Pesticide Resistance and Related Mutation Frequencies of Tetranychus urticae in Hainan, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tetranychus urticae Populations
2.2. Toxicity Bioassay
2.3. DNA Extraction and Determination of Mutation Frequencies
2.4. Data Analysis
3. Results
3.1. Resistance of T. urticae to Abamectin and Traditional Pesticides
3.2. Resistance of T. urticae to Recently Developed Pesticides
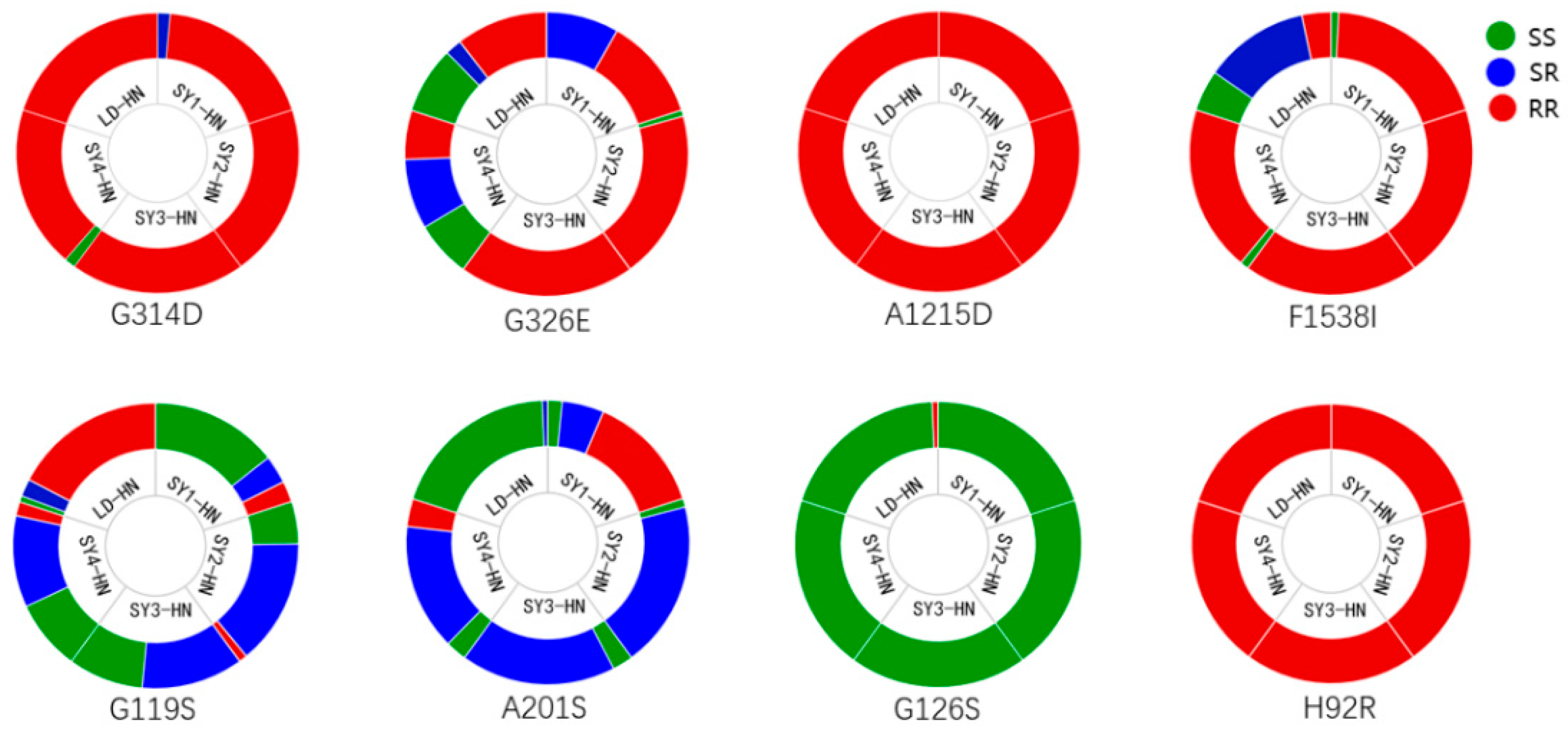
3.3. Mutation Frequencies of Target Sites
4. Discussion
4.1. Extremely High Resistance to Abamectin Accompanied with High Frequencies of Point Mutations
4.2. Prudent Application of Traditional Pesticides due to Medium to High Resistance
4.3. Alternative Use of Novel Pesticides for T. urticae Control
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Leeuwen, T.; Vontas, J.; Tsagkarakou, A.; Dermauw, W.; Tirry, L. Acaricide resistance mechanisms in the two-spotted spider mite Tetranychus urticae and other important Acari: A review. Insect Biochem. Mol. Biol. 2010, 40, 563–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Namin, H.H.; Zhurov, V.; Spenler, J.; Grbic, M.; Grbic, V.; Scott, I.M. Resistance to pyridaben in Canadian greenhouse populations of two-spotted spider mites, Tetranychus urticae (Koch). Pestic. Biochem. Physiol. 2020, 170, 104677. [Google Scholar] [CrossRef] [PubMed]
- Bajda, S.; Dermauw, W.; Panteleri, R.; Sugimoto, N.; Douris, V.; Tirry, L.; Osakabe, M.; Vontas, J.; Van Leeuwen, T. A mutation in the PSST homologue of complex I (NADH: Ubiquinone oxidoreductase) from Tetranychus urticae is associated with resistance to METI acaricides. Insect Biochem. Mol. Biol. 2017, 80, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Solmaz, E.; Cevik, B.; Ay, R. Abamectin resistance and resistance mechanisms in Tetranychus urticae populations from cut flowers greenhouses in Turkey. Int. J. Acarol. 2020, 46, 94–99. [Google Scholar] [CrossRef]
- Xue, W.X.; Snoeck, S.; Njiru, C.; Inak, E.; Dermauw, W.; Van Leeuwen, T. Geographical distribution and molecular insights into abamectin and milbemectin cross-resistance in European field populations of Tetranychus urticae. Pest Manag. Sci. 2020, 76, 2569–2581. [Google Scholar] [CrossRef]
- Herron, G.A.; Langfield, K.L.; Chen, Y.; Wilson, L.J. Development of abamectin resistance in Tetranychus urticae in Australian cotton and the establishment of discriminating doses for T. lambi. Exp. Appl. Acarol. 2021, 83, 325–341. [Google Scholar] [CrossRef]
