Abstract
Synthetic chemicals are used to protect crops and agricultural products, thereby producing high yields. However, intensive use of these synthetic chemicals significantly affects the environment and sustainable agriculture production. Moreover, direct or indirect exposure to these synthetic chemicals may cause acute or chronic toxicity in humans and animals. Due to their biodegradability, low toxicity, and being environmentally friendly, secondary metabolites derived from plant sources are being studied as a sustainable approach. Apiaceae family crops are a good source of bioactive phytochemicals. Many studies have found that Apiaceae extracts and essential oils possess various biocidal activities: antibacterial, antifungal, herbicidal, insecticidal or repellent, and larvicidal activities, among others. These various potent bioactivities make the Apiaceae an excellent alternative source for synthetic chemicals. In this context, the present review highlights the biocidal activities of some Apiaceae species and their potential applications in agriculture to protect the plant and agricultural products against pests, weeds, phytopathogens, and foodborne and food spoilage microorganisms.
Keywords:
Antibacterial; antifungal; biocides; essential oil; herbicides; insecticides; pesticides; phytopathogens; Umbelliferae 1. Introduction
Apiaceae (formerly Umbelliferae) is one of the largest plant families in the order Apiales. Commonly known as parsley or carrot family, it consists of nearly 3780 species belonging to 434 genera distributed throughout many regions in the world [1]. The Apiaceae family includes some of the widely used vegetables and aromatic herbs such as carrot (Daucus carota), parsnip (Pastinaca sativa), celery (Apium graveolens), Gotu kola (Centella asiatica), parsley (Petroselinum crispum), coriander (Coriandrum sativum), dill (Anethum graveolens), fennel (Foeniculum vulgare), cumin (Cuminum cyminum), anise (Pimpinella anisum), caraway (Carum carvi), as well as poisonous species like water hemlock (Cicuta maculata), northern water hemlock (Cicuta virosa), poison hemlock (Conium maculatum), Aethusa cynapium (fool’s parsley), and hemlock water-dropwort (Oenanthe crocata). Plants of the Apiaceae family are grown, not only as a nutritional source, but also as a source of flavour, fragrance, and medicine. Different parts, such as the root, leaf, leaf stalk, pseudo-bulb, and seed, serve these purposes. All parts of Apiaceae plants contain secretory glands that are important in storing essential oils (EOs), giving rise to the distinct flavour of each species [2,3,4]. Moreover, EO plays a vital role in plants: defence against bacteria, viruses, fungi, insects, and herbivores, and as an attractant to pollinators [5].
Agrochemicals, such as pesticides, fertilizers, and chemical growth agents are widely used to increase crop yield and manage weeds, pests, and diseases. Extensive use of these synthetic agrochemicals poses potential risks to human health and the environment, including agrochemical poisoning, negative impact on biocontrol agents, reduced biodiversity and ecosystem functions, residues in the environment and water and soil pollution, food, and drinking water, accumulation in the food chain, high and acute toxicity in humans, occupational and non-occupational health impacts, and pest-resistance development. Therefore, the development of non-toxic, biodegradable, and eco-friendly natural agrochemicals as an alternative to synthetic biocides gained much attention [6,7,8].
In recent years, the extraction of active compounds (secondary metabolites) from plant sources gained interest among scientists. Plants synthesize secondary metabolites in response to defence mechanisms against biotic or abiotic stress. These secondary metabolites protect plants from phytopathogens (fungi, bacteria, viruses, nematodes, protozoa), predators, UV-B radiation, and drought, and play a role in growth and development. Plants produce several secondary metabolites, such as phenolics, terpenes, carotenoids, alkaloids, and other nitrogen/sulphur-containing compounds [9,10]. Various plant extracts and secondary metabolites are known to have various bioactivities, such as herbicidal, insecticidal, and antimicrobial activities [11]. Medicinal aromatic plants contain high concentrations of these secondary metabolites or bioactive compounds and can be used in pharmaceuticals, agrochemicals, food additives, and as ingredients in cosmetics [11].
Apiaceae species are a good source of secondary metabolite compounds with diverse biological activities, such as apoptosis inducers, antibacterial, antifungal, phytotoxic activities, and cyclooxygenase inhibitory activity [12,13]. Thus, Apiaceae can be used in agriculture as a biopesticide, sprouting inhibitor, antifungal agent, and insect repellent [14]. In this respect, this current review focuses on the Apiaceae as a source of biocidal components and their potential uses in agriculture. Relevant articles published in English were searched in different databases, specifically, Google Scholar, Scopus, Wiley Online Library, Semantic Scholar, and Pub Med. This review cites data from 150 articles, published between 2005 and 2021, and compiles the biocidal potential of 54 Apiaceae species.
2. Phytochemicalsin Apiaceae Extracts
Several Apiaceae species are an excellent source of EOs, which contain more than 760 different chemical components [1,2]. The chemical composition varies with the plant part used for extraction, various extraction methods, phenological stages of the plant, harvesting season, plant age, soil nature, and environmental conditions. These variations in chemical composition have a direct effect on their biological activities [15,16]. Knowledge of these chemical constituents is vital to make use of this economically important plant family, not only for medicinal benefits, but also for environmental applications. Essential oils and their components, mainly monoterpenoids and sesquiterpenoids, show antimicrobial, repellent, chemosterilant, antifeeding, and other biocidal activities [17,18]. Table 1 shows some Apiaceae species and their major components, while Figure 1 shows the chemical structure of several main components.

Table 1.
Phytochemicals in Apiaceae plant extracts.
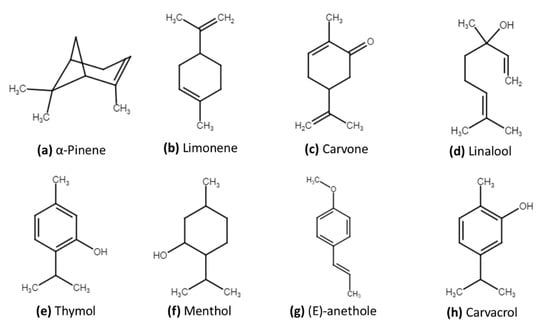
Figure 1.
Chemical structure of several major components with potential biocidal activity;
3. Biocidal Effects of Apiaceae Extracts
The biocidal activities of phytochemicals, such as monoterpene, phthalides, terpenoids, phenylpropanoids (coumarins and phenylpropenes), and polyacetylenes, are commonly found in Apiaceae plants [4,5]. Compared with single components, oil in its complete composition showed high antimicrobial activity due to the synergistic effect of minor and major constituents of oil [21]. Table 2 highlights the biocidal potential of several Apiaceae species.

Table 2.
Phytochemical constituents of Apiaceae plant extracts.
Botanical insecticides affect the pest with little or no side effects. These botanical insecticides kill the insects or affect their physiology in various ways, such as survival, behaviour, development, reproduction, and metabolic pathways [7].
The mechanism of insecticidal activity in secondary metabolites, including EOs, occurs via fumigant, contact, repellent, antifeedant, ovicidal, oviposition deterrent and larvicidal activity, and by inhibiting/altering neurotransmitters [acetylcholinesterase enzymes (AChE) and octopamine) and the neurotransmitter inhibitor (γ-aminobutyric acid (GABA)], impairment of the antioxidative defence systems, and cellular respiration [116,117,118,119]. Several earlier studies reported the inhibition of AChE by plant EOs. EOs components such as α-pinene and β-pinene, β-phellandrene, carvacrol, limonene, menthol, menthone, 1,8-cineole, cis-ocimene, niloticin, and (L)-fenchone inhibited the AChE of insect pests [7,117]. Thymol and eugenol disrupt the functions of GABA and octopamine [118].
Phytotoxic plant extracts or metabolites delay or inhibit seed germination, as well as retarding the growth of weeds. The phytotoxic or herbicidal effect of EOs is associated with various mechanisms, including inhibition of DNA synthesis and cell proliferation, decreasing mitochondrial respiration, inducing reactive oxygen species (ROS) production, inhibition of enzymes, photosynthesis, seed germination, and seedling growth, altering membrane permeability and respiration. Oxygenated monoterpenes play a major role in most of the mechanisms. Monoterpenes, 1,8-cineole and camphor inhibit DNA synthesis, cell proliferation, and elongation [120,121,122].
The common antimicrobial mechanism of many EOs is attributed to the effect of membrane permeability or functioning, leading to cell death in fungi or bacteria. Other than these, antifungal mechanisms of secondary metabolites, including EOs, involve inhibiting the fungi cell wall formation, inhibiting the mitochondrial electron transport, cell division, RNA or DNA and/or protein synthesis, and efflux pumps [123]. The sites and number of hydroxyl groups on the phenolic compounds (simple phenols, phenolic acids, quinones, flavones, flavonoids, flavonols, tannins, and coumarins) may be related to their relative toxicity to fungi. The antifungal mechanism of terpenoids (diterpenes, triterpenes, tetraterpenes, hemiterpenes, and sesquiterpenes) is related to membrane disruption by the lipophilic compounds, while the mechanisms of alkaloids (heterocyclic nitrogen compounds) are attributed to their ability to intercalate with DNA [12].
4. Potential Use of Apiaceae Extracts as Agrochemicals
Synthetic agrochemicals are used to manage weeds, pests, and phytopathogens, thus increasing productivity and food safety. However, long-time usage of these synthetic chemicals may lead to environmental contamination, accumulation of toxic residues and biomagnification through the food chain, development of insect resistance, and threats to humans and non-target organisms [9,103]. These synthetic chemicals produce acute or chronic toxicity in living beings, depending on physicochemical characteristics, concentration and exposure time, route of entry, toxicodynamics and toxicokinetics (absorption, distribution, half-life, metabolism, and elimination), a combination of various pesticides, and the components of their formulation [9]. Secondary metabolites, including EOs, from aromatic plants, drew much attention due to their low toxicity to animals and non-target insects, fast degradation in the environment, and local availability. Other than the many health benefits, the biocidal potential of Apiaceae has great importance in agriculture for use as a natural pesticide, herbicide or phytotoxin, and antimicrobial [124].
