Abstract
As it was previously reported, black spot development in the skin of Hass avocado has been related to a decreased antioxidant defense system. The aim of this study was to investigate the effect of different postharvest storage conditions on controlling black spot development targeting their effect on the antioxidant system (non-enzymatic and enzymatic) of the skin. Four postharvest treatments (T1: regular air storage (RA) at 5 °C for 40 d; T2: controlled atmosphere storage (CA) of 4 kPa O2 and 6 kPa CO2 at 5 °C for 40 d; T3: 10 d RA + 30 d CA and T4: 5 µM methyl jasmonate (MeJA) for 30 s + 10 RA + 30 d CA) were tested on controlling black spot incidence in fruit from six orchards from different agroclimatic zones and harvests. Then, on two selected orchards and harvests, the evolution of total phenolics (TPC), antioxidant capacity (AC) and antioxidant enzymes (catalase (CAT), polyphenol oxidase (PPO), superoxide dismutase (SOD), peroxidase (POD), phenylalanine ammonia lyase (PAL)) was monitored. Results revealed that incidence of black spot disorder was not associated to an agroclimatic zone and harvest stage. Immediate application of CA (T2) controlled black spot development during prolonged storage (40 d) and under these conditions TPC content remained higher compared to the other treatments. No clear role of CAT, PPO, SOD, POD and PAL on controlling black spot was observed. The results obtained are of value for the Hass avocado supply chain since a clear performance of CA was evidenced that will result in reduction of postharvest losses associated to this problem.
1. Introduction
The avocado mesocarp is recognized by its highly nutritional oil which is rich in monounsaturated fatty acids such as oleic acid. In addition, its composition includes vitamin E, proteins, bioactive compounds and 10% of unsaponifiable compounds such as sterols and volatile acids [1]. The main phenolic compounds found in the epicarp and seed are epicatechin derivatives and flavonoids such as quercetin derivatives [2].
Originally from Central America, the cultivation of avocado (Persea americana Mill) has spread to various regions of the world, mainly towards tropical, subtropical and Mediterranean climates. Mexico is the main producer of avocados in the world, contributing 34%, while Chile produces 2.5%. The predominant variety in Chile is Hass, which represents 90% of the national production concentrated between the Norte Chico and the Central Zone of the country [3,4].
In recent years, the exportation of avocados has been challenged because a defect has been found in the fruit epicarp known as a black spot, manifested as a dark circular spot on the skin during storage, and that mainly affects the perception of the quality of the fruit. This disorder is confused with anthracnose, which is a disease caused by a fungus that develops dark and irregular spots on the epicarp but even compromises the mesocarp of the fruit. Moreover, other disorders mimic black spot symptoms such as chilling injury during storage and lenticel breakdown. Recent research distinguishes black spot from anthracnose, indicating that the possible factors associated with the incidence of black spots on the epicarp of avocados are produced by cold storage for more than 10 days; in addition, to a drastic decrease in the enzymatic defense system of the epicarp [5]. Pre-harvest factors such as maximum temperatures and relative humidity during growth and development have also been reported to be negatively associated with black spot development [5]. Similarly, Lindh et al. [6] reported that lenticel damage does not result in black spots, and the harvest system did not correlate to the incidence of black spots. Controlled atmosphere (CA) has also been studied showing a significant reduction in black spot incidence, even though this disorder is orchard dependent [5,7].
In the need to meet fruit quality standards for exports, growers have taken measures to prevent anthracnose with the application of fungicides both at preharvest and postharvest stages. Some recent studies indicate that the application of methyl jasmonate (MeJA) reduces cold damage during avocado storage and prevents anthracnose by altering its fatty acid content and compounds related to stress responses [8,9,10]. However, no application of MeJA has been studied to control black spot. Based on the previous antecedents that indicate that black spot is correlated with a drastic decrease in the defense system of the epicarp during the first 10 days, substances of the GRAS (generally recognized as safe) type such as MeJA and others could help to control the appearance of this physiological disorder. Regarding this, the central objectives of this research were to evaluate the effect of different postharvest treatments on: (i) the black spot incidence in orchards of three agroclimatic zones and two harvests; and (ii) the enzymatic and non-enzymatic defense system of the epicarp and its evolution during storage.
2. Materials and Methods
2.1. Selection of Orchards and Plant Material
Five thousand avocado cv. Hass fruits were harvested in the 2019/2020 season from six orchards located in three agroclimatic zones of the Region of Valparaíso. Two maturity stages were considered: early harvest (23–27% dry matter) and middle harvest (>27–30% dry matter). The orchards from the coastal zone (orchards A and B) were located at a distance of less than 10 km from the sea and between 100–250 m above sea level. Those in the intermediate zone (orchards C and D) were located at a distance between 20 and 45 km from the sea and between 300–400 m above sea level, and those in the interior zone (orchards E and F) were located at a distance greater than 45 km from the sea and between 300–900 m above the sea level.
2.2. Postharvest Treatments
Four hundred fruits were selected from each orchard per harvest and were split into four treatments: (T1) 40 d of storage in regular air (21 kPa O2 and 0.04 kPa CO2) at 5 °C, (T2) 40 d of storage in a controlled atmosphere of 4 kPa O2 and 6 kPa CO2 at 5 °C, (T3) 10 d of storage in regular air at 5 °C plus 30 d in a controlled atmosphere at 5 °C and 4 kPa O2 and 6 kPa CO2, (T4) application of 5 µM MeJA for 30 s, fruit drying at room temperature and application of 10 d of storage in regular air at 5 °C followed by 30 d in a controlled atmosphere at 5 °C and 4 kPa O2 and 6 kPa CO2.
In addition, five fruits were sampled per treatment from the orchards that presented the highest incidence of black spot (B and E), at times 0, 3, 6, 10 and 40 d, respectively. The epicarp or skin of each fruit was removed and immediately frozen and stored at −80 °C for further analyses. The remaining fruits were subjected to a shelf-life period (20 °C and 65–60% RH) until the fruit reached the ready-to-eat stage (RTE, 4–14 N firmness).
2.3. Quality Parameters
The incidence of black spot was determined by visual inspection of the epicarp after prolonged postharvest storage under the different treatments and furthermore at the RTE stage, where 0 = without incidence and 1 = with incidence. The results were expressed as a percentage of the fruits per treatment [5].
