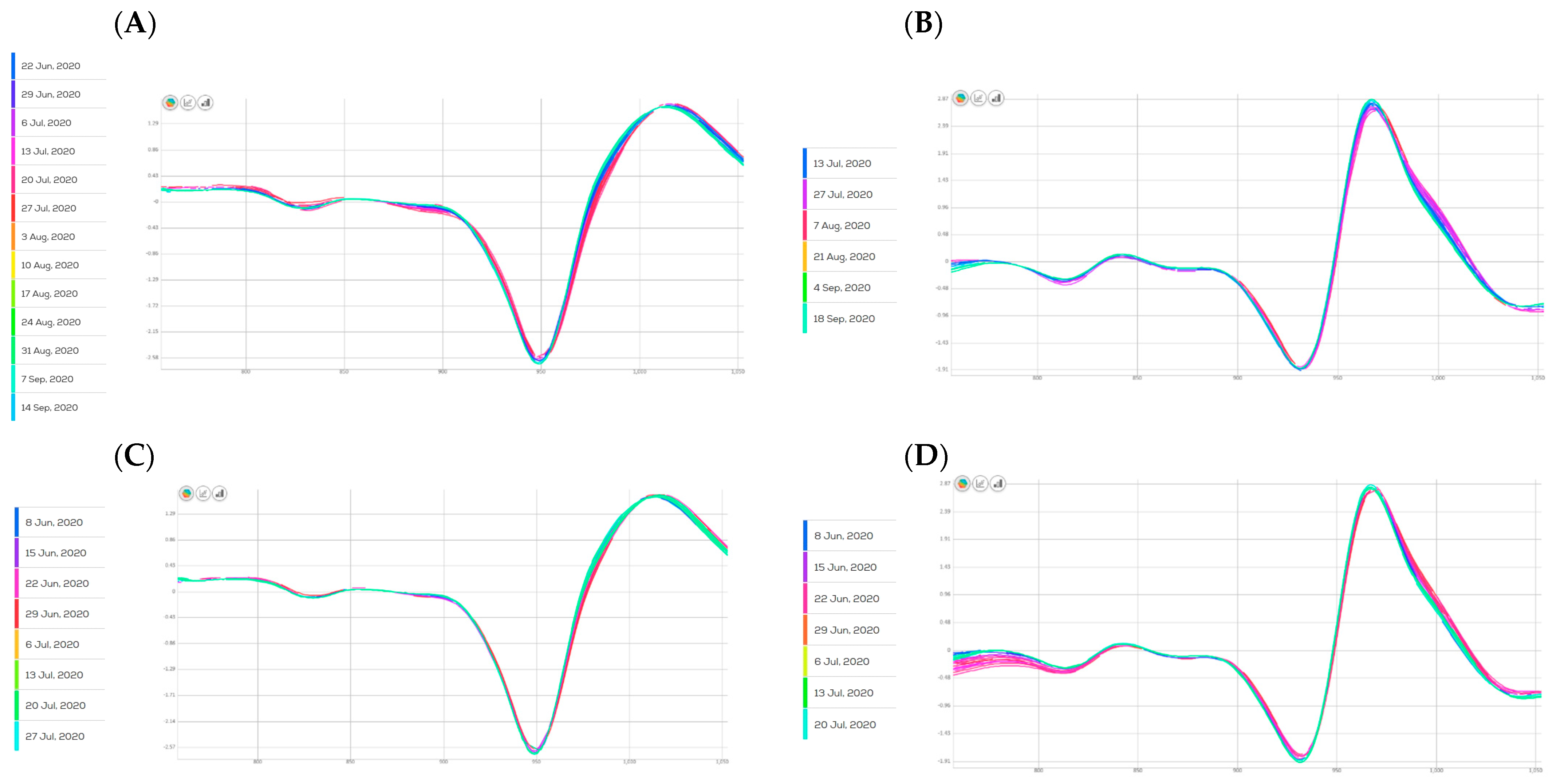
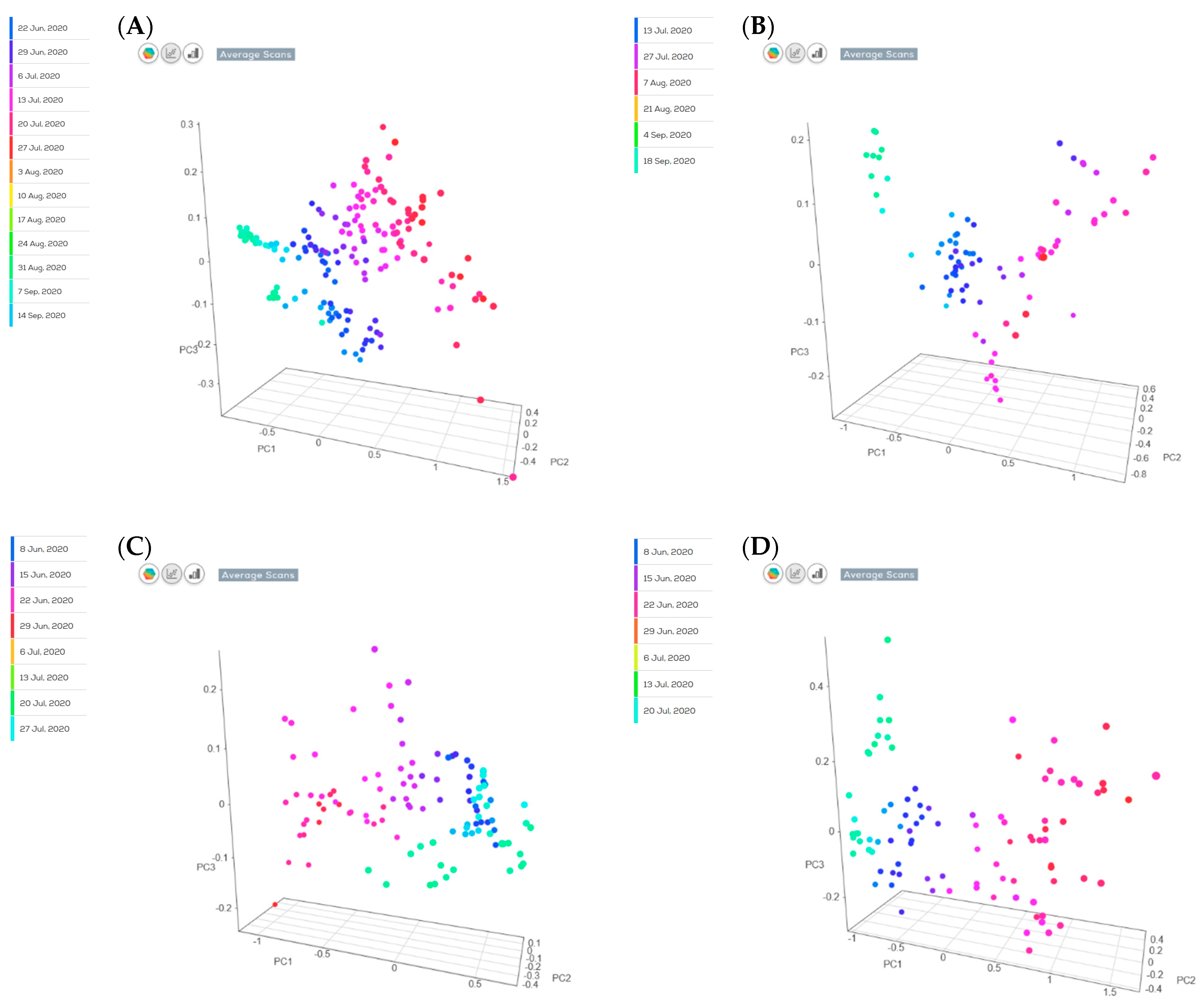
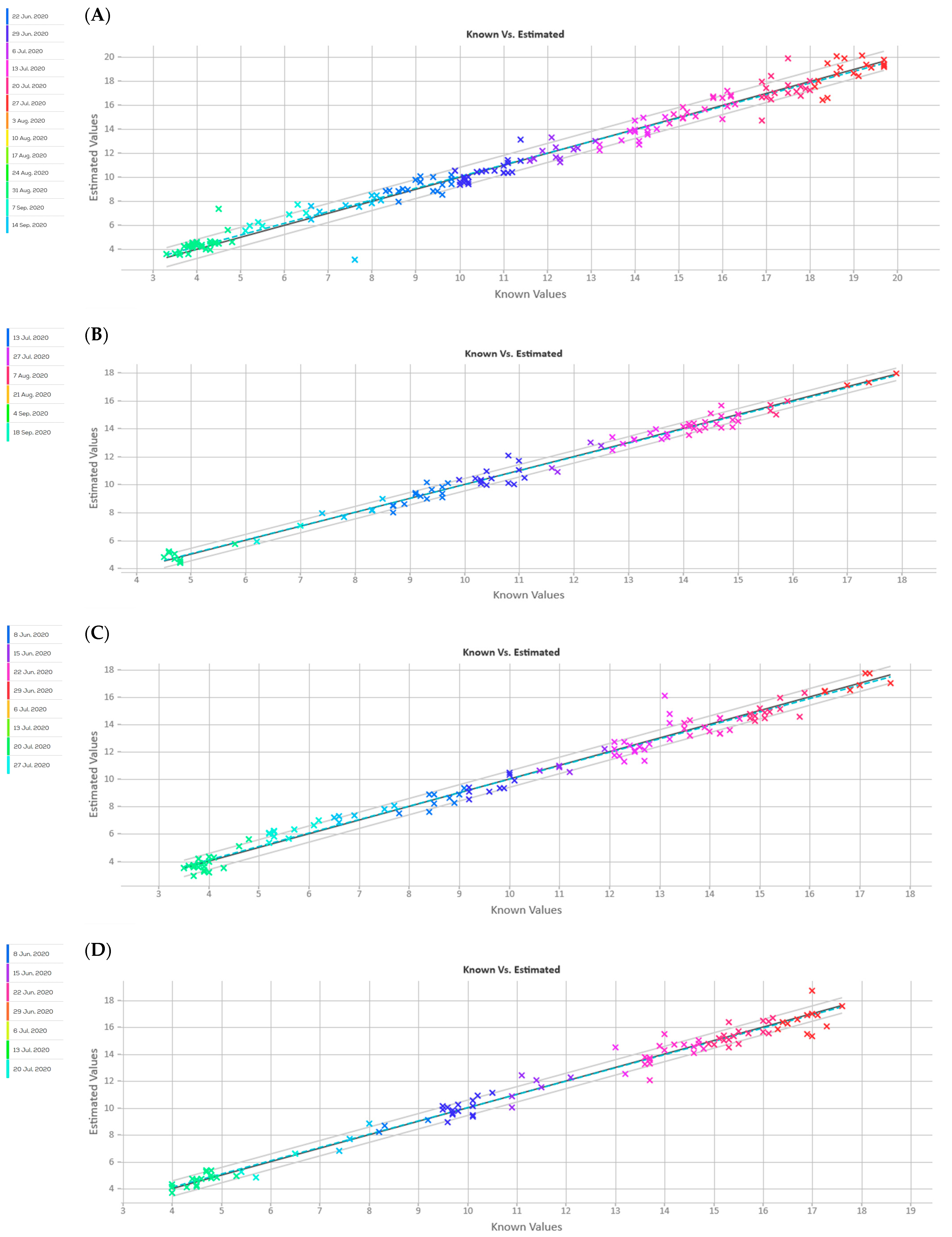
| Parameter | STD | Pre-Processing Combination | RMSE | R2 | RPD | N Latent Variables |
|---|---|---|---|---|---|---|
| TSS | 2.63 | Raw Spectra | 0.69 | 0.93 | 3.80 | 10 |
| LOGT, FD, SNV | 0.68 | 0.93 | 3.90 | 10 | ||
| LOGT, SD, SNV | 0.69 | 0.93 | 3.83 | 6 | ||
| FD, SNV | 0.68 | 0.93 | 3.88 | 10 | ||
| SD, SNV | 0.96 | 0.93 | 2.74 | 7 | ||
| pH | 0.32 | Raw Spectra | 0.07 | 0.95 | 4.37 | 10 |
| LOGT, FD, SNV | 0.06 | 0.96 | 4.98 | 12 | ||
| LOGT, SD, SNV | 0.07 | 0.95 | 4.62 | 6 | ||
| FD, SNV | 0.06 | 0.95 | 5.06 | 12 | ||
| SD, SNV | 0.07 | 0.96 | 4.76 | 7 | ||
| TA | 2.38 | Raw Spectra | 0.47 | 0.96 | 5.06 | 12 |
| LOGT, FD, SNV | 0.51 | 0.96 | 4.72 | 9 | ||
| LOGT, SD, SNV | 0.52 | 0.95 | 4.59 | 6 | ||
| FD, SNV | 0.49 | 0.96 | 4.90 | 10 | ||
| SD, SNV | 0.52 | 0.95 | 4.59 | 10 |
| Parameter | STD | Pre-Processing Combination | RMSE | R2 | RPD |