- Tang, X.F.; Zhang, Y.J.; Wu, Q.J.; Xie, W.; Wang, S.L. Stage -Specific Expression of Resistance to Different Acaricides in Four Field Populations of Tetranychus urticae (Acari: Tetranychidae). J. Econ. Entomol. 2014, 107, 1900–1907. [Google Scholar] [CrossRef]
- Xu, D.D.; He, Y.Y.; Zhang, Y.J.; Xie, W.; Wu, Q.J.; Wang, S.L. Status of pesticide resistance and associated mutations in the two-spotted spider mite, Tetranychus urticae, in China. Pestic. Biochem. Physiol. 2018, 150, 89–96. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, D.D.; Zhang, Y.J.; Wu, Q.J.; Xie, W.; Guo, Z.J.; Wang, S.L. Frequencies and mechanisms of pesticide resistance in Tetranychus urticae field populations in China. Insect Sci. 2022, 29, 827–839. [Google Scholar] [CrossRef]
- Inak, E. Geographical distribution and origin of acetylcholinesterase mutations conferring acaricide resistance in Tetranychus urticae populations from Turkey. Exp. Appl. Acarol. 2022, 86, 49–59. [Google Scholar] [CrossRef]
- Xu, Z.F.; Zhu, W.Y.; Liu, Y.C.; Liu, X.; Chen, Q.S.; Peng, M.; Wang, X.Z.; Shen, G.M.; He, L. Analysis of insecticide resistance-related genes of the carmine spider mite Tetranychus cinnabarinus based on a de novo assembled transcriptome. PLoS ONE 2014, 9, e94779. [Google Scholar] [CrossRef] [Green Version]
- Dermauw, W.; Ilias, A.; Riga, M.; Tsagkarakou, A.; Grbić, M.; Tirry, L.; Van Leeuwen, T.; Vontas, J. The cys-loop ligand-gated ion channel gene family of Tetranychus urticae: Implications for acaricide toxicology and a novel mutation associated with abamectin resistance. Insect Biochem. Mol. Biol. 2012, 42, 455–465. [Google Scholar] [CrossRef]
- Kwon, D.H.; Clark, J.M.; Lee, S.H. Cloning of a sodium channel gene and identification of mutations putatively associated with fenpropathrin resistance in Tetranychus urticae. Pestic. Biochem. Physiol. 2010, 97, 93–100. [Google Scholar] [CrossRef]
- Binyang, A.J.; Elanga -Ndille, E.; Tene-Fossog, B.; Ndo, C.; Nouage, L.; Assatse, T.; Fotso -Toguem, Y.; Tabue, R.; Zeukeng, F.; Nguiffo, D.N.; et al. Distribution of acetylcholinesterase (Ace-1(R)) target-site G119S mutation and resistance to carbamates and organophosphates in Anopheles gambiae sensu lato populations from Cameroon. Parasit. Vectors 2022, 15, 53. [Google Scholar] [CrossRef]