4.1. Insecticidal Activity for Crops and Stored Producs Protection
4.1.1. Insecticidal Activity to Protect Crops
Several Apiaceae genera are toxic to various insects [124]. Tuta absoluta, a major pest of tomato cropping, can be controlled by the EO of Carum capticum (Ajwain) [7]. Thymol, γ-terpinene, and ρ-cymene are the major components of C. capticum oil that showed AChE inhibition [7].
Ammi visnaga (toothpick-plant) seeds possess acaricidal activity against Tetranychus urticae Koch, which is a polyphagous species causing damage to numerous plants (around 1200 species), including major food crops and ornamental plants [125].
Cotton leafworm, Spodoptera littoralis, causes serious damage to important economic crops, such as maize, cotton, cereals, potatoes, tobacco, tomato, vegetables, and ornamental plants [47,86]. Apiaceae species, C. carvi, F. vulgare, C. cyminum, C. caraway, C. sativum, D. carota [86], Helosciadium nodiflorum (water celery) [126], and Crithmum maritimum [47] were demonstrated to possess toxicity against S. littoralis larvae. Benelli et al. [49] also suggest that cumin (C. cyminum) and anise (P. anisum) EOs can be used to protect crops from pests or to control insect vectors, as they have potential insecticidal activity against the peach-potato aphid (Myzus persicae) and the tobacco cutworm/cotton leafworm (S. littoralis), and insect vectors, such as the common housefly (Musca domestica). The crop pest Spodoptera litura is a threat to many crops and develops resistance to synthetic pesticides. This pest can be counteracted by sea fennel (C. maritimum) EOs [48].
4.1.2. Insecticidal Activity to Protect Stored Products
Several species of storage insect pests infest agricultural products and stored granaries, causing substantial weight losses (around 10%) and quality [15]. They may carry pathogens and contaminate food [127]. Sitophilus oryzae (rice weevil), Sitophilus zeamais (maize weevil), Tyrophagus putrescentiae (mould or cheese mite), Tribolium confusum (confused flour beetle), Callosobruchus maculatus (cowpea weevil), Periplaneta americana (American cockroach), Tribolium castaneum (red flour beetle) are some of the pests that affect stored cereals, grains, flour, and other dried products [127]. Research is focused on producing safe and low-risk natural compounds for pest control [43]. Several Apiaceae species were reported to carry insecticidal activities and have potential to protect stored grains from insect infestation.
Monoterpenes, such as (S)-(−)-linalool and (S)-(−)-menthone showed the most contact toxicity and fumigant activities, respectively, while α- pinene, menthone, citronellal, and linalool exhibited repellent activities against the rice weevil (S. oryzae) [128]. Cuminaldehyde and (S)-carvone showed strong repellent activities, fumigation toxicity, and AChE inhibition [129].
Apiaceae species were demonstrated to possess insecticidal and repellent activity. EOs from F. vulgare, Petroselinum hortense, C. sativum, and P. anisum showed stronger fumigant toxicity against the storage pest, red flour beetle (T. castaneum) than against the rice weevil (S. oryzae) [43]. C. carvi EO and two isolated main compounds, (R)-carvone and D-limonene, exhibited strong contact and fumigant toxicity against the maize weevil (S. zeamais) and the red flour beetle (T. castaneum) [33]. A. graveolens (dill), C. cyminum (cumin), and P. crispum (parsley) EOs were reported as having contact toxicity, fumigant, and repellent activities against the stored grain pest S. zeamais. Moreover, AChE inhibition was also reported with P. crispum and C. cyminum EOs [129]. Trachyspermum ammi EO also showed repellent and fumigant activity against the rice weevil (S. oryzae). In addition, T. ammi EO showed significant inhibition of AChE activity in rice weevils [15].
Insecticidal activity of EO derived from A. graveolens (dill) seeds was reported against T. confusum (confused flour beetle), C. maculatus (cowpea weevils), M. domestica, P. americana (American cockroach), and T. castaneum (red flour beetles)] [130].
Plodia interpunctella (Indian meal moth), Sitotroga cerealella (grain moth), and T. putrescentiae (mould or cheese mite) affecting stored products, such as grains, flours, feeds, dried nuts, and fruits, globally. P. interpunctella moth continuously produces a silken web on the food; S. cerealella larvae invade grains and complete the larval and pupal stages within the grains, thus decreasing grain weight and nutritional value; and T. putrescentiae mites disseminate toxic fungi and induce allergic reactions among workers engaged in agriculture and food industries. Camphor and linalool found in C. sativum EO, extracted by steam distillation, exhibited acaricidal and insecticidal properties against P. interpunctella, S. cerealella, and T. putrescentiae [41].
4.2. Herbicidal/Phytotoxic Activity against Weeds
Many studies were undertaken to investigate the herbicidal potential of Apiaceae species against weeds. Enzymes such as α- amylase, catalase, and peroxidase enzymes play a key role in the physiological functions of the seeds and plants. Application of EOs increases oxidative stress in plant cells, which inhibits these enzymes and causes subsequent cell death. Moreover, the detrimental effect on the DNA and RNA of the cell results in decreased cell division, growth, and elongation [63].
Herbicidal effects of EOs derived from C. sativum, C. carvi, F. vulgare, and P. anisum were reported against various weeds [122]. Eryngium triquetrum EO and its major constituent falcarinol showed strong herbicidal activity against Lepidium, while Smyrnium olusatrum EO showed moderate herbicidal activity. Therefore, E. triquetrum and S. olusatrum EOs can be used in crop protection to inhibit photosynthesis in weeds [55].
Caraway (C. carvi) EO (oil in water emulsion) are rich in oxygenated monoterpenes and exhibited herbicidal activity against Echinochloa crus-galli (barnyard grass, a typical maize weed) [29]. C. carvi EO was found to inhibit the germination of seeds of common weeds, including Amaranthus retroflexus, Centaurea salsotitialis, Raphanus raphanistrum, Rumex nepalensis, Sonchus oleraceus, and Sinapis arvensis [131].
Fennel (F. vulgare) EO strongly inhibited the seed germination and seedling growth of grass weeds, Phalaris minor, Avena ludoviciana, broad-leaved weeds, Rumex dentatus and Medicago denticulate, and can, therefore, be used in the biological management of weeds in wheat (Triticum aestivum) crops [63]. Fennel (F. vulgare) EO was also reported for its phytotoxic activity against field bindweed (Convolvulus arvensis) with significant inhibition of germination and early growth in field bindweed seedlings [132].
4.3. Antimicrobial Activity against Phytopathogens
Plant pathogens, including fungi, bacteria, viruses, oomycetes, and nematodes, can cause diseases or damage to plants and result in significant crop yield and quality losses, of which fungi are the predominant pathogens, causing almost 30% of crop diseases during their cultivation or after harvest. Fungal disease symptoms on fruits, leaves, stems, and other plant parts include scabs, mouldy coatings, rust, smuts, powdery mildew, blotches, and rotted tissues. Plant diseases caused by phytopathogenic bacteria and fungi threaten human health and food security [13,120,133,134].
Various phytopathogenic bacteria, including Xanthomonas spp. (bacterial spots and blights), Erwinia spp. (soft rot, fire blight), Pseudomonas spp. (soft rot, bacterial canker), Agrobacterium spp. (crown gall), as well as Ralstonia solanacearum (bacterial wilt in banana), affect various agricultural plants [123].
Moreover, Fusarium oxysporum (vascular wilt of the banana tree), Macrophomina phaseolina (damping-off, seedling blight, and rot in peanuts, cabbage, pepper, chickpeas, soybeans, sweet potatoes, sesame, potatoes, sorghum, wheat, corn, etc.), Alternaria alternata (rot), Penicillium digitatum (green mould in citrus), and Aspergillus flavus (damping-off in peanut) are some of the fungi responsible for many diseases in various plants [134,135].
Apiaceae family plants were widely investigated for their potential biocidal activity against phytopathogens.
Apiaceae, such as Torilis anthriscus, Aegopodium podagraria, D. carota, Heracleum sphondyilium, Pimpinella saxifrage, P. sativa, Angelica silvestris, and F. vulgare exhibited antimicrobial activity against phytopathogenic bacteria Agrobacterium radiobacter pv tumefaciens, Erwinia carotowora, Pseudomonas fluorescens, and Pseudomonas glycinea [13]. The EO of C. maculatum (poison hemlock), a poisonous Apiaceae species, has antibacterial activity against Pseudomonas aeruginosa [136].
E. triquetrum EO exhibited strong antibacterial activities against potato blackleg disease, Pectobacterium atrosepticum, and the soil bacterium Pseudomonas cichorii, which is responsible for disease in lettuce, celery, and chrysanthemum, while S. olusatrum EO showed moderate antibacterial activity [55].
The Apiaceae Ferulago angulate exhibited toxicity in variable degrees against phytopathogenic bacteria (Erwinia amylovora, Xanthomonas oryzae, Pseudomonas syringae, Pectobacterium carotovorum, R. solanacearum, Bacillus thuringiensis), and fungi (A. alternata, Culvularia fallax, M. phaseolina, F. oxysporum, Cytospora sacchari, Colletotrichum tricbellum) [57].
A. alternata and P. digitatum are two postharvest pathogens in tomato fruits. Based on in vivo studies, Carum copticum and F. vulgare EOs have the potential to control postharvest decay in tomatoes caused by A. alternata and P. digitatum [31].