|---|---|---|---|---|---|
| °Brix | 7.88 | LOGT, FD, SNV | 0.60 | 0.95 | 13.10 |
| FD, SNV | 0.62 | 0.94 | 12.70 | ||
| pH | 1.82 | LOGT, FD, SNV | 0.07 | 0.96 | 26.05 |
| FD, SNV | 0.07 | 0.96 | 26.05 | ||
| TA | 2.92 | Raw Spectra | 0.46 | 0.97 | 6.35 |
| FD, SNV | 0.40 | 0.97 | 7.30 |
| Parameter | STD | Pre-Processing Combination | RMSE | R2 | RPD | N Latent Variables |
|---|---|---|---|---|---|---|
| Cotton Candy™ | 4.37 | Raw Spectra | 0.67 | 0.98 | 6.53 | 6 |
| LOGT, FD, SNV | 0.60 | 0.98 | 7.29 | 3 | ||
| LOGT, SD, SNV | 0.63 | 0.98 | 6.88 | 3 | ||
| FD, SNV | 0.59 | 0.98 | 7.42 | 3 | ||
| SD, SNV | 0.61 | 0.98 | 7.21 | 4 | ||
| Allison™ | 5.07 | Raw Spectra | 0.96 | 0.97 | 5.26 | 7 |
| LOGT, FD, SNV | 0.77 | 0.98 | 6.56 | 4 | ||
| LOGT, SD, SNV | 0.87 | 0.97 | 5.82 | 4 | ||
| FD, SNV | 0.88 | 0.97 | 5.77 | 5 | ||
| SD, SNV | 0.91 | 0.97 | 5.57 | 5 | ||
| Autumncrisp® | 3.43 | Raw Spectra | 0.61 | 0.97 | 5.64 | 6 |