- Fotoukkiaii, S.M.; Tan, Z.; Xue, W.X.; Wybouw, N.; Van Leeuwen, T. Identification and characterization of new mutations in mitochondrial cytochrome b that confer resistance to bifenazate and acequinocyl in the spider mite Tetranychus urticae. Pest Manag. Sci. 2020, 76, 1154–1163. [Google Scholar] [CrossRef]
- Grbić, M.; Van Leeuwen, T.; Clark, R.M.; Rombauts, S.; Rouzé, P.; Grbić, V.; Osborne, E.J.; Dermauw, W.; Ngoc, P.C.; Ortego, F.; et al. The genome of Tetranychus urticae reveals herbivorous pest adaptations. Nature 2011, 479, 487–492. [Google Scholar] [CrossRef] [Green Version]
- Lin, Z.F.; Ji, X.C.; Pan, F.; Liang, Y.P.; Xiao, T.B.; Xie, S.H. Investigation and analysis of the current status of pesticide application in winter melon and vegetable pest control in Hainan Province, southern China. Acta Entomol. Sinica 2016, 59, 1282–1290. [Google Scholar]
- Ferreira, C.B.S.; Andrade, F.H.N.; Rodrigues, A.R.S.; Siqueira, H.A.A.; Gondim, M.G.C. Resistance in field populations of Tetranychus urticae to acaricides and characterization of the inheritance of abamectin resistance. Crop Prot. 2015, 67, 77–83. [Google Scholar] [CrossRef]
- Emre, İ.; Sultan, Ç.; Ahmet, G.F. Monitoring of acaricide resistance and target site mutations in Tetranychus urticae Koch (Acari: Tetranychidae) populations collected from bean fields in Central Anatolia. Int. J. Acarol. 2022, 48, 279–285. [Google Scholar]
- Kwon, D.H.; Yoon, K.S.; Clark, J.M.; Lee, S.H. A point mutation in a glutamate-gated chloride channel confers abamectin resistance in the two-spotted spider mite, Tetranychus urticae Koch. Insect Mol. Biol. 2010, 19, 583–591. [Google Scholar] [CrossRef]
- Mermans, C.; Dermauw, W.; Geibel, S.; Van Leeuwen, T. A G326E substitution in the glutamate-gated chloride channel 3 (GluCl3) of the two-spotted spider mite Tetranychus urticae abolishes the agonistic activity of macrocyclic lactones. Pest Manag. Sci. 2017, 73, 2413–2418. [Google Scholar] [CrossRef] [PubMed]
- Riga, M.; Tsakireli, D.; Ilias, A.; Morou, E.; Myridakis, A.; Stephanou, E.G.; Nauen, R.; Dermauw, W.; Van Leeuwen, T.; Paine, M.; et al. Abamectin is metabolized by CYP392A16, a cytochrome P450 associated with high levels of acaricide resistance in Tetranychus urticae. Insect Biochem. Mol. Biol. 2014, 46, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.D.; Zhang, Y.; Zhang, Y.J.; Wu, Q.J.; Guo, Z.J.; Xie, W.; Zhou, X.M.; Wang, S.L. Transcriptome profiling and functional analysis suggest that the constitutive overexpression of four cytochrome P450s confers resistance to abamectin in Tetranychus urticae from China. Pest Manag. Sci. 2021, 77, 1204–1213. [Google Scholar] [CrossRef] [PubMed]