E. triquetrum and S. olusatrum EOs have potent antifungal and fumigant activity against Fusarium graminearum (cereal fusarium) and moderate activity against Botrytis cinerea (grey rot on tomatoes, strawberries). S. olusatrum EO showed strong antifungal activity against F. graminearum and Zymoseptoria tritici, which are responsible for the septoria blight [55].
A. graveolens (dill) seeds EO mainly consists of carvone, limonene, α-phellandrene, β-phellandrene and p-cymene and were proved to have antifungal (Aspergillus niger, Aspergillus oryzae, A. flavus, A. alternata) and antibacterial (Escherichia coli, P. aeruginosa, Bacillus subtilis, Staphylococcus aureus) activities [130].
Food poisoning is the most common cause of illness and death in both developed and developing countries. Most food poisoning diseases are caused by bacterial contamination. Pathogenic and food spoilage bacteria, such as Salmonella typhi, E. coli, P. aeruginosa, S. aureus, Listeria monocytogenes, and Bacillus cereus are the causal agents of foodborne diseases or food spoilage. Plant extracts are a good source of antimicrobial agents that can be used as food preservatives [137,138].
Apiaceae C. cyminum was reported to be effective against S. aureus [138]. Coriander (C. sativum) EO and its major compound linalool showed antibacterial activity against Campylobacter jejuni and Campylobacter coli, pathogens that cause foodborne diseases [139].
Coriander (C. sativum) was also reported to have strong antimicrobial activity against bacteria, B. subtilis, followed by Stenotropomonas maltophilia, and Penicillium expansum (fungi producing mycotoxin). Moreover, the strongest antibiofilm activity of coriander EO was also reported against S. maltophilia [140].
Carum nigrum (black caraway) seed EO has antibacterial activity against foodborne bacteria, B. cereus and P. aeruginosa, and antifungal activity against foodborne fungi, such as P. purpurogenum, Acrophialophora fusispora, A. flavus, and A. niger [35].
The antimicrobial activity of T. ammi (Ajwain) EO was demonstrated against various food spoilage and foodborne bacteria (B. cereus, S. aureus, L. monocytogenes, Salmonella typhimurium, E. coli) and fungi (Penicillium citrinum, Penicillium chrysogenum, A. flavus, A. niger, Aspergillus parasiticus) [70].
Moreover, certain fungi, including Aspergillus spp., Fusarium spp., and Alternaria spp., produce mycotoxins that can be harmful to human health due to hepatotoxic, nephrotoxic, and carcinogenic effects, or even cause death [120]. Mycotoxins are toxic secondary metabolites produced by certain fungal species in various agricultural and other food products, either in the field or during storage. Mycotoxin contamination in crops is potentially harmful to animals and human health. Aflatoxins B1, B2, G1, and G2 are the four major toxins generated in foods, of which the aflatoxin B1, secreted by A. flavus, A. parasiticus and Aspergillus nomius, is the most toxic and has potent teratogenic, mutagenic, hepatotoxic, and immune suppressive activities [141,142,143].
Apiaceae, F. vulgare EO extracted from flowers and roots, significantly inhibited the growth of A. parasiticus and the production of aflatoxins B1 and G1 [144]. Carvone and linalool compounds found in C. carvi and C. sativum seed EOs, respectively, showed an antifungal and aflatoxin-inhibition ability against A. flavus. Thus, they can be used as a preservative, particularly in post-harvest processing and the storage of agricultural products that are susceptible to aflatoxin contamination, such as cereals, dried fruits, and spices [143]. EOs of other Apiaceae plants, including C. cyminum [145], C. carvi [28,146], C. sativum [28], and C. copticum (T. ammi) were also reported to inhibit both growth and/or mycotoxin production. Table 3 summarizes the potential use of Apiaceae extracts as agrochemicals.

Table 3.
Potential use of Apiaceae extracts as agrochemicals.
5. Future Trends
Although EOs are an excellent alternative for synthetic chemicals, low water solubility, low persistence in the environment, chemical variability, low yield, and easy degradation under heat, humidity, light, and oxygen may limit the application of essential oils as biocides. To overcome these drawbacks, nanotechnologies are modern and eco-friendly approaches that enhance the effectiveness of natural products [48,63,118].
Nanoemulsion is a novel approach that increases the physical stability of active substances, decreases volatility, enhances solubility, and prevents interactions with the environment [48,63]. Nanoemulsions are oil-in-water dispersions with extremely small droplet diameters in the range of 10−100 nm and extended stability [48]. Size reduction to a nanoscale can improve the properties of materials, such as enhanced infusibility, effective release of the active ingredient, rapid interaction, enhanced bioactivity towards the target, and physical stability [63,147]. In agricultural applications, nanoemulsions have significant herbicidal and antimicrobial potential against weeds and pathogens that are resistant to synthetic biocides. This may be attributed to increased chemical stability and solubility, decreased evaporation, and the degradation of active essential oil components [63,148]. Nanotechnology also enhances efficacy and reduces the number of active ingredients currently employed [149].
The germination of P. minor, A. ludoviciana, R. dentatus, and Medicago denticulate was completely suppressed by nanoemulsions of F. vulgare EO at low concentrations. Further, the storage stability of F. vulgare nanoemulsion at ambient temperature was increased [63]. Significant antimicrobial activity of cumin (C. cyminum) and fennel (F. vulgare) EO nanoemulsions showed antibacterial/anti-biofilm properties against the foodborne pathogens K. pneumoniae, C. violaceum, and S. typhimurium [150]. Repellent activity by aniseed oil against Rhopalosiphum padi was also reported [149].
Other than nanotechnology, advanced extraction techniques, chemical synthesis of novel compounds, metabolic engineering, biotechnology, DNA sequencing, and recombinant DNA technologies can also enhance plant product effectiveness and commercial applications [118].
6. Conclusions
In conclusion, plant-based biocides can be a suitable candidate to substitute synthetic chemicals as they are cheap, safe, sustainable, eco-friendly, and biodegradable substances. In this context, secondary metabolites of Apiaceae species are widely used to control most agricultural pests, weeds, pathogenic foodborne, and food spoilage microbes to increase agriculture production and ensure food security. Several studies on various Apiaceae species for their potential biocidal activity proved their potent insecticidal, herbicidal, antibacterial, and antifungal activities. Therefore, Apiaceae-derived secondary metabolites, including EOs, can be used as a natural source in biocontrol products in agriculture, bio-pesticide, bio-herbicide, bio-fungicide, and antibacterial agents in organic farming and crop protection. However, there are several limitations in the application of secondary metabolites, including EOs, mainly due to their fast degradation and low stability in heat, light, or oxygen. Recently, nanotechnology research has attempted to overcome these limitations of natural products used as biocides.
Author Contributions
Writing—original draft, P.T., N.G., A.G., O.M. and T.M. Writing—review and editing, P.T., A.G., N.G., O.M. and T.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ahmad, B.S.; Talou, T.; Saad, Z.; Hijazi, A.; Ahmad, B.S.; Talou, T.; Saad, Z.; Hijazi, A.; Merah, O.; Apiaceae, T. The Apiaceae: Ethnomedicinal Family as Source for Industrial Uses. Ind. Crops Prod. 2017, 109, 661–671. [Google Scholar] [CrossRef]