Fruit weight was measured individually at harvest, after postharvest treatments, and at the RTE stage. The values were then used to calculate the percentage of weight loss. Dry matter was determined in the epicarp and mesocarp at harvest using 20 fruits per orchard. The epicarp was separated from the mesocarp and the seed was discarded, they were chopped and placed separately in an oven at 100 °C for 24 h. The dry matter content was expressed as a percentage (g of dry matter per 100 g of epicarp or mesocarp).
Non-destructive firmness was evaluated at harvest and after postharvest treatments in five fruits for each treatment as described by Ochoa-Ascencio et al. [11] with minor modifications. A texture analyzer (Model TA.XT plusC, Stable Micro Systems Ltd., Surrey, United Kingdom equipped with a 10 mm Ø cylindrical probe, with an initial force of 0.5 N and a speed of 8 mm s−1 was used. The compressive force was recorded in Newton (N) at 2 mm deformation.
2.4. Analysis of Epicarp Non-Enzymatic Defense System
Total Phenolic Compounds and Antioxidant Capacity
For the extraction of total phenolic compounds and antioxidant capacity, the method described by Saavedra et al. [12] was used. Briefly, 50 mg of lyophilized epicarp were homogenized with 2 mL of acetone: water (70:30 v/v) and mixed in a shaker (VorTemp 56, Labnet International, Inc., New York, NY, USA) at 1300 rpm and 3 mm shaker orbit for 1 h. Then, it was centrifuged at 10,000× g and 4 °C for 20 min (Labnet International, Inc., New York, NY, USA). Two hundred µL of the supernatant was evaporated with nitrogen gas and the remaining pellet was resuspended with 400 µL of methanol and stored (−20 °C) for subsequent analysis of total phenolics and antioxidant capacity.
Total phenolic compounds were quantified by mixing 240 μL of water (HPLC grade), 20 μL of 1 N Folin-Ciocalteu reagent, 20 μL of 5% (w/v) sodium carbonate, and 20 μL of the extract in microplates. Samples were incubated for 2 h in darkness at room temperature and the absorbance was measured at 765 nm in a UV-Vis microplate spectrophotometer (Multiscan GO type, Thermo Fisher Scientific, Vantaa, Findland). Total phenolic compounds were calculated using gallic acid as a standard curve (50–200 μg mL−1, y = 0.0031x − 0.012, r2 = 0.995) and the results were expressed as μg gallic acid equivalent (GAE) per mg of dry weight (DW) sample.
The antioxidant capacity was obtained by mixing 20 µL of extract and 125 µL of 60 µM of 2,2-diphenyl-1-picrylhydrazyl (DPPH). The mixture was incubated for 30 min in darkness and the absorbance was measured at 517 nm. The antioxidant capacity was calculated using a trolox as a standard curve (20–140 μM mL−1, y = 0.41x − 1.107, r2 = 0.998) and expressed as μM of trolox equivalent (TE) per mg of sample (DW).
2.5. Analysis of Epicarp Enzymatic Defense System
2.5.1. Enzyme Extraction
The extraction and enzymatic activity methods were performed as described by Uarrota et al. [5] with minor modifications. One hundred mg powdered samples were mixed with 500 µL of extraction buffer pH 7.0 (potassium phosphate buffer (50 mM, pH 7.0, with 25 µM ethylenediaminetetraacetic acid (EDTA) and 0.025% Triton X-100) and 20 µL of 125 mM of phenylmethylsulfonyl fluoride (PMSF). The sample was vortexed and centrifuged at 10,000× g for 10 min at 4 °C (Z216 MK refrigerated microcentrifuge, Ø = 11 mm). The supernatant was kept at −20 °C.
The content of total soluble proteins was determined by the Bradford method using bovine serum albumin (BSA) as the standard curve [13]. Total soluble protein was expressed μg g−1 of fresh avocado epicarp.
2.5.2. Catalase (CAT) Activity
The protein extract (2.5 µL) was mixed with 250 µL of potassium phosphate buffer (50 mM, pH 7.0) and 22.5 µL of 50 mM hydrogen peroxide. The absorbance was measured at 240 nm every 10 s for 5 min. The catalase activity was calculated using Equation (1). Where, “k” is the decay constant calculated as the natural logarithm (ln) of the ratio of the absorbance measured at each moment and the absorbance at the starting point of the measurement, “df” is the dilution factor, t is the reaction time, “ε” the molar extinction coefficient and, “c” the protein concentration in the samples. The CAT activity was expressed as mmol of hydrogen peroxide min−1 g−1 of protein.
2.5.3. Polyphenol Oxidase (PPO) Activity
To 100 mg of pulverized sample, 500 μL of extraction buffer (0.1 M sodium phosphate buffer, pH 6.5, and 2% polyvinylpyrrolidone (PVP)) was added. The mixture was vortexed and then centrifuged (10,000× g at 4 °C per 10 min). The enzymatic assay was performed by adding to a microplate 240 μL of buffer, 20 μL of the extract, and 40 μL of 0.1 M catechol. The formation of oxidized catechol polymer was monitored for 30 min by measuring the change in absorbance at 410 nm every 2 min. The PPO activity was calculated based on the slope of the linear portion of the curve and it was expressed as mmol of catechol min−1 g−1 of protein.
2.5.4. Superoxide Dismutase (SOD) Activity
The extract was prepared with 100 mg of sample and 0.5 mL of 0.1 M potassium phosphate buffer (pH 7.8 and containing 0.1 mM EDTA), then homogenized and centrifuged (13,000× g for 10 min at 0–5 °C). Fifty µL of the extract were mixed with 250 µL of a working solution prepared as follows: 194 mg of 13 mM methionine, 6 mg of 75 µM nitro blue tetrazolium (NBT), and 3.73 mL of 1.3 µM riboflavin. The samples were illuminated with a fluorescent lamp (Sylvania FC 12 T10 CW RS, JUC, Spain) for 15 min. Solvents without sample extract were used as control and non-illuminated solutions were used as blank. The absorbance was measured at 560 nm after illumination. One unit of SOD (U) was defined as the amount of enzyme necessary to inhibit 50% of NBT and it was calculated according to Equations (2) and (3).