| LOGT, FD, SNV | 0.44 | 0.98 | 7.75 | 4 | ||
| LOGT, SD, SNV | 0.43 | 0.98 | 8.06 | 4 | ||
| FD, SNV | 0.54 | 0.98 | 6.38 | 4 | ||
| SD, SNV | 0.50 | 0.98 | 6.83 | 4 | ||
| Summer Royal | 4.41 | Raw Spectra | 0.72 | 0.97 | 6.14 | 7 |
| LOGT, FD, SNV | 0.57 | 0.97 | 7.69 | 4 | ||
| LOGT, SD, SNV | 0.59 | 0.98 | 7.55 | 5 | ||
| FD, SNV | 0.61 | 0.98 | 7.30 | 4 | ||
| SD, SNV | 0.66 | 0.98 | 7.70 | 5 |
| Cultivar | STD | Pre-Processing Combination | RMSE | R2 | RPD |
|---|---|---|---|---|---|
| Cotton Candy™ | 5.09 | LOGT, FD, SNV | 0.68 | 0.97 | 7.48 |
| FD, SNV | 0.69 | 0.97 | 7.37 | ||
| Allison™ | 6.26 | LOGT, FD, SNV | 0.72 | 0.97 | 8.70 |
| LOGT, SD, SNV | 0.84 | 0.96 | 7.45 | ||
| Autumncrisp® | 5.83 | LOGT, FD, SNV | 0.66 | 0.95 | 8.84 |
| LOGT, SD, SNV | 0.60 | 0.96 | 9.72 | ||
| Summer Royal | 5.91 | LOGT, FD, SNV | 0.88 | 0.95 | 6.72 |