- Aćimović, M.G. Nutraceutical Potential of Apiaceae. In Bioactive Molecules in Food; Mérillon, J.-M., Ramawat, K.G., Eds.; Reference Series in Phytochemistry; Springer International Publishing: Cham, Switzerland, 2019; pp. 1311–1341. ISBN 978-3-319-78030-6. [Google Scholar]
- Aćimović, M.G.; Milićb, N.B. Perspectives of the Apiaceae Hepatoprotective Effects—A Review. Nat. Prod. Commun. 2017, 12, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Sousa, R.M.O.F.; Cunha, A.C.; Fernandes-Ferreira, M. The Potential of Apiaceae Species as Sources of Singular Phytochemicals and Plant-Based Pesticides. Phytochemistry 2021, 187, 112714. [Google Scholar] [CrossRef] [PubMed]
- Christensen, L.P.; Brandt, K. Bioactive Polyacetylenes in Food Plants of the Apiaceae Family: Occurrence, Bioactivity and Analysis. J. Pharm. Biomed. Anal. 2006, 41, 683–693. [Google Scholar] [CrossRef]
- Damalas, C.A.; Eleftherohorinos, I.G. Pesticide Exposure, Safety Issues, and Risk Assessment Indicators. Int. J. Environ. Res. Public Health 2011, 8, 1402–1419. [Google Scholar] [CrossRef]
- Piri, A.; Sahebzadeh, N.; Zibaee, A.; Sendi, J.J.; Shamakhi, L.; Shahriari, M. Toxicity and Physiological Effects of Ajwain (Carum copticum, Apiaceae) Essential Oil and Its Major Constituents against Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Chemosphere 2020, 256, 127103. [Google Scholar] [CrossRef]
- Rajput, P.; Thakur, A.; Devi, P. Chapter 5—Emerging Agrochemicals Contaminants: Current Status, Challenges, and Technological Solutions. In Agrochemicals Detection, Treatment and Remediation; Prasad, M.N.V., Ed.; Butterworth-Heinemann: Oxford, UK, 2020; pp. 117–142. ISBN 978-0-08-103017-2. [Google Scholar]
- Ramírez-Gómez, X.S.; Jiménez-García, S.N.; Campos, V.B.; Campos, M.L.G. Plant Metabolites in Plant Defense Against Pathogens; IntechOpen: London, UK, 2019; ISBN 978-1-78985-116-8. [Google Scholar]
- Zaynab, M.; Fatima, M.; Abbas, S.; Sharif, Y.; Umair, M.; Zafar, M.H.; Bahadar, K. Role of Secondary Metabolites in Plant Defense against Pathogens. Microb. Pathog. 2018, 124, 198–202. [Google Scholar] [CrossRef]
- Naboulsi, I.; Aboulmouhajir, A.; Kouisni, L.; Bekkaoui, F.; Yasri, A. Plants Extracts and Secondary Metabolites, Their Extraction Methods and Use in Agriculture for Controlling Crop Stresses and Improving Productivity: A Review. Acad. J. Med. Plants 2018, 6, 223–240. [Google Scholar]
- Boulogne, I.; Petit, P.; Ozier-lafontaine, H.; Loranger-merciris, G.; Boulogne, I.; Petit, P.; Ozier-lafontaine, H.; Desfontaines, L.; Loranger-Merciris, G. Insecticidal and Antifungal Chemicals Produced by Plants: A Review. Environ. Chem. Lett. 2012, 10, 325–347. [Google Scholar] [CrossRef]
- Duško, B.L.; Comiæ, L.; Sukdolak, S. Antibacterial Activity of Some Plants from Family Apiaceae in Relation to Selected Phytopathogenic Bacteria. Kragujev. J. Sci. 2006, 28, 65–72. [Google Scholar]
- Ferrie, A.M.R.; Caswell, K.L. Chapter 13—Applications of Doubled Haploidy for Improving Industrial Oilseeds. In Industrial Oil Crops; McKeon, T.A., Hayes, D.G., Hildebrand, D.F., Weselake, R.J., Eds.; AOCS Press: Champaign, IL, USA, 2016; pp. 359–378. ISBN 978-1-893997-98-1. [Google Scholar]
- Chaubey, M.K. Biological Effects of Essential Oils Against Rice Weevil Sitophilus oryzae L. (Coleoptera: Curculionidae). J. Essent. Oil Bear. Plants 2012, 15, 809–815. [Google Scholar] [CrossRef]
- Petretto, G.L.; Fancello, F.; Bakhy, K.; Faiz, C.A.; Sibawayh, Z.; Chessa, M.; Zara, S.; Sanna, M.L.; Maldini, M.; Rourke, J.P.; et al. Chemical Composition and Antimicrobial Activity of Essential Oils from Cuminum cyminum L. Collected in Different Areas of Morocco. Food Biosci. 2018, 22, 50–58. [Google Scholar] [CrossRef]
- El Karkouri, J.; Bouhrim, M.; Al Kamaly, O.M.; Mechchate, H.; Kchibale, A.; Adadi, I.; Amine, S.; Alaoui Ismaili, S.; Zair, T. Chemical Composition, Antibacterial and Antifungal Activity of the Essential Oil from Cistus ladanifer L. Plants 2021, 10, 2068. [Google Scholar] [CrossRef]
- Gazim, Z.C.; Demarchi, I.G.; Lonardoni, M.V.C.; Amorim, A.C.L.; Hovell, A.M.C.; Rezende, C.M.; Ferreira, G.A.; de Lima, E.L.; de Cosmo, F.A.; Cortez, D.A.G. Acaricidal Activity of the Essential Oil from Tetradenia riparia (Lamiaceae) on the Cattle Tick Rhipicephalus (Boophilus) microplus (Acari; Ixodidae). Exp. Parasitol. 2011, 129, 175–178. [Google Scholar] [CrossRef]
- Abdulmanea, K.; Prokudina, E.A.; Lanková, P.; Vaníčková, L.; Koblovská, R.; Zelený, V.; Lapčík, O. Immunochemical and HPLC Identification of Isoflavonoids in the Apiaceae Family. Biochem. Syst. Ecol. 2012, 45, 237–243. [Google Scholar] [CrossRef]
- Sadaoui, N.; Bec, N.; Barragan-Montero, V.; Kadri, N.; Cuisinier, F.; Larroque, C.; Arab, K.; Khettal, B. The Essential Oil of Algerian Ammodaucus leucotrichus Coss. & Dur. and Its Effect on the Cholinesterase and Monoamine Oxidase Activities. Fitoterapia 2018, 130, 1–5. [Google Scholar] [CrossRef]
- Nikolić, M.; Marković, T.; Ćirić, A.; Glamočlija, J.; Marković, D.; Soković, M. Susceptibility of Oral candida Spp.: Reference Strains and Clinical Isolates to Selected Essential Oils of Apiaceae Species. Lek. Sirovine 2016, 35, 151–162. [Google Scholar] [CrossRef]
- Singh, G.; Maurya, S.; de Lampasona, M.P.; Catalan, C. Chemical Constituents, Antimicrobial Investigations, and Antioxidative Potentials of Anethum graveolens L. Essential Oil and Acetone Extract: Part 52. J. Food Sci. 2005, 70, M208–M215. [Google Scholar] [CrossRef]
- Aćimović, M.G.; Pavlović, S.D.; Varga, A.O.; Filipović, V.M.; Cvetković, M.T.; Stanković, J.M.; Čabarkapa, I.S. Chemical Composition and Antibacterial Activity of Angelica Archangelica Root Essential Oil. Nat. Prod. Commun. 2017, 12, 205–206. [Google Scholar] [CrossRef]
- Sipailiene, A.; Venskutonis, P.R.; Sarkinas, A.; Cypiene, V. Composition and Antimicrobial Activity of Celery (Apium graveolens) Leaf and Root Extracts Obtained with Liquid Carbon Dioxide. Acta Hortic. 2005, 677, 71–77. [Google Scholar] [CrossRef]
- Alsalman, A.-H.; Aboalhaija, N.; Talib, W.; Abaza, I.; Afifi, F. Evaluation of the Single and Combined Antibacterial Efficiency of the Leaf Essential Oils of Four Common Culinary Herbs: Dill, Celery, Coriander and Fennel Grown in Jordan. J. Essent. Oil Bear. Plants 2021, 24, 317–328. [Google Scholar] [CrossRef]
- Maggi, F.; Giuliani, C.; Fico, G.; Ricciutelli, M.; Bramucci, M.; Quassinti, L.; Petrelli, D.; Vitali, L.A.; Cianfaglione, K.; Tirillini, B.; et al. Secondary Metabolites, Secretory Structures and Biological Activity of Water Celery (Apium nodiflorum (L.) Lag.) Growing in Central Italy. Plant Biosyst.—Int. J. Deal. All Asp. Plant Biol. 2019, 153, 325–335. [Google Scholar] [CrossRef]
- López, S.; Lima, B.; Aragón, L.; Espinar, L.A.; Tapia, A.; Zacchino, S.; Zygadlo, J.; Feresin, G.E.; López, M.L. Essential Oil of Azorella Cryptantha Collected in Two Different Locations from San Juan Province, Argentina: Chemical Variability and Anti-Insect and Antimicrobial Activities. Chem. Biodivers. 2012, 9, 1452–1464. [Google Scholar] [CrossRef] [PubMed]
- Abou El-Soud, N.H.; Deabes, M.M.; Abou El-Kassem, L.; Khalil, M.Y. Antifungal Activity of Family Apiaceae Essential Oils. J. Appl. Sci. Res. 2012, 8, 4964–4973. [Google Scholar]
- Synowiec, A.; Możdżeń, K.; Krajewska, A.; Landi, M.; Araniti, F. Carum carvi L. Essential Oil: A Promising Candidate for Botanical Herbicide against Echinochloa crus-galli (L.) P. Beauv. in Maize Cultivation. Ind. Crops Prod. 2019, 140, 111652. [Google Scholar] [CrossRef]
- Hajlaoui, H.; Arraouadi, S.; Noumi, E.; Aouadi, K.; Adnan, M.; Khan, M.A.; Kadri, A.; Snoussi, M. Antimicrobial, Antioxidant, Anti-Acetylcholinesterase, Antidiabetic, and Pharmacokinetic Properties of Carum carvi L. and Coriandrum sativum L. Essential Oils Alone and in Combination. Molecules 2021, 26, 3625. [Google Scholar] [CrossRef]