2.5.5. Peroxidase (POD) Activity
The peroxidase activity was carried out according to Bi et al. [14] with modifications. To 100 mg of pulverized sample, 1.5 mL of extraction buffer (0.1 mM sodium phosphate buffer, pH 7.0 containing 0.1 mM EDTA and 0.1% Triton X-100), 0.5% (w/v) PVP and 15 µL of 0.1 M PMSF were added. The mixture was vortexed, sonicated at low temperature for 10 min and centrifuged (13,000× g at 4 °C per 20 min). The POD assay was performed by mixing 160 µL of 20 mM Guaiacol in microplates with 40 µL of extract followed by incubation for 5 min at 30 °C. Then, 80 µL of 50 mM hydrogen peroxide was added. The formation of the oxidized tetra guaiacol polymer was monitored by measuring the change in absorbance at 460 nm every 30 s for 5 min. POD activity was calculated based on the slope of the linear portion of the curve and it was expressed as mmol of oxidized tetraguaiacol min−1 g−1 of protein.
2.5.6. Phenylalanine Ammonia Lyase (PAL) Activity
The extract was prepared by homogenizing 100 mg of the powdered sample with 1 mL of 0.1 M sodium borate buffer, pH 8.8, containing 5 mM β-mercaptoethanol, 2 mM EDTA, and 2% (w/v) PVP in an ice bath. The mixture was centrifuged (10,000× g for 15 min at 4 °C) and the supernatant recovered. Ten µL of extract, 230 µL of sodium borate buffer pH 8.8, and 50 µL of the substrate (60 mM L-phenylalanine) were mixed and incubated at 37 °C for 30 min. The reaction was stopped by adding 10 µL of 1 M trichloroacetic acid (TCA) and the absorbance was measured at 290 nm. PAL activity was determined by cinnamate production and calculated as in Equation (4). The sample and control OD absorbances along with the dilution factor (df), reaction time (t), molar extinction coefficient (ε), and protein concentration (c) in the sample.
2.6. Statistical Analysis
Analysis of variance (ANOVA) followed by Tukey’s test was performed using Statgraphics Centurion XVI (StatPoint, Rockville, MD, USA) at 95% confidence.
3. Results and Discussion
3.1. Incidence of Black Spot
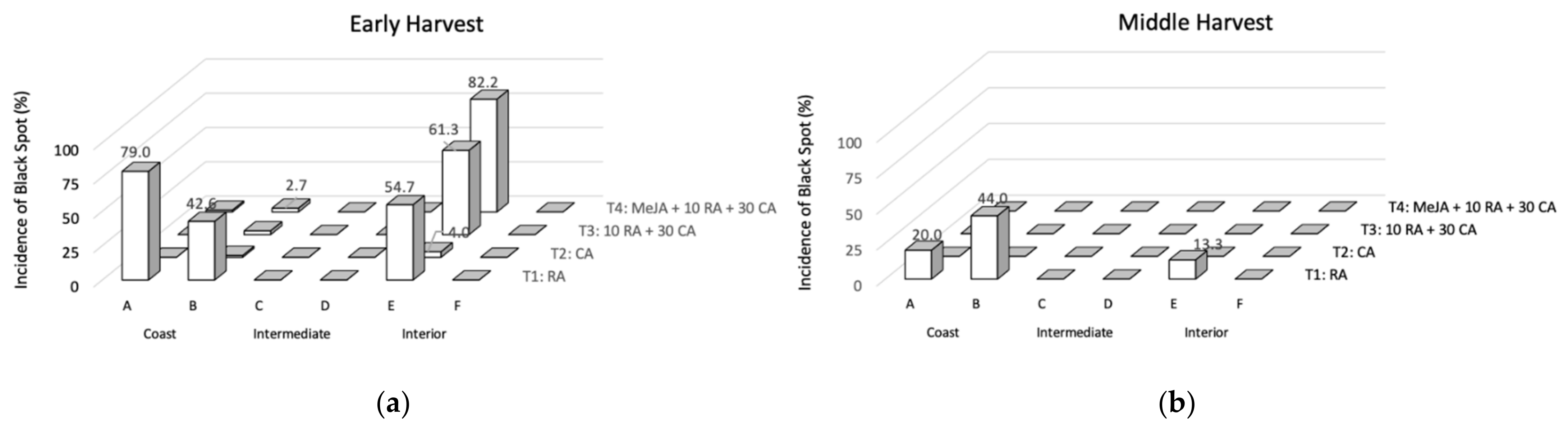
The incidence of the disorder on avocados from an early and middle harvest after 40 d of cold storage subjected to different treatments (T1-T4) is shown in Figure 1. Only three orchards presented fruit with black spots (A, B, and E) in both harvests. No fruit with the presence of anthracnose was found. Fruit stored in regular air (T1) showed a higher incidence of black spot and it was the only treatment affecting fruit from the middle harvest. On the other hand, fruit immediately stored under a controlled atmosphere after the harvest (T2) showed the lowest black spot incidence. These results are in concordance with the incidence observed on avocados from the 2017/2018 and 2018/2019 seasons, where the orchards A, B, and E showed higher black spot incidence [5,7]. For the early harvest fruit, all the treatments with CA (T2, T3, and T4) almost totally controlled the incidence of black spot when compared to T1, except for one of the orchards in the interior zone (orchard E). For orchards with a recurring history of black spot, the 10 d in regular air can be critical to promote the disorder development and time should be reduced to fully control the incidence of black spot as T2. Commercially, Chilean Hass avocados destined for export remain over 7 d in regular air before the containers are consolidated and the controlled atmosphere is applied. The effect of CA (reduced oxygen and increased carbon dioxide concentrations) beyond delaying ripening [15], avoids the oxidation process of the fruits preventing the appearance of black spot. In addition, reduced oxygen favors the maintenance of pigments and antioxidant compounds [16].
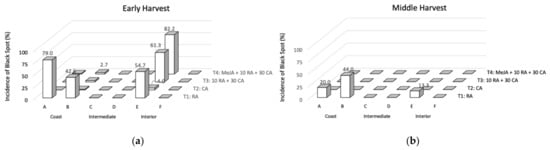
Figure 1.
Evolution of the incidence of black spots for the different postharvest treatments evaluated in early (a) and middle (b) harvests in different orchards after prolonged cold storage. T1: 40 d in regular air (RA) at 5 °C; T2: 40 d in a controlled atmosphere (CA) of 4 kPa O2 and 6 kPa CO2 at 5 °C; T3: 10 d in RA at 5 °C plus 30 d in CA 4 kPa O2 and 6 kPa CO2 at 5 °C; and T4: 5 µM MeJA plus 10 d in RA at 5 °C plus 30 d in CA 4 kPa O2 and 6 kPa CO2 at 5 °C.