- Simma, E.A.; Hailu, B.; Jonckheere, W.; Rogiers, C.; Duchateau, L.; Dermauw, W.; Van Leeuwen, T. Acaricide resistance status and identification of resistance mutations in populations of the two-spotted spider mite Tetranychus urticae from Ethiopia. Exp. Appl. Acarol. 2020, 82, 475–491. [Google Scholar] [CrossRef]
- Ilias, A.; Vassiliou, V.A.; Vontas, J.; Tsagkarakou, A. Molecular diagnostics for detecting pyrethroid and abamectin resistance mutations in Tetranychus urticae. Pestic. Biochem. Physiol. 2017, 135, 9–14. [Google Scholar] [CrossRef]
- Riga, M.; Bajda, S.; Themistokleous, C.; Papadaki, S.; Palzewicz, M.; Dermauw, W.; Vontas, J.; Van Leeuwen, T. The relative contribution of target-site mutations in complex acaricide resistant phenotypes as assessed by marker assisted backcrossing in Tetranychus urticae. Sci. Rep. 2017, 7, 9202. [Google Scholar] [CrossRef] [Green Version]
- Ilias, A.; Vontas, J.; Tsagkarakou, A. Global distribution and origin of target site insecticide resistance mutations in Tetranychus urticae. Insect Biochem. Mol. Biol. 2014, 48, 17–28. [Google Scholar] [CrossRef]
- Liu, Z.X.; Zhou, L.J.; Yao, Q.; Liu, Y.Q.; Bi, X.Y.; Huang, J.G. Laboratory selection, resistance risk assessment, multi-resistance, and management of Tetranychus urticae Koch to bifenthrin, bifenazate and cyflumetofen on cowpea. Pest Manag. Sci. 2020, 76, 1912–1919. [Google Scholar] [CrossRef]
- Khajehali, J.; Van Leeuwen, T.; Grispou, M.; Morou, E.; Alout, H.; Weill, M.; Tirry, L.; Vontas, J.; Tsagkarakou, A. Acetylcholinesterase point mutations in European strains of Tetranychus urticae (Acari: Tetranychidae) resistant to organophosphates. Pest Manag. Sci. 2010, 66, 220–228. [Google Scholar] [CrossRef]
- Itoh, Y.; Shimotsuma, Y.; Jouraku, A.; Dermauw, W.; Van Leeuwen, T.; Osakabe, M. Combination of target site mutation and associated CYPs confers high-level resistance to pyridaben in Tetranychus urticae. Pestic. Biochem. Physiol. 2022, 181, 105000. [Google Scholar] [CrossRef]
- Qiao, X.F.; Xu, D.D.; Zhang, Y.J.; Xu, B.Y.; Wang, S.L. Effects of bifenazate·etoxazole on the oviposition and development of dominant mite species on vegetables and its control efficacy. J. Plant Prot. 2019, 46, 1310–1315. [Google Scholar]