- Abdolahi, A.; Hassani, A.; Ghosta, Y.; Javadi, T.; Meshkatalsadat, M.H. Essential Oils as Control Agents of Postaharvest Alternaria and Penicillium Rots on Tomato Fruits. J. Food Saf. 2010, 30, 341–352. [Google Scholar] [CrossRef]
- Khalil, N.; Ashour, M.; Fikry, S.; Singab, A.N.; Salama, O. Chemical Composition and Antimicrobial Activity of the Essential Oils of Selected Apiaceous Fruits. Future J. Pharm. Sci. 2018, 4, 88–92. [Google Scholar] [CrossRef]
- Fang, R.; Jiang, C.H.; Wang, X.Y.; Zhang, H.M.; Liu, Z.L.; Zhou, L.; Du, S.S.; Deng, Z.W. Insecticidal Activity of Essential Oil of Carum carvi Fruits from China and Its Main Components against Two Grain Storage Insects. Molecules 2010, 15, 9391–9402. [Google Scholar] [CrossRef]
- Simic, A.; Rančic, A.; Sokovic, M.D.; Ristic, M.; Grujic-Jovanovic, S.; Vukojevic, J.; Marin, P.D. Essential Oil Composition of Cymbopogon Winterianus. and Carum carvi. and Their Antimicrobial Activities. Pharm. Biol. 2008, 46, 437–441. [Google Scholar] [CrossRef]
- Singh, G.; Marimuthu, P.; de Heluani, C.S.; Catalan, C.A.N. Antioxidant and Biocidal Activities of Carum Nigrum (Seed) Essential Oil, Oleoresin, and Their Selected Components. J. Agric. Food Chem. 2006, 54, 174–181. [Google Scholar] [CrossRef]
- Oyedeji, O.A.; Afolayan, A.J. Chemical Composition and Antibacterial Activity of the Essential Oil of Centella asiatica Growing in South Africa. Pharm. Biol. 2005, 43, 249–252. [Google Scholar] [CrossRef]
- Petrović, G.M.; Stamenković, J.G.; Kostevski, I.R.; Stojanović, G.S.; Mitić, V.D.; Zlatković, B.K. Chemical Composition of Volatiles; Antimicrobial, Antioxidant and Cholinesterase Inhibitory Activity of Chaerophyllum aromaticum L. (Apiaceae) Essential Oils and Extracts. Chem. Biodivers. 2017, 14, e1600367. [Google Scholar] [CrossRef]
- Lakušić, B.; Slavkovska, V.; Pavlović, M.; Milenković, M.; Stanković, J.A.; Couladis, M. Chemical Composition and Antimicrobial Activity of the Essential Oil from Chaerophyllum aureum L. (Apiaceae). Nat. Prod. Commun. 2009, 4, 115–118. [Google Scholar] [CrossRef]
- Hayta, S.; Celikezen, F.C. Evaluation of Essential Oil Composition, Antioxidant and Antimicrobial Properties of Chaerophyllum Crinitum Boiss (Apiaceae) from Turkey: A Traditional Medicinal Herb. J. Biol. Sci. 2016, 16, 72–76. [Google Scholar] [CrossRef][Green Version]
- Shafaghat, A. Comparison of the Antimicrobial Activity and Chemical Constituents of the Essential Oil and Hexanic Extract from Chaerophyllum Macropodum. J. Essent. Oil Bear. Plants 2017, 20, 835–843. [Google Scholar] [CrossRef]
- Lee, M.-J.; Lee, S.-E.; Kang, M.-S.; Park, B.; Lee, S.-G.; Lee, H.-S. Acaricidal and Insecticidal Properties of Coriandrum sativum Oils and Their Major Constituents Extracted by Three Different Methods against Stored Product Pests. Appl. Biol. Chem. 2018, 61, 481–488. [Google Scholar] [CrossRef]
- Khani, A.; Rahdari, T. Chemical Composition and Insecticidal Activity of Essential Oil from Coriandrum sativum Seeds against Tribolium confusum and Callosobruchus maculatus. ISRN Pharm. 2012, 2012, 263517. [Google Scholar] [CrossRef]
- Amini, S.; Tajabadi, F.; Khani, M.; Labbafi, M.R.; Tavakoli, M. Identification of the Seed Essential Oil Composition of Four Apiaceae Species and Comparison of Their Biological Effects on Sitophilus oryzae L. and Tribolium castaneum (Herbst.). J. Med. Plants 2018, 17, 68–76. [Google Scholar]
- Alves-Silva, J.M.; Guerra, I.; Gonçalves, M.J.; Cavaleiro, C.; Cruz, M.T.; Figueirinha, A.; Salgueiro, L. Chemical Composition of Crithmum maritimum L. Essential Oil and Hydrodistillation Residual Water by GC-MS and HPLC-DAD-MS/MS, and Their Biological Activities. Ind. Crops Prod. 2020, 149, 112329. [Google Scholar] [CrossRef]
- D’Agostino, G.; Giambra, B.; Palla, F.; Bruno, M.; Badalamenti, N. The Application of the Essential Oils of Thymus vulgaris L. and Crithmum maritimum L. as Biocidal on Two Tholu Bommalu Indian Leather Puppets. Plants 2021, 10, 1508. [Google Scholar] [CrossRef] [PubMed]
- Pasias, I.N.; Ntakoulas, D.D.; Raptopoulou, K.; Gardeli, C.; Proestos, C. Chemical Composition of Essential Oils of Aromatic and Medicinal Herbs Cultivated in Greece—Benefits and Drawbacks. Foods 2021, 10, 2354. [Google Scholar] [CrossRef] [PubMed]
- Pavela, R.; Maggi, F.; Lupidi, G.; Cianfaglione, K.; Dauvergne, X.; Bruno, M.; Benelli, G. Efficacy of Sea Fennel (Crithmum maritimum L., Apiaceae) Essential Oils against Culex quinquefasciatus Say and Spodoptera littoralis (Boisd.). Ind. Crops Prod. 2017, 109, 603–610. [Google Scholar] [CrossRef]
- Suresh, U.; Murugan, K.; Panneerselvam, C.; Aziz, A.T.; Cianfaglione, K.; Wang, L.; Maggi, F. Encapsulation of Sea Fennel (Crithmum maritimum) Essential Oil in Nanoemulsion and SiO2 Nanoparticles for Treatment of the Crop Pest Spodoptera Litura and the Dengue Vector Aedes Aegypti. Ind. Crops Prod. 2020, 158, 113033. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Petrelli, R.; Cappellacci, L.; Canale, A.; Senthil-Nathan, S.; Maggi, F. Not Just Popular Spices! Essential Oils from Cuminum cyminum and Pimpinella anisum Are Toxic to Insect Pests and Vectors without Affecting Non-Target Invertebrates. Ind. Crops Prod. 2018, 124, 236–243. [Google Scholar] [CrossRef]
- Abbdellaoui, M.; Bouhlali, E.D.T.; Rhaffari, L.E. Chemical Composition and Antioxidant Activities of the Essential Oils of Cumin (Cuminum cyminum) Conducted Under Organic Production Conditions. J. Essent. Oil Bear. Plants 2019, 22, 1500–1508. [Google Scholar] [CrossRef]
- Attia, S.; Grissa, K.L.; Lognay, G.; Heuskin, S.; Mailleux, A.C.; Hance, T. Chemical Composition and Acaricidal Properties of Deverra Scoparia Essential Oil (Araliales: Apiaceae) and Blends of Its Major Constituents against Tetranychus urticae (Acari: Tetranychidae). J. Econ. Entomol. 2011, 104, 1220–1228. [Google Scholar] [CrossRef]
- Glamoclija, J.M.; Sokovic, M.D.; Šiljegovic, J.D.; Ristic, M.S.; Ciric, A.D.; Grubišic, D.V. Chemical Composition and Antimicrobial Activity of Echinophora spinosa L. (Apiaceae) Essential Oil. Rec. Nat. Prod. 2011, 5, 319–323. [Google Scholar]
- Kremer, D.; Zovko Končić, M.; Kosalec, I.; Košir, I.J.; Potočnik, T.; Čerenak, A.; Srečec, S.; Dunkić, V.; Vuko, E. Phytochemical Traits and Biological Activity of Eryngium amethystinum and E. alpinum (Apiaceae). Horticulturae 2021, 7, 364. [Google Scholar] [CrossRef]
- Casiglia, S.; Bruno, M.; Rosselli, S.; Senatore, F. Chemical Composition and Antimicrobial Activity of the Essential Oil from Flowers of Eryngium triquetrum (Apiaceae) Collected Wild in Sicily. Nat. Prod. Commun. 2016, 11, 1019–1024. [Google Scholar] [CrossRef]
- Merad, N.; Andreu, V.; Chaib, S.; de Carvalho Augusto, R.; Duval, D.; Bertrand, C.; Boumghar, Y.; Pichette, A.; Djabou, N. Essential Oils from Two Apiaceae Species as Potential Agents in Organic Crops Protection. Antibiotics 2021, 10, 636. [Google Scholar] [CrossRef]
- Karakaya, S.; Göger, G.; Bostanlik, F.D.; Demirci, B.; Duman, H.; Kiliç, C.S. Comparison of the Essential Oils of Ferula orientalis L., Ferulago Sandrasica Peşmen and Quézel, and Hippomarathrum microcarpum Petrov and Their Antimicrobial Activity. Turk. J. Pharm. Sci. 2019, 16, 69–75. [Google Scholar] [CrossRef]
- Moghaddam, M.; Mehdizadeh, L.; Mirzaei Najafgholi, H.; Ghasemi Pirbalouti, A. Chemical Composition, Antibacterial and Antifungal Activities of Seed Essential Oil of Ferulago Angulata. Int. J. Food Prop. 2018, 21, 158–170. [Google Scholar] [CrossRef]
- Karakaya, S.; Koca, M.; Sytar, O.; Dursunoglu, B.; Ozbek, H.; Duman, H.; Guvenalp, Z.; Kılıc, C.S. Antioxidant and Anticholinesterase Potential of Ferulago Cassia with Farther Bio-Guided Isolation of Active Coumarin Constituents. South Afr. J. Bot. 2019, 121, 536–542. [Google Scholar] [CrossRef]