3.2. Quality Parameters
Dry matter content of epicarp and mesocarp and the non-destructive firmness are shown in Table 1. The dry matter of the epicarp varied between 21% and 26%, the highest values were found in the intermediate zone (orchards C and D) and the lowest in the coastal zone (orchards A and B). Similar dry matter contents in mesocarp were found and ranged between 22% and 28% in both harvests. No statistical difference was observed in the dry matter between early and middle harvests for both tissues although the expected differences should be 3–4% between the harvest seasons. For orchards C and D, similar contents of dry matter were attributed to a close harvest time. In general, the orchards that presented black spot incidence after 40 d of cold storage in regular air, showed significantly lower epicarp dry matter contents (A, B, and E with ranges from 21.1% to 22.4%) than those that did not present the disorder (C, D, F with ranges from 23.4% to 26.1%). Besides showing lower dry matter, the epicarp was thinner and less rough (observed data; not shown) in addition to presenting a higher incidence of black spot. Therefore, probably a higher concentration of some compounds in avocado epicarp could contribute to the control of black spot development. A previous study on Hass avocado fruit produced in regions of Mexico with a semi-warm and temperate climate revealed higher production of lignin and total phenolics and a lower concentration of chlorophylls [17].

Table 1.
Quality parameters (dry matter and firmness) of avocado fruit at harvest (before application of postharvest treatments) in orchards from the coast (A and B), intermediate (C and D), and interior (E and F) zones.
Regarding mesocarp dry matter, no correlation was observed with the incidence of black spot. Similar results were reported by Uarrota et al. ([5] and unpublished data) who evaluated two seasons (2017/2018 and 2018/2019) and two harvests (early and middle).
The non-destructive firmness was evaluated at harvest and after application of evaluated postharvest treatments. No significant differences or trends were observed between harvest time and orchards. On the other hand, no significant changes were observed in firmness after cold storage for all treatments. However, a desirable decrease in firmness at 20 °C was observed, since the softening of the mesocarp is one of the main effects of the ripening process explained by modifications in the composition and structure of the fruit cell wall due to the enzymatic activity associated with the loss of turgor [18,19,20].
Regarding weight loss (Figure S1), fruit stored in regular air showed a greater loss for both harvests (5–7%), and slightly higher values were observed in orchards from early harvest. Similar results were reported by Escobar et al. [21], where weight loss ranged 5 to 6% under the same storage conditions. On the other hand, a lower weight loss was observed in all fruit stored in CA, which did not exceed 2.2%, mainly due to the higher water vapor pressure within CA containers. A significant increase in fruit weight loss occurred during ripening related to the increase in temperature which generate an increase in the transpiration rate of the fruit. Therefore, the CA treatment, in addition, presented favorable conditions for preventing fruit weight loss.
3.3. Total Phenolic Compounds (TPC) and Antioxidant Capacity (AC)
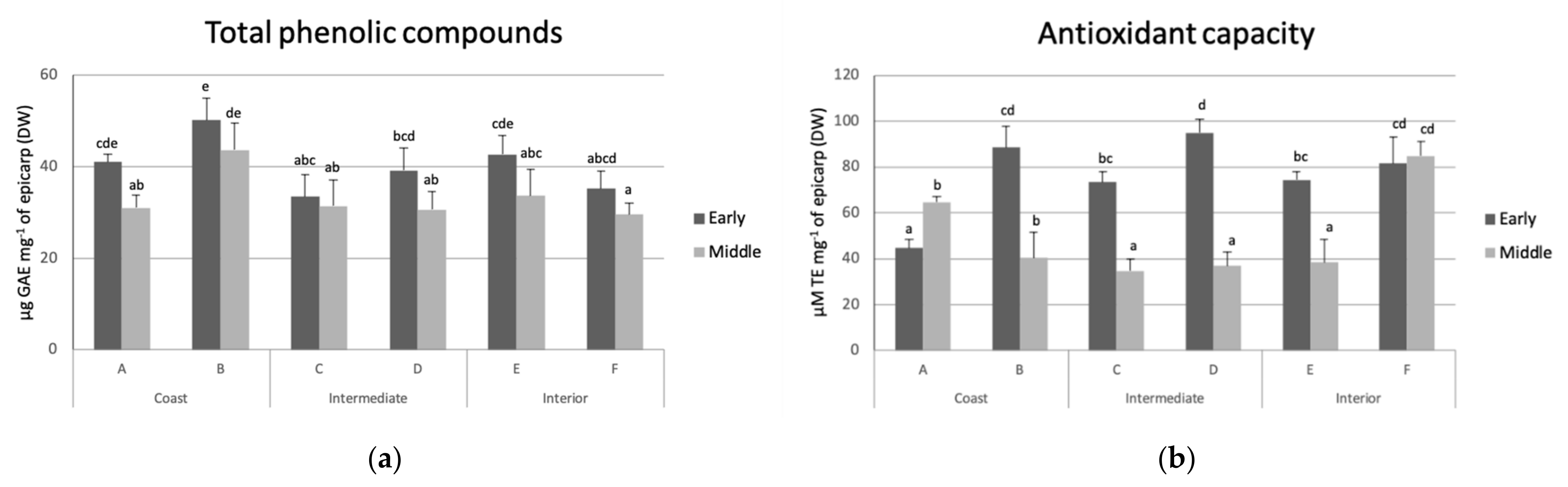
TPC and AC were evaluated at harvest (Figure 2). No association was found between TPC and AC with the incidence of black spot. However, fruit from early harvest showed higher mean contents of phenolics and AC than those of middle harvest.
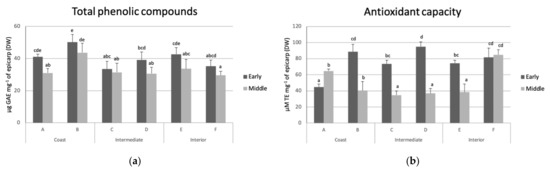
Figure 2.
(a) Total phenolic compounds (TPC) expressed as gallic acid equivalents (GAE) and (b) antioxidant capacity (CA) expressed as trolox equivalents (TE) in avocado fruit from orchards of coast (A and B), intermediate (C and D), and interior (E and F) zones of early and middle harvests. Different lower-case letters in bars show statistical differences among orchards and each harvest time (95% confidence).