| LOGT, SD, SNV | 0.83 | 0.96 | 7.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrara, G.; Marcotuli, V.; Didonna, A.; Stellacci, A.M.; Palasciano, M.; Mazzeo, A. Ripeness Prediction in Table Grape Cultivars by Using a Portable NIR Device. Horticulturae 2022, 8, 613. https://doi.org/10.3390/horticulturae8070613
Ferrara G, Marcotuli V, Didonna A, Stellacci AM, Palasciano M, Mazzeo A. Ripeness Prediction in Table Grape Cultivars by Using a Portable NIR Device. Horticulturae. 2022; 8(7):613. https://doi.org/10.3390/horticulturae8070613
Chicago/Turabian StyleFerrara, Giuseppe, Valerio Marcotuli, Angelo Didonna, Anna Maria Stellacci, Marino Palasciano, and Andrea Mazzeo. 2022. "Ripeness Prediction in Table Grape Cultivars by Using a Portable NIR Device" Horticulturae 8, no. 7: 613. https://doi.org/10.3390/horticulturae8070613
APA StyleFerrara, G., Marcotuli, V., Didonna, A., Stellacci, A. M., Palasciano, M., & Mazzeo, A. (2022). Ripeness Prediction in Table Grape Cultivars by Using a Portable NIR Device. Horticulturae, 8(7), 613. https://doi.org/10.3390/horticulturae8070613