- Dikpınar, T.; Süzgeç-Selçuk, S.; Çelik, B.Ö.; Uruşak, E.A. Antimicrobial Activity of Rhizomes of Ferulago Trachycarpa Boiss. and Bioguided Isolation of Active Coumarin Constituents. Ind. Crops Prod. 2018, 123, 762–767. [Google Scholar] [CrossRef]
- Tavakoli, S.; Yassa, N.; Delnavazi, M.R.; Akhbari, M.; Hadjiakhoondi, A.; Hajimehdipoor, H.; Khalighi-Sigaroodi, F.; Hajiaghaee, R. Chemical Composition and Biological Activities of the Essential Oils from Different Parts of Ferulago trifida Boiss. J. Essent. Oil Res. 2017, 29, 407–419. [Google Scholar] [CrossRef]
- Sayed Ahmad, B.; Talou, T.; Saad, Z.; Hijazi, A.; Cerny, M.; Kanaan, H.; Chokr, A.; Merah, O. Fennel Oil and By-Products Seed Characterization and Their Potential Applications. Ind. Crops Prod. 2018, 111, 92–98. [Google Scholar] [CrossRef]
- Teke, M.A.; Mutlu, Ç. Insecticidal and Behavioral Effects of Some Plant Essential Oils against Sitophilus granarius L. and Tribolium castaneum (Herbst). J. Plant Dis. Prot. 2021, 128, 109–119. [Google Scholar] [CrossRef]
- Kaur, P.; Gupta, S.; Kaur, K.; Kaur, N.; Kumar, R.; Bhullar, M.S. Nanoemulsion of Foeniculum Vulgare Essential Oil: A Propitious Striver against Weeds of Triticum Aestivum. Ind. Crops Prod. 2021, 168, 113601. [Google Scholar] [CrossRef]
- Torbati, M.; Nazemiyeh, H.; Lotfipour, F.; Asnaashari, S.; Nemati, M.; Fathiazad, F. Composition and Antibacterial Activity of Heracleum Transcaucasicum and Heracleum Anisactis Aerial Parts Essential Oil. Adv. Pharm. Bull. 2013, 3, 415–418. [Google Scholar] [CrossRef][Green Version]
- Moradalizadeh, M.; Akhgar, M.R.; Rajaei, P.; Faghihi-Zarandi, A. Chemical Composition of the Essential Oils of Levisticum Officinale Growing Wild in Iran. Chem. Nat. Compd. 2012, 47, 1007–1009. [Google Scholar] [CrossRef]
- Liu, Z.L.; Chu, S.S.; Jiang, G.H. Insecticidal Activity and Composition of Essential Oil of Ostericum sieboldii (Apiaceae) against Sitophilus zeamais and Tribolium castaneum. Rec. Nat. Prod. 2011, 5, 74–81. [Google Scholar]
- Brusotti, G.; Ibrahim, M.F.; Dentamaro, A.; Gilardoni, G.; Tosi, S.; Grisoli, P.; Dacarro, C.; Guglielminetti, M.L.; Hussain, F.H.S.; Caccialanza, G.; et al. Chemical Composition and Antimicrobial Activity of the Volatile Fractions from Leaves and Flowers of the Wild Iraqi Kurdish Plant Prangos Peucedanifolia FENZL. Chem. Biodivers. 2013, 10, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Stojković, D.; Mahomoodally, M.F.; Jugreet, B.S.; Paksoy, M.Y.; Ivanov, M.; Gašić, U.; Gallo, M.; Montesano, D. Comprehensive Biological and Chemical Evaluation of Two Seseli Species (S. Gummiferum and S. Transcaucasicum). Antioxidants 2021, 10, 1510. [Google Scholar] [CrossRef] [PubMed]
- Rosato, A.; Maggi, F.; Cianfaglione, K.; Conti, F.; Ciaschetti, G.; Rakotosaona, R.; Fracchiolla, G.; Clodoveo, M.L.; Franchini, C.; Corbo, F. Chemical Composition and Antibacterial Activity of Seven Uncommon Essential Oils. J. Essent. Oil Res. 2018, 30, 233–243. [Google Scholar] [CrossRef]
- Gandomi, H.; Abbaszadeh, S.; Jebellijavan, A.; Sharifzadeh, A. Chemical Constituents, Antimicrobial and Antioxidative Effects of Trachyspermum ammi Essential Oil. J. Food Processing Preserv. 2014, 38, 1690–1695. [Google Scholar] [CrossRef]
- Demirci, B.; Yasdikcioǧlu, G.K.; Başer, K.H.C. Sesquiterpene Hydrocarbons of the Essential Oil of Actinolema Macrolema Boiss. Turk. J. Chem. 2013, 37, 917–926. [Google Scholar] [CrossRef]
- Stefanovic, O.; Comic, L.; Stanojevic, D.; Solujic-Sukdolak, S. Antibacterial Activity of Aegopodium podagraria L. Extracts and Interaction between Extracts and Antibiotics. Turk. J. Biol. 2009, 33, 145–150. [Google Scholar] [CrossRef]
- Pavela, R. Larvicidal Effects of Various Euro-Asiatic Plants against Culex quinquefasciatus Say Larvae (Diptera: Culicidae). Parasitol. Res. 2008, 102, 555–559. [Google Scholar] [CrossRef]
- Fairouz, B.; Nora, C.; Salima, K.G. Insecticidal Effect of Ammi visnaga L. (Apiaceae: Apial) Methanolic Extract against a Citrus Pest, Toxoptera aurantii (Aphididae: Homoptera) under Controlled Conditions. J. Entomol. Zool. Stud. 2016, 4, 230–235. [Google Scholar]
- Meepagala, K.M.; Estep, A.S.; Becnel, J.J. Mosquitocidal Activity of Extracts from Ammi visnaga (Apiaceae) Seeds. J. Agric. Chem. Environ. 2016, 05, 170–178. [Google Scholar] [CrossRef][Green Version]
- Ghoneim, K.; Mohammad, A.; Al-Daly, A.; Amer, M.; Khadrawy, F.; Mahmoud, M.A. Metabolic Responsiveness of Desert Locust Schistocerca gregaria (Forskal) (Orthoptera: Acrididae) to the Khella Plant Ammi visnaga L. (Apiaceae) Extracts. Int. J. Adv. Life Sci. 2014, 7, 204–216. [Google Scholar]
- Sebaa, A.; Marouf, A.; Kambouche, N.; Derdour, A. Phytochemical Composition, Antioxidant and Antimicrobial Activities of Ammodaucus leucotrichus Fruit from Algerian Sahara. Orient. J. Chem. 2018, 34, 519–525. [Google Scholar] [CrossRef]
- Chaubey, M.K. Insecticidal Activity of Trachyspermum ammi (Umbelliferae), Anethum graveolens (Umbelliferae) and Nigella Sativa (Ranunculaceae) Essential Oils against Stored-Product Beetle Tribolium castaneum Herbst (Coleoptera: Tenebrionidae). Afr. J. Agric. Res. 2007, 2, 596–600. [Google Scholar] [CrossRef]
- Kostić, I.; Lazarević, J.; Šešlija Jovanović, D.; Kostić, M.; Marković, T.; Milanović, S. Potential of Essential Oils from Anise, Dill and Fennel Seeds for the Gypsy Moth Control. Plants 2021, 10, 2194. [Google Scholar] [CrossRef]
- Evergetis, E.; Michaelakis, A.; Haroutounian, S.A. Essential Oils of Umbelliferae (Apiaceae) Family Taxa as Emerging Potent Agents for Mosquito Control. In Integrated Pest Management and Pest Control—Current and Future Tactics; Soloneski, S., Ed.; InTech: London, UK, 2012; pp. 613–638. ISBN 978-953-51-0050-8. [Google Scholar]
- Karakas, M. Use of Aromatic Plant Extracts as Bio-Insecticides for the Control of Stored-Product Insect, Sitophilus granarius. Int. J. Entomol. Res. 2017, 2, 27–29. [Google Scholar]
- Din, Z.U.; Shad, A.A.; Bakht, J.; Ullah, I.; Jan, S. In Vitro Antimicrobial, Antioxidant Activity and Phytochemical Screening of Apium graveolens. Pak. J. Pharm. Sci. 2015, 28, 1699–1704. [Google Scholar]
- Sousa, R.M.O.F.; Rosa, J.S.; Oliveira, L.; Cunha, A.; Fernandes-Ferreira, M. Activities of Apiaceae Essential Oils against Armyworm, Pseudaletia unipuncta (Lepidoptera: Noctuidae). J. Agric. Food Chem. 2013, 61, 7661–7672. [Google Scholar] [CrossRef]
- Ebadollahi, A.; Mahboubi, M. Insecticidal Activity of the Essential Oil Isolated from Azilia Eryngioides (PAU) Hedge et Lamond against Two Beetle Pests. Chil. J. Agric. Res. 2011, 71, 406–411. [Google Scholar] [CrossRef][Green Version]
- Lima, B.; Sanchez, M.; Agüero, M.B.; Tapia, A.; Palermo, J.A.; Feresin, G.E. Antibacterial Activity of Extracts and Compounds Isolated from the Andean Medicinal Plant Azorella Cryptantha (Clos) Reiche, Apiaceae. Ind. Crops Prod. 2015, 64, 152–157. [Google Scholar] [CrossRef]
- Ben-Khalifa, N.E.; Chaieb, I.; Laarif, A.; Haouala, R. Insecticidal Activity of Six Apiaceae Essential Oils against Spodoptera littoralis Biosduval (Lepidoptera: Noctuidae). J. New Sci. 2018, 55, 3603–3609. [Google Scholar]
- Rajkumar, S.; Jebanesan, A. Larvicidal and Adult Emergence Inhibition Effect of Centella asiatica Brahmi (Umbelliferae) against Mosquito Culex quinquefasciatus Say (Diptera: Culicidae). Afr. J. Biomed. Res. 2005, 8, 31–33. [Google Scholar] [CrossRef]
- Idris, F.N.; Nadzir, M.M. Antimicrobial Activity of Centella asiatica on Aspergillus Niger and Bacillus Subtilis. Chem. Eng. Trans. 2017, 56, 1381–1386. [Google Scholar] [CrossRef]
- Shafaghat, A. Antibacterial Activity and Composition of Essential Oils from Flower, Leaf and Stem of Chaerophyllum Macropodum Boiss. from Iran. Nat. Prod. Commun. 2009, 4, 861–864. [Google Scholar] [CrossRef] [PubMed]