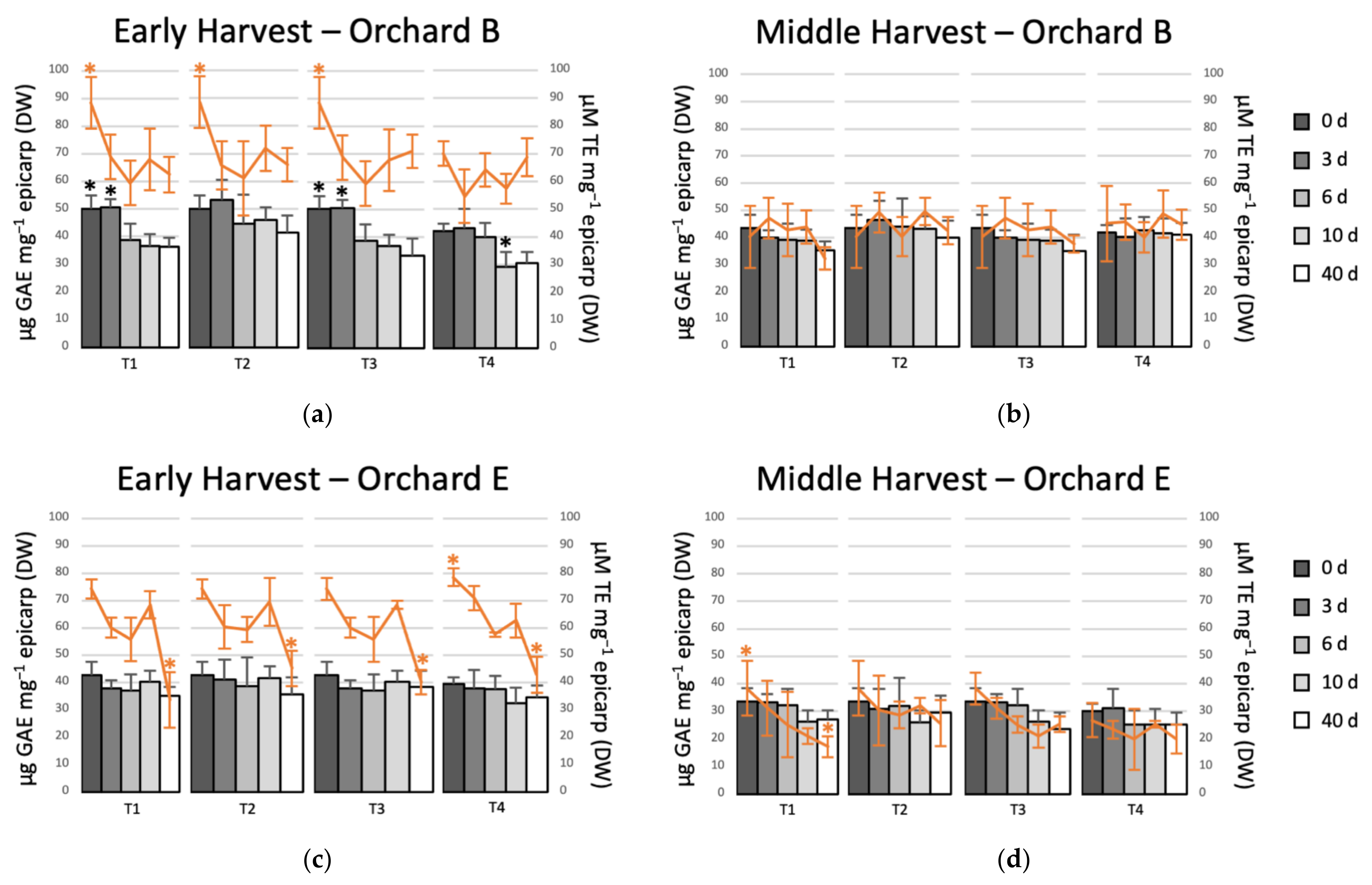
Based on our results and those reported by Uarrota et al. [5], the orchards with higher black spot incidence (B and E) were chosen to evaluate the effect of postharvest treatments on the total phenolic content and antioxidant capacity (Figure 3). At the beginning of cold storage, TPC was higher in avocado epicarp of early harvest, which ranged from 40 to 50 µg GAE mg−1 epicarp (DW), whereas for middle harvest TPC ranged from 30 to 43 µg GAE mg−1. Similar results were reported by Uarrota et al. [5]. The controlled atmosphere applied immediately after harvest (T2), besides fully controlling the incidence of black spot, maintained higher levels of TPC after 40 d of cold storage (Figure 3a,b). Similar behavior, but less marked, was observed for middle harvest, where the fruit from orchard B with T2 showed the highest levels of total phenolics, mainly in the first days of storage (Figure 3c). For the interior zone orchard (E) in early harvest, it was observed that the only treatment that totally controlled the black spot during prolonged storage was T2, i.e., the controlled atmosphere applied immediately after harvest. The treatments that included 10 d in regular air or MeJA plus 10 d in regular air (T3 and T4) showed a higher incidence of black spot at 40 d. The control of black spot by T2 was related to a higher content of phenolic compounds in this treatment compared to the other treatments. A similar trend was observed for the middle harvest, the incidence of black spot was much lower, but only T2 fully controlled the incidence of black spot and showed higher content of total phenolics than the other treatments after 40 d of storage (Figure 3c,d). Saxena et al. [22] evaluated the effect of a controlled atmosphere at different concentrations of O2 and CO2 on the content of total phenolics in jackfruit. They reported that at high CO2 and low O2 concentrations the phenolic compounds were maintained to a greater extent than under other conditions.
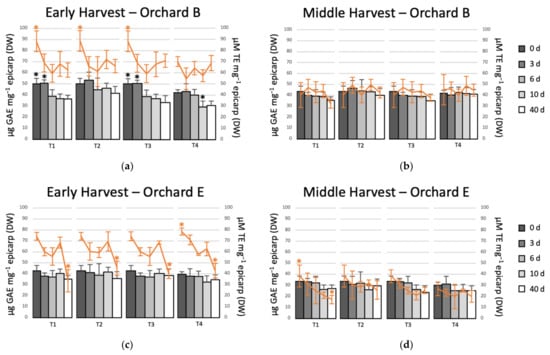
Figure 3.
Evolution of the total phenolic content (TPC) expressed as gallic acid equivalents (GAE) and the antioxidant capacity (AC) expressed as trolox equivalents (TE) of avocado fruit epicarp during different postharvest treatments. (a) Early harvest orchard B; (b) middle harvest orchard B; (c) early harvest orchard E; and, (d) middle harvest orchard E. T1: 40 d in regular air (RA) at 5 °C; T2: 40 d in controlled atmosphere (CA) 4 kPa O2 and 6 kPa CO2 at 5 °C; T3: 10 d in RA at 5 °C plus 30 d in CA 4 kPa O2 and 6 kPa CO2 at 5 °C; and, T4: 5 µM MeJA plus 10 d in RA at 5 °C plus 30 d in CA 4 kPa O2 and 6 kPa CO2 at 5 °C. TPC is represented by bars and AC by lines. The asterisk showed statistical differences in each treatment (95% confidence).