- Li, J.J.; Hu, T.Y.; Wang, G.B.; Nie, P.C.; Xiao, H.C.; Shang, S.Q. Toxicity and control efficacy of 9 acaricides on the Tetranychus urticae in strawberry. Acta Agric. Boreali -Occident. Sin. 2020, 29, 921–927. [Google Scholar]
- Xue, W.X.; Wybouw, N.; Van Leeuwen, T. The G126S substitution in mitochondrially encoded cytochrome b does not confer bifenazate resistance in the spider mite Tetranychus urticae. Exp. Appl. Acarol. 2021, 85, 161–172. [Google Scholar] [CrossRef]
- Shang, S.Q.; Chang, Y.; Li, W.Z.; Wang, C.Q.; Nie, P.C. Effects of B-azolemiteacrylic on life-history traits and demographic parameters of two-spotted spider mite, Tetranychus urticae (Acari: Tetranychidae). Exp. Appl. Acarol. 2022, 86, 61–71. [Google Scholar] [CrossRef]
- Papapostolou, K.M.; Riga, M.; Charamis, J.; Skoufa, E.; Souchlas, V.; Ilias, A.; Dermauw, W.; Ioannidis, P.; Van Leeuwen, T.; Vontas, J. Identification and characterization of striking multiple-insecticide resistance in a Tetranychus urticae field population from Greece. Pest Manag. Sci. 2021, 77, 666–676. [Google Scholar] [CrossRef]
| Population | Collection Location | Collection Date | Collection Site | Host Plant |
|---|---|---|---|---|
| SY-HN1 | Sanya, Hainan | 21 February 2021 | E109°10′, N18°21′ | Muskmelon |
| SY-HN2 | Sanya, Hainan | 21 February 2021 | E109°5′, N18°25′ | Watermelon |
| SY-HN3 | Sanya, Hainan | 21 February 2021 | E109°5′, N18°25′ | Hami melon |
| SY-HN4 | Sanya, Hainan | 21 February 2021 | E109°5′, N18°25′ | Gourd melon |
| LD-HN | Ledong, Hainan | 30 December 2021 | E109°1′, N18°74′ | Watermelon |
| Pesticide | Population | N a | Slope ± SE | LC50 (95% FL b; mg/L) | χ2 (df) c | RF d |
|---|---|---|---|---|---|---|
| Abamectin | IPP-SS | 533 | 2.39 ± 0.18 | 0.05 (0.04–0.05) | 1.62 (4) | 1.00 |
| SY-HN1 | 293 | 2.04 ± 0.25 | 433.35 (320.99–684.74) | 4.06 (4) | 9420.74 | |
| SY-HN2 | 352 | 1.67 ± 0.16 | 220.97 (180.41–273.30) | 1.018 (4) | 4803.78 | |
| SY-HN3 | 435 | 1.33 ± 0.16 | 456.05 (340.34–691.16) | 2.589 (3) | 9914.07 | |
| SY-HN4 | 406 | 0.86 ± 0.18 | 329.30 (203.879–912.21) | 1.722 (3) | 7158.61 | |
| LD-HN | 444 | 1.42 ± 0.17 | 283.77 (193.79–538.77) | 3.3246 (3) | 6168.87 | |
| Bifenthrin | IPP-SS | 508 | 1.82 ± 0.15 | 1.61 (1.27–1.96) | 2.02 (3) | 1.00 |
| SY-HN1 | 350 | 2.19 ± 0.19 | 346.25 (287.35–412.23) | 1.25 (4) | 215.60 | |
| SY-HN2 | 366 | 1.07 ± 0.15 | 504.32 (359.13–823.13) | 2.48 (4) | 314.02 | |
| SY-HN3 | 378 | 0.99 ± 0.15 | 366.76 (268.13–546.91) | 2.35 (4) | 228.37 | |