- Matasyoh, J.C.; Maiyo, Z.C.; Ngure, R.M.; Chepkorir, R. Chemical Composition and Antimicrobial Activity of the Essential Oil of Coriandrum sativum. Food Chem. 2009, 113, 526–529. [Google Scholar] [CrossRef]
- Pereira, C.G.; Moraes, C.B.; Franco, C.H.; Feltrin, C.; Grougnet, R.; Barbosa, E.G.; Panciera, M.; Correia, C.R.D.; Rodrigues, M.J.; Custódio, L. In Vitro Anti-Trypanosoma Cruzi Activity of Halophytes from Southern Portugal Reloaded: A Special Focus on Sea Fennel (Crithmum maritimum L.). Plants 2021, 10, 2235. [Google Scholar] [CrossRef]
- Chaubey, M.K. Evaluation of Insecticidal Properties of Cuminum cyminum and Piper Nigrum Essential Oils against Sitophilus zeamais. J. Entomol. 2017, 14, 148–154. [Google Scholar] [CrossRef]
- Hrudová, E.; Kocourková, B.; Zelená, V. Insecticidal Effect of Carrot (Daucus Carota) and Lovage (Levisticum Officinale) (Apiaceae) Extracts against Tribolium confusum Jacquelin Du Duval, 1868 (Coleoptera, Tenebrionidae). Acta Univ. Agric. Silvic. Mendel. Brun. 2014, 54, 165–168. [Google Scholar] [CrossRef]
- Mouloud, G.; Rabah, B.; Khellaf, R. Antimicrobial and Antioxidant Activity of Methanol Extract of Echinophora spinosa L. from Jijel, Algeria. Alger. J. Biosci. 2020, 1, 24–29. [Google Scholar] [CrossRef]
- Pavela, R.; Maggi, F.; Cianfaglione, K.; Canale, A.; Benelli, G. Promising Insecticidal Efficacy of the Essential Oils from the Halophyte Echinophora spinosa (Apiaceae) Growing in Corsica Island, France. Environ. Sci. Pollut. Res. 2020, 27, 14454–14464. [Google Scholar] [CrossRef]
- Lingaraju, D.P.; Sudarshana, M.S.; Mahendra, C.; Rao, K.P. Phytochemical Screening and Antimicrobial Activity of Leaf Extracts of Eryngium foetidum L. (Apiaceae). Indo Am. J. Pharm. Res. 2016, 6, 4339–4344. [Google Scholar]
- Sumitha, K.V.; Prajitha, V.; Sandhya, V.N.; Anjana, S.; Thoppil, J.E. Potential Larvicidal Principles in Eryngium foetidum L. (Apiaceae), An Omnipresent Weed, Effective Against Aedes Albopictus Skuse. J. Essent. Oil-Bear. Plants 2014, 17, 1279–1286. [Google Scholar] [CrossRef]
- Zengin, G.; Mahomoodally, M.F.; Paksoy, M.Y.; Picot-Allain, C.; Glamocilja, J.; Sokovic, M.; Diuzheva, A.; Jekő, J.; Cziáky, Z.; Rodrigues, M.J.; et al. Phytochemical Characterization and Bioactivities of Five Apiaceae Species: Natural Sources for Novel Ingredients. Ind. Crops Prod. 2019, 135, 107–121. [Google Scholar] [CrossRef]
- Kahraman, C.; Topcu, G.; Bedir, E.; Tatli, I.I.; Ekizoglu, M.; Akdemir, Z.S. Phytochemical Screening and Evaluation of the Antimicrobial and Antioxidant Activities of Ferula Caspica M. Bieb. Extracts. Saudi Pharm. J. 2019, 27, 525–531. [Google Scholar] [CrossRef]
- Ramezanipour, O.; Yakhcha, M. Study on the Effect of Ferula Pseudalliacea (Family: Apiacea) Extract on Varroa Destructor (Acari: Varroidae) Infestation in Honeybee (Hymenoptera: Apidae, Apis Melifera). Iran. Vet. J. 2020, 15, 85–92. [Google Scholar] [CrossRef]
- Zengin, G.; Sinan, K.I.; Ak, G.; Mahomoodally, M.F.; Paksoy, M.Y.; Picot-Allain, C.; Glamocilja, J.; Sokovic, M.; Jekő, J.; Cziáky, Z.; et al. Chemical Profile, Antioxidant, Antimicrobial, Enzyme Inhibitory, and Cytotoxicity of Seven Apiaceae Species from Turkey: A Comparative Study. Ind. Crops Prod. 2020, 153, 112572. [Google Scholar] [CrossRef]
- Goodarzi, S.; Tavakoli, S.; Abai, M.R.; Amini, Z.; Vatandoost, H.; Yassa, N.; Hadjiakhoondi, A.; Tofighi, Z. Strong Insecticidal Potential of Methanol Extract of Ferulago trifida Fruits against Anopheles Stephensi as Malaria Vector. Environ. Sci. Pollut. Res. 2019, 26, 7711–7717. [Google Scholar] [CrossRef]
- Ebadollahi, A. Susceptibility of Two Sitophilus Species (Coleoptera: Curculionidae) to Essential Oils from Foeniculum Vulgare and Satureja hortensis. Ecol. Balk. 2011, 3, 1–8. [Google Scholar]
- Modise, S.A.; Ashafa, A.O.T. Larvicidal, Pupicidal and Insecticidal Activities of Cosmos Bipinnatus, Foeniculum Vulgare and Tagetes Minuta against Culex quinquefasciatus Mosquitoes. Trop. J. Pharm. Res. 2016, 15, 965–972. [Google Scholar] [CrossRef]
- Al-Mekhlafi, F.A.; Abutaha, N.; Al-Doaiss, A.A.; Ahmed Al- Keridis, L.; Alsayadi, A.I.; Ali El Hadi Mohamed, R.; Wadaan, M.A.; Elfaki Ibrahim, K.; Al-Khalifa, M.S. Target and Non-Target Effects of Foeniculum Vulgare and Matricaria Chamomilla Combined Extract on Culex Pipiens Mosquitoes. Saudi J. Biol. Sci. 2021, 28, 5773–5780. [Google Scholar] [CrossRef]
- Torbati, M.; Nazemiyeh, H.; Lotfipour, F.; Nemati, M.; Asnaashari, S.; Fathiazad, F. Chemical Composition and in Vitro Antioxidant and Antibacterial Activity of Heracleum Transcaucasicum and Heracleum Anisactis Roots Essential Oil. BioImpacts 2014, 4, 69–74. [Google Scholar] [PubMed]
- Sedaghat, M.M.; Sanei Dehkordi, A.; Abai, M.R.; Khanavi, M.; Mohtarami, F.; Salim Abadi, Y.; Rafi, F.; Vatandoost, H. Larvicidal Activity of Essential Oils of Apiaceae Plants against Malaria Vector, Anopheles Stephensi. Iran. J. Arthropod-Borne Dis. 2011, 5, 51–59. [Google Scholar] [PubMed]
- Izakmehri, K.; Saber, M.; Mehrvar, A.; Hassanpouraghdam, M.B.; Vojoudi, S. Lethal and Sublethal Effects of Essential Oils from Eucalyptus Camaldulensis and Heracleum persicum against the Adults of Callosobruchus maculatus. J. Insect Sci. 2013, 13, 152. [Google Scholar] [CrossRef] [PubMed]
- Rančić, A.; Soković, M.; Vukojević, J.; Simić, A.; Marin, P.; Duletić-Laušević, S.; Djoković, D. Chemical Composition and Antimicrobial Activities of Essential Oils of Myrrhis odorata (L.) Scop, Hypericum perforatum L. and Helichrysum arenarium (L.) Moench. J. Essent. Oil Res. 2005, 17, 341–345. [Google Scholar] [CrossRef]
- Matejić, J.S.; Džamić, A.M.; Mihajilov-krstev, T.; Ranđelović, V.N. Antimicrobial Potential of Essential Oil from Pastinaca sativa L. Biol. Nyssana 2014, 5, 31–35. [Google Scholar]
- Vitalini, S.; Palmioli, A.; Orlando, F.; Scarì, G.; Airoldi, C.; De Noni, I.; Bocchi, S.; Iriti, M. Phytotoxicity, Nematicidal Activity and Chemical Constituents of Peucedanum ostruthium (L.) W.D.J.Koch (Apiaceae). Ind. Crops Prod. 2021, 166, 113499. [Google Scholar] [CrossRef]
- Mohamed, H.S.A.A.; Abdelgadir, W.S.; Almagboul, A.Z.I. In Vitro Antimicrobial Activity of Anise Seed (Pimpinella anisum L.). Int. J. Adv. Res. 2015, 3, 359–367. [Google Scholar]
- Soni, R.; Sharma, G.; Jasuja, N.D. Essential Oil Yield Pattern and Antibacterial and Insecticidal Activities of Trachyspermum ammi and Myristica Fragrans. Scientifica 2016, 2016, 1428194. [Google Scholar] [CrossRef]
- Chaubey, M.K. Study of Insecticidal Properties of Trachyspermum ammi and Mentha Arvensis Essential Oils against Sitophilus zeamais L. (Coleoptera: Curculionidae). Curr. Life Sci. 2017, 4, 10–17. [Google Scholar] [CrossRef]
- Bisrat, D.; Jung, C. Insecticidal Toxicities of Three Main Constituents Derived from Trachyspermum ammi (L.) Sprague Ex Turrill Fruits against the Small Hive Beetles, Aethina Tumida Murray. Molecules 2020, 25, 1100. [Google Scholar] [CrossRef]
- Chaudhari, A.K.; Singh, V.K.; Kedia, A.; Das, S.; Dubey, N.K. Essential Oils and Their Bioactive Compounds as Eco-Friendly Novel Green Pesticides for Management of Storage Insect Pests: Prospects and Retrospects. Environ. Sci. Pollut. Res. 2021, 28, 18918–18940. [Google Scholar] [CrossRef]
- Jankowska, M.; Rogalska, J.; Wyszkowska, J.; Stankiewicz, M. Molecular Targets for Components of Essential Oils in the Insect Nervous System—A Review. Molecules 2017, 23, 34. [Google Scholar] [CrossRef]
- Prakash, B.; Kumar, A.; Singh, P.P.; Das, S.; Dubey, N.K. Chapter 16—Prospects of Plant Products in the Management of Insect Pests of Food Grains: Current Status and Future Perspectives. In Natural Bioactive Compounds; Sinha, R.P., Häder, D.-P., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 317–335. ISBN 978-0-12-820655-3. [Google Scholar]
- Rattan, R.S. Mechanism of Action of Insecticidal Secondary Metabolites of Plant Origin. Crop Prot. 2010, 29, 913–920. [Google Scholar] [CrossRef]