In the case of the evolution of the antioxidant activity (line graphs in Figure 3), the results were not as clear as for the phenolic compounds. For orchard B, the AC remained slightly higher for T2 than the other treatments for both harvests. However, the differences were not significant (Figure 3a). For orchard E, no differences were observed between treatments (Figure 3b). The AC decreased in middle harvest when compared to early, which ranged between 70–90 and 26–45 µM TE mg−1 epicarp (DW) in early and middle harvests, respectively. Similar results were reported by Uarrota et al. [5]. The AC of avocados of orchards of middle harvest was relatively stable during cold storage. However, the fruit of early harvest showed a greater decrease in AC after 40 d. Although phenolic compounds are substances that are involved in the stability of AC, there are other compounds such as flavonoids, amino acids, tocopherols, and pigments that can be associated with AC [23]. On the other hand, López et al. [16] reported that the effect of controlled atmospheres (4 kPa O2 + 96 kPa N2) in ripe Imperial tomato was more effective than the storage at low temperature. The main antioxidant compound in tomatoes is ascorbic acid, which is higher when stored at CA rather than RA. The ascorbic acid can be degraded by the enzyme ascorbic acid oxidase, which acts in the presence of oxygen. The higher levels of ascorbic acid in tomatoes stored in controlled atmospheres are due to the fact that they degraded to a lesser extent due to the absence of oxygen.
3.4. Analysis of Epicarp Enzymatic Defense System
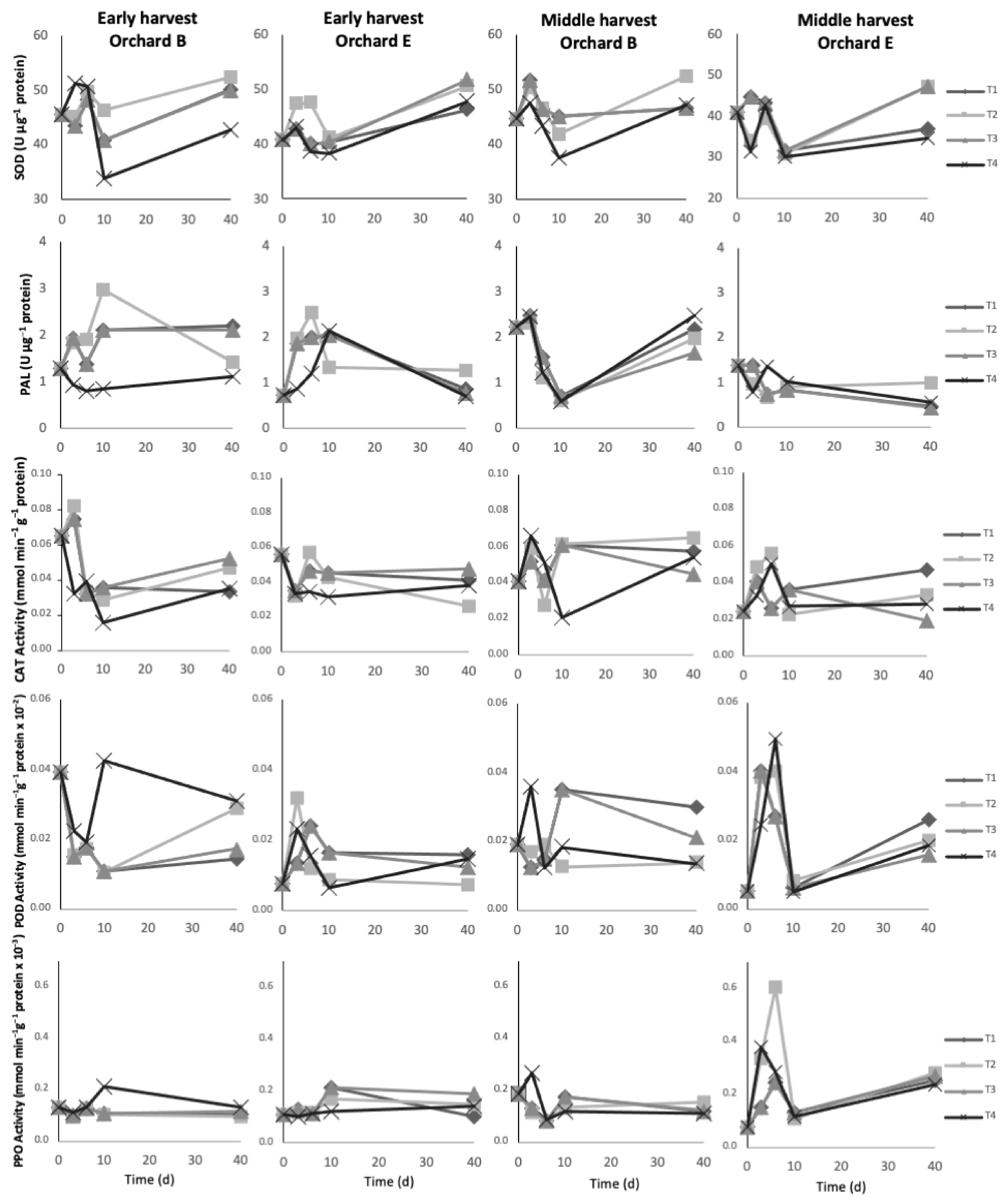
The evolution of enzymatic activities of SOD, PAL, CAT, POD, and PPO in the epicarp of fruit from early and middle harvests during different postharvest treatments is shown in Figure 4.
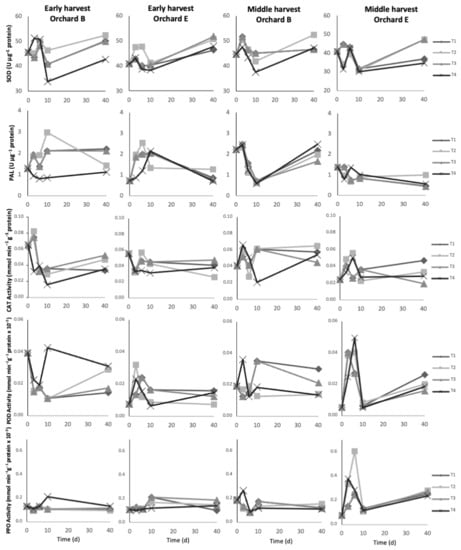
Figure 4.