| SY-HN4 | 322 | 1.10 ± 0.17 | 498.10 (347.45–859.13) | 1.03 (4) | 310.15 | |
| LD-HN | 322 | 2.10 ± 0.24 | 1259.39 (1065.43–1519.59) | 3.91 (4) | 784.18 | |
| Pyridaben | IPP-SS | 597 | 1.04 ± 0.10 | 66.74 (51.36–91.72) | 1.10 (5) | 1.00 |
| SY-HN1 | 444 | 1.54 ± 0.23 | 2774.12 (2284.88–3335.05) | 0.75 (3) | 41.57 | |
| SY-HN2 | 369 | >5000 | ||||
| SY-HN3 | 410 | >5000 | ||||
| SY-HN4 | 378 | >5000 | ||||
| LD-HN | 362 | >5000 | ||||
| Profenofos | IPP-SS | 536 | 0.89 ± 0.11 | 18.87 (13.91–27.60) | 0.60 (4) | 1.00 |
| SY-HN1 | 439 | 1.81 ± 0.16 | 256.22 (215.75–308.00) | 3.17 (4) | 13.58 | |
| SY-HN2 | 324 | 0.92 ± 0.15 | 428.42 (238.90–1387.83) | 4.92 (3) | 22.71 | |
| SY-HN3 | 292 | 1.74 ± 0.21 | 1080.66 (876.66–1370.91) | 1.38 (3) | 57.27 | |
| SY-HN4 | 376 | 1.24 ± 0.14 | 271.50 (209.54–361.62) | 0.68 (3) | 14.39 | |
| LD-HN | 398 | 2.01 ± 0.24 | 1434.85 (1007.81–2768.04) | 5.77 (3) | 76.05 |
| Pesticide | Population | N a | Slope ± SE | LC50 (95% FL b; mg/L) | χ2 (df) c | RF d |
|---|---|---|---|---|---|---|
| Bifenazate | IPP-SS | 603 | 1.08 ± 0.11 | 7.49 (5.12–10.07) | 1.32 (6) | 1.00 |
| SY-HN1 | 327 | 0.97 ± 0.15 | 91.73 (63.11–156.46) | 0.14 (4) | 12.25 | |
| SY-HN2 | 276 | 2.45 ± 0.41 | 46.10 (38.21–53.36) | 1.06 (3) | 6.16 | |
| SY-HN3 | 378 | 1.29 ± 0.17 | 50.21 (39.46–64.68) | 1.15 (3) | 6.70 | |
| SY-HN4 | 292 | 1.35 ± 0.20 | 52.09 (29.71–108.88) | 4.56 (3) | 6.95 | |
| LD-HN | 472 | 1.24 ± 0.12 | 249.91 (196.85–330.40) | 2.35 (4) | 33.37 | |
| Cyflumetofen | IPP-SS | 496 | 1.93 ± 0.20 | 0.63 (0.53–0.77) | 0.97 (3) | 1.00 |
| SY-HN1 | 391 | 1.27 ± 0.14 | 321.17 (251.33–418.30) | 3.45 (4) | 510.61 | |
| SY-HN2 | 290 | 1.03 ± 0.19 | 125.88 (79.67–176.52) | 1.28 (3) | 200.13 | |
| SY-HN3 | 297 | 1.12 ± 0.16 | 38.44 (27.04–52.46) | 1.51 (4) | 61.12 | |
| SY-HN4 | 314 | 1.39 ± 0.17 | 80.10 (37.71–134.33) | 4.69 (3) | 127.18 | |
| LD-HN | 440 | 1.40 ± 0.14 | 660.27 (527.90–860.34) | 3.00 (4) | 1049.71 | |
| B-azolemiteacrylic | IPP-SS | 517 | 1.68 ± 0.13 | 0.48 (0.40–0.58) | 0.97 (4) | 1.00 |
| SY-HN1 | 322 | 1.81 ± 0.18 | 1.04 (0.85–1.28) | 0.71 (3) | 2.17 | |
| SY-HN2 | 292 | 1.27 ± 0.19 | 0.76 (0.57–1.01) | 2.17 (3) | 1.58 | |
| SY-HN3 | 462 | 1.67 ± 0.17 | 1.12 (0.94–1.39) | 2.53 (3) | 2.34 | |
| SY-HN4 | 412 | 1.24 ± 0.17 | 0.60 (0.48–0.80) | 1.31 (3) | 1.25 | |
| LD-HN | 334 | 1.57 ± 0.19 | 6.14 (3.81–13.22) | 4.97 (3) | 12.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, T.; Wu, M