- Ivănescu, B.; Burlec, A.F.; Crivoi, F.; Roșu, C.; Corciovă, A. Secondary Metabolites from Artemisia Genus as Biopesticides and Innovative Nano-Based Application Strategies. Molecules 2021, 26, 3061. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Alqarawi, A.A.; Abd_Allah, E.F. Bioherbicides: Current Knowledge on Weed Control Mechanism. Ecotoxicol. Environ. Saf. 2018, 158, 131–138. [Google Scholar] [CrossRef]
- Verdeguer, M.; Sánchez-Moreiras, A.M.; Araniti, F. Phytotoxic Effects and Mechanism of Action of Essential Oils and Terpenoids. Plants 2020, 9, 1571. [Google Scholar] [CrossRef]
- Raveau, R.; Fontaine, J.; Lounès-Hadj Sahraoui, A. Essential Oils as Potential Alternative Biocontrol Products against Plant Pathogens and Weeds: A Review. Foods 2020, 9, 365. [Google Scholar] [CrossRef]
- Ebadollahi, A. Plant Essential Oils from Apiaceae Family as Alternatives to Conventional Insecticides. Ecol. Balk. 2013, 5, 149–172. [Google Scholar]
- Pavela, R. Acaricidal Properties of Extracts and Major Furanochromenes from the Seeds of Ammi visnaga Linn. against Tetranychus urticae Koch. Ind. Crops Prod. 2015, 67, 108–113. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Ricciutelli, M.; Lupidi, G.; Maggi, F. Efficacy of the Volatile Oil from Water Celery (Helosciadium nodiflorum, Apiaceae) against the Filariasis Vector Culex quinquefasciatus, the Housefly Musca Domestica, and the African Cotton Leafworm Spodoptera littoralis. Chem. Biodivers. 2017, 14, e1700376. [Google Scholar] [CrossRef]
- Bell, C.H. 15—Pest Control of Stored Food Products: Insects and Mites. In Hygiene in Food Processing, 2nd ed.; Lelieveld, H.L.M., Holah, J.T., Napper, D., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2014; pp. 494–538. ISBN 978-0-85709-429-2. [Google Scholar]
- Fouad, H.A.; de Souza Tavares, W.; Zanuncio, J.C. Toxicity and Repellent Activity of Monoterpene Enantiomers to Rice Weevils (Sitophilus oryzae). Pest Manag. Sci. 2021, 77, 3500–3507. [Google Scholar] [CrossRef] [PubMed]
- Rosa, J.S.; Oliveira, L.; Sousa, R.M.O.F.; Escobar, C.B.; Fernandes-Ferreira, M. Bioactivity of Some Apiaceae Essential Oils and Their Constituents against Sitophilus zeamais (Coleoptera: Curculionidae). Bull. Entomol. Res. 2020, 110, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Kaur, V.; Kaur, R.; Bhardwaj, U. A Review on Dill Essential Oil and Its Chief Compounds as Natural Biocide. Flavour Fragr. J. 2021, 36, 412–431. [Google Scholar] [CrossRef]
- Azirak, S.; Karaman, S. Allelopathic Effect of Some Essential Oils and Components on Germination of Weed Species. Acta Agric. Scand. Sect. B Soil Plant Sci. 2008, 58, 88–92. [Google Scholar] [CrossRef]
- Sabzi Nojadeh, M.; Pouresmaeil, M.; Younessi-Hamzekhanlu, M.; Venditti, A. Phytochemical Profile of Fennel Essential Oils and Possible Applications for Natural Antioxidant and Controlling Convolvulus arvensis L. Nat. Prod. Res. 2021, 35, 4164–4168. [Google Scholar] [CrossRef] [PubMed]
- Pujari, J.D.; Yakkundimath, R.; Byadgi, A.S. Image Processing Based Detection of Fungal Diseases in Plants. Procedia Comput. Sci. 2015, 46, 1802–1808. [Google Scholar] [CrossRef]
- Silva, R.N.; Monteiro, V.N.; Steindorff, A.S.; Gomes, E.V.; Noronha, E.F.; Ulhoa, C.J. Trichoderma/Pathogen/Plant Interaction in Pre-Harvest Food Security. Fungal Biol. 2019, 123, 565–583. [Google Scholar] [CrossRef]
- Carmona-Hernandez, S.; Reyes-Pérez, J.J.; Chiquito-Contreras, R.G.; Rincon-Enriquez, G.; Cerdan-Cabrera, C.R.; Hernandez-Montiel, L.G. Biocontrol of Postharvest Fruit Fungal Diseases by Bacterial Antagonists: A Review. Agronomy 2019, 9, 121. [Google Scholar] [CrossRef]
- Di Napoli, M.; Varcamonti, M.; Basile, A.; Bruno, M.; Maggi, F.; Zanfardino, A. Anti-Pseudomonas Aeruginosa Activity of Hemlock (Conium maculatum, Apiaceae) Essential Oil. Nat. Prod. Res. 2019, 33, 3436–3440. [Google Scholar] [CrossRef]
- Mith, H.; Duré, R.; Delcenserie, V.; Zhiri, A.; Daube, G.; Clinquart, A. Antimicrobial Activities of Commercial Essential Oils and Their Components against Food-Borne Pathogens and Food Spoilage Bacteria. Food Sci. Nutr. 2014, 2, 403–416. [Google Scholar] [CrossRef]
- Mostafa, A.A.; Al-Askar, A.A.; Almaary, K.S.; Dawoud, T.M.; Sholkamy, E.N.; Bakri, M.M. Antimicrobial Activity of Some Plant Extracts against Bacterial Strains Causing Food Poisoning Diseases. Saudi J. Biol. Sci. 2018, 25, 361–366. [Google Scholar] [CrossRef]
- Duarte, A.; Luís, Â.; Oleastro, M.; Domingues, F.C. Antioxidant Properties of Coriander Essential Oil and Linalool and Their Potential to Control Campylobacter Spp. Food Control 2016, 61, 115–122. [Google Scholar] [CrossRef]
- Kačániová, M.; Galovičová, L.; Ivanišová, E.; Vukovic, N.L.; Štefániková, J.; Valková, V.; Borotová, P.; Žiarovská, J.; Terentjeva, M.; Felšöciová, S.; et al. Antioxidant, Antimicrobial and Antibiofilm Activity of Coriander (Coriandrum sativum L.) Essential Oil for Its Application in Foods. Foods 2020, 9, 282. [Google Scholar] [CrossRef]
- Das, S.; Kumar Singh, V.; Kumar Dwivedy, A.; Kumar Chaudhari, A.; Deepika; Kishore Dubey, N. Nanostructured Pimpinella anisum Essential Oil as Novel Green Food Preservative against Fungal Infestation, Aflatoxin B1 Contamination and Deterioration of Nutritional Qualities. Food Chem. 2021, 344, 128574. [Google Scholar] [CrossRef]
- El-Soud, N.H.A.; Deabes, M.; El-Kassem, L.A.; Khalil, M. Chemical Composition and Antifungal Activity of Ocimum basilicum L. Essential Oil. Open Access Maced. J. Med. Sci. 2015, 3, 374. [Google Scholar] [CrossRef]
- Lasram, S.; Zemni, H.; Hamdi, Z.; Chenenaoui, S.; Houissa, H.; Saidani Tounsi, M.; Ghorbel, A. Antifungal and Antiaflatoxinogenic Activities of Carum carvi L., Coriandrum sativum L. Seed Essential Oils and Their Major Terpene Component against Aspergillus Flavus. Ind. Crops Prod. 2019, 134, 11–18. [Google Scholar] [CrossRef]
- Alinezhad, S.; Kamalzadeh, A.; Shams-Ghahfarokhi, M.; Rezaee, M.-B.; Jaimand, K.; Kawachi, M.; Zamani, Z.; Tolouei, R.; Razzaghi-Abyaneh, M. Search for Novel Antifungals from 49 Indigenous Medicinal Plants: Foeniculum Vulgare and Platycladus Orientalis as Strong Inhibitors of Aflatoxin Production by Aspergillus Parasiticus. Ann. Microbiol. 2011, 61, 673–681. [Google Scholar] [CrossRef]
- Kedia, A.; Prakash, B.; Mishra, P.K.; Dubey, N.K. Antifungal and Antiaflatoxigenic Properties of Cuminum cyminum (L.) Seed Essential Oil and Its Efficacy as a Preservative in Stored Commodities. Int. J. Food Microbiol. 2014, 168–169, 1–7. [Google Scholar] [CrossRef]
- Maurya, A.; Kumar, S.; Singh, B.K.; Chaudhari, A.K.; Dwivedy, A.K.; Prakash, B.; Dubey, N.K. Mechanistic Investigations on Antifungal and Antiaflatoxigenic Activities of Chemically Characterised Carum carvi L. Essential Oil against Fungal Infestation and Aflatoxin Contamination of Herbal Raw Materials. Nat. Prod. Res. 2021, 1–6. [Google Scholar] [CrossRef]
- Hazrati, H.; Saharkhiz, M.J.; Niakousari, M.; Moein, M. Natural Herbicide Activity of Satureja hortensis L. Essential Oil Nanoemulsion on the Seed Germination and Morphophysiological Features of Two Important Weed Species. Ecotoxicol. Environ. Saf. 2017, 142, 423–430. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils—Present Status and Future Perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Villalobos, M.J.; Cantó-Tejero, M.; Vallejo, R.; Guirao, P.; Rodríguez-Rojo, S.; Cocero, M.J. Use of Nanoemulsions of Plant Essential Oils as Aphid Repellents. Ind. Crops Prod. 2017, 110, 45–57. [Google Scholar] [CrossRef]
- Venkadesaperumal, G.; Rucha, S.; Sundar, K.; Shetty, P.H. Anti-Quorum Sensing Activity of Spice Oil Nanoemulsions against Food Borne Pathogens. LWT Food Sci. Technol. 2016, 66, 225–231. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).