Evolution of enzymatic activity of superoxide dismutase (SOD), phenylalanine ammonia-lyase (PAL), catalase (CAT), peroxidase (POD) and polyphenol oxidase (PPO) of avocado fruit epicarp during different postharvest treatments. The (first) column shows fruit from the early harvest of orchards B; the (second), from the early harvest of orchards E; the (third), from the middle harvest of orchard B; and, the (last) column from the middle harvest of orchard E. T1: 40 d in regular air (RA) at 5 °C; T2: 40 d in controlled atmosphere (CA) 4 kPa O2 and 6 kPa CO2 at 5 °C; T3: 10 d in RA at 5 °C plus 30 d in CA 4 kPa O2 and 6 kPa CO2 at 5 °C; and, T4: 5 µM MeJA plus 10 d in RA at 5 °C plus 30 d in CA 4 kPa O2 and 6 kPa CO2 at 5 °C.
Regarding early harvest, only SOD showed differences between orchards, where B showed higher activity than E. This difference was greater in the first 6 d of storage and it is in agreement with those observed for TPC and AC. Superoxide dismutase is efficient at scavenging reactive oxygen species (ROS), which may contribute to the antioxidant defense system [5,24]. PAL is an entry-point enzyme in the phenylpropanoid pathway, which is one of the most important pathways for the synthesis of phenolics and flavonoids [25]. Cold storage stimulates the activity of PAL in fruits such as cucumber, grapes, and walnuts [25,26,27]. In our study, increasing activity of PAL was observed in the first days of cold storage (T1, T2, and T3). However, PAL was significantly lower with the application of 5 µM MeJA (T4). Glowacz et al. [8] reported that PAL activity increased in avocados treated with 100 µM MeJA, but no changes were observed at 10 µM. The low activity of PAL with the application of MeJA is consistent with the low contents of phenolics found in avocados with the same treatment. On the other hand, all four postharvest treatments showed non-significant differences in the activities of SOD, CAT, POD, and PPO. Nevertheless, enzymes such as SOD and PPO showed slightly increasing activity during storage, whereas PAL and CAT decreased.
The avocado fruit from the middle harvest showed more marked differences in enzymatic activity between orchards. Orchard B showed higher enzymatic activity than E, except for POD. POD enzymes are important ROS scavengers [25]. Most enzymes showed increasing activities during storage, except for CAT which was not significant. CAT, as well as SOD, are sensitive to oxidation, thus, antioxidant molecules (i.e., phenolics) are important to counteract the effect of ROS [28]. All treatments applied did not show differences among the enzyme’s activities studied. The previous study by Uarrota et al. [5] showed higher black spot incidence in the orchards presenting decreases in SOD, CAT, POD, PAL activities, and phenolics. As air conditions (regular air storage) favor oxidation of certain antioxidant enzymes, thus, the non-enzymatic defense system (i.e., phenolics) becomes crucial against black spot development.
4. Conclusions
Results of this study revealed that regular air storage cold conditions favor black spot development in fruit from orchards displaying a history of this disorder. The disorder could not be associated with a specific agroclimatic zone or harvest. Immediate application of controlled atmosphere conditions of 4 kPa O2 and 6 kPa CO2 at 5 °C controlled black spot disorder up to the evaluated 40 d. These conditions resulted in fruit skin with a higher content of total phenolics compared to the other evaluated treatments during the prolonged storage period. The activity of antioxidant enzymes (CAT, PPO, SOD, POD, PAL) did not show a clear trend in relation to controlling black spot disorder in the skin of Hass avocados.
The results obtained are of practical application to the Hass avocado supply chain and contribute to decreasing avocado food losses. Further studies will focus on deeply understanding how CA controls black spot development at different omics levels.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/horticulturae8050369/s1, Figure S1: Weight loss (%) of avocado fruit during different postharvest treatments at the end of cold storage (a,b) and at ready-to-eat (RTE) (c,d) from early and middle harvest.
Author Contributions
Conceptualization, R.P.; methodology, J.V., V.U., E.P. and C.F.; software, C.F.; formal analysis, J.V., C.F., B.G.D. and R.P.; investigation, J.V., C.Z. and E.P.; resources, R.P. and B.G.D.; writing—original draft preparation, C.F. and J.V.; writing—review and editing, R.P., V.U. and B.G.D.; visualization, C.F.; supervision, R.P.; project administration, R.P.; funding acquisition, R.P. and B.G.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Comité de Paltas Chile and associated producers and exporters (Santa Cruz, El Parque, Jorge Schmidt, Baika, Subsole).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All relevant data is shown in Figures.
Acknowledgments
We would like to thank Vicente Lindh for his technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chil, I.; Molina, S.; Ortiz, L.; Dutok, C.; Souto, R. Estado del Arte de la especie Persea americana Mill (aguacate). Rev. Amaz. Investig. 2019, 8, 73–86. [Google Scholar]
- Melgar, B.; Dias, M.J.; Ciric, A.; Sokovic, M.; Garcia-Castello, E.; Rodriguez-Lopez, A.; Barros, L.; Ferreira, I. Bioactive characterization of Persea americana Mill. by-products: A rich source of inherent antioxidants. Ind. Crops Prod. 2018, 111, 212–218. [Google Scholar] [CrossRef] [Green Version]
- FAOSTAT. Food and Agriculture Statistical Division. Crops and Livestock Products. 2020. Available online: https://www.fao.org/faostat/en/#data/QCL/visualize (accessed on 5 January 2022).
- ODEPA. Oficina de Estudios y Políticas Agrarias. Ministerio de Agricultura. La palta Chilena en los Mercados Internacionales. 2018. Available online: https://www.odepa.gob.cl/wp-content/uploads/2018/12/palta2018rev1.pdf (accessed on 5 January 2022).
- Uarrota, V.; Hernandez, I.; Ponce, E.; Vidal, J.; Fuentealba, C.; Defilippi, B.; Lindh, V.; Zulueta, C.; Chirinos, R.; Campos, D.; et al. Unravelling factors associated with ‘blackspot’ disorder in stored Hass avocado (Persea americana Mill) fruit. J. Hortic. Sci. 2020, 95, 804–815. [Google Scholar] [CrossRef]
- Lindh, V.; Uarrota, V.; Zulueta, C.; Alvaro, J.E.; Valdenegro, M.; Cuneo, I.F.; Mery, D.; Pedreschi, R. Image analysis reveals that lenticel damage does not result in black spot development but enhances dehydration in Persea americana Mill. cv. Hass during prolonged storage. Agronomy 2021, 11, 1699. [Google Scholar] [CrossRef]
- Hernández, I.; Uarrota, V.; Paredes, D.; Fuentealba, C.; Defilippi, B.G.; Campos-Vargas, R.; Meneses, C.; Hertog, M.; Pedreschi, R. Can metabolites at harvest be used as physiological markers for modelling the softening behaviour of Chilean “Hass” avocados destined to local and distant markets? Postharvest Biol. Technol. 2021, 174, 111457. [Google Scholar] [CrossRef]
- Glowacz, M.; Roets, N.; Sivakumar, D. Control of anthracnose disease via increased activity of defence related enzymes in ‘Hass’ avocado fruit treated with methyl jasmonate and methyl salicylate. Food Chem. 2017, 234, 163–167. [Google Scholar] [CrossRef]
- Silvankalyani, V.; Feygenberg, O.; Maorer, D.; Zaaroor, M.; Fallik, E.; Alkan, N. Combined treatments reduce chilling injury and maintain fruit quality in avocado fruit during cold quarantine. PLoS ONE 2015, 10, e0140522. [Google Scholar]
- Munhuweyi, K.; Mpai, S.; Sivakumar, D. Extension of avocado fruit postharvest quality using non-chemical treatments. Agronomy 2020, 10, 212. [Google Scholar] [CrossRef] [Green Version]
- Ochoa-Ascencio, S.; Hertog, M.; Nicolaï, B. Modelling the transient effect of 1-MCP on ‘Hass’ avocado softening: A Mexican comparative study. Postharvest Biol. Technol. 2009, 51, 62–72. [Google Scholar] [CrossRef]
- Saavedra, J.; Córdova, A.; Navarro, R.; Díaz-Calderón, P.; Fuentealba, C.; Astudillo-Castro, C.; Toledo, L.; Enrione, J.; Galvez, L. Industrial avocado waste: Functional compounds preservation by convective drying process. J. Food Eng. 2017, 198, 81–90. [Google Scholar] [CrossRef]
- Bradford, M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar]
- Bi, X.; Hemar, Y.; Balaban, M.; Liao, X. The effect of ultrasound on particle size, color, viscosity and polyphenol oxidase activity of diluted avocado puree. Ultrason. Sonochem. 2015, 27, 567–575. [Google Scholar] [CrossRef]
- Olivares, D.; Alvarez, E.; Véliz, D.; García-Rojas, M.; Díaz, C.; Defilippi, B. Effects of 1-methylcyclopropane and controlled atmosphere on ethylene synthesis and quality attributes of avocado cvs Edranol and Fuerte. J. Food Qual. 2020, 2020, 5075218. [Google Scholar] [CrossRef]
- López, J.; Valverde, F.; Mejía, S.; López, G.; Vega, M. Effect of controlled atmosphere storage on the postharvest and nutritional quality of tomato fruit. Rev. Chapingo Ser. Hortic. 2011, 17, 115–128. [Google Scholar] [CrossRef]
- Medina-Carrillo, R.; Salazar-García, S.; Bonilla-Cárdenas, J.; Herrera-González, J.; Ibarra-Estrada, M.; Alvarez-Bravo, A. Secondary metabolites and lignin in “Hass” avocado fruit skin during fruit development in three producing regions. HortScience 2017, 52, 852–858. [Google Scholar] [CrossRef]
- Bower, J.; Cutting, J. Avocado fruit development and ripening physiology. Hortic. Rev. 1988, 10, 229–271. [Google Scholar]
- Silveira, A. Fisiología y Bioquímica de los Productos MPF. V Congreso Iberoamericano de Tecnología Postcosecha y Agroexportaciones, Cartagena, España, 2007th ed.; Universidad Politécnica de Cartagena: Cartagena, Spain, 1655. [Google Scholar]
- Goulao, L.; Oliveira, C. Cell wall modifications during fruit ripening. When a fruit is not the fruit. Trends Food Sci. Technol. 2008, 19, 4–25. [Google Scholar] [CrossRef] [Green Version]
- Escobar, J.; Rodriguez, P.; Cortes, M.; Correa, G. Influence of dry matter as a harvest index and cold storage time on cv. Hass avocado quality produced in high tropic region. Inf. Technol. 2019, 30, 199–210. [Google Scholar]
- Saxena, A.; Saxena, T.M.; Raju, P.S.; Bawa, A.S. Effect of controlled atmosphere storage and chitosan coating on quality of fresh-cut jackfruit bulbs. Food Bioproc. Technol. 2013, 6, 2182–2189. [Google Scholar]
- Chirinos, R.; Campos, D.; Martínez, S.; Llanos, S.; Betalleluz-Pallardel, I.; García-Ríos, D.; Pedreschi, R. The effect of hydrothermal treatment on metabolite composition of Hass avocados stored in a controlled atmosphere. Plants 2021, 10, 2427. [Google Scholar] [CrossRef]
- Fukai, T.; Ushio-Fukai, M. Superoxide dismutases: Role in redox signaling, vascular function and diseases. Antioxid. Redox Signal. 2011, 15, 1583–1606. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Wu, C.; Wang, G.; He, J.; Zhu, S. Transcriptomic analysis reveals a role of phenylpropanoid pathway in the enhancement of chilling tolerance by pre-storage cold acclimation in cucumber fruit. Sci. Hortic. 2021, 288, 110282. [Google Scholar] [CrossRef]
- Sanchez-Ballesta, M.T.; Romero, I.; Jiménez, J.B.; Orea, J.M.; González-Ureña, A.; Escribano, M.I.; Merodio, C. Involvement of the phenylpropanoid pathway in the response of table grapes to low temperature and high CO2 levels. Postharvest Biol. Technol. 2007, 46, 29–35. [Google Scholar] [CrossRef] [Green Version]
- Christopoulos, M.V.; Tsantili, E. Participation of phenylalanine ammonia-lyase (PAL) in increased phenolic compounds in fresh cold stressed walnut (Juglans regia L.) kernels. Postharvest Biol. Technol. 2015, 104, 17–25. [Google Scholar] [CrossRef]
- Tesfay, S.; Bertling, I.; Bower, J. Antioxidant levels in various tissues during the maturation of Hass avocado (Persea americana Mill.). J. Hortic. Sci. Biotechnol. 2010, 85, 106–112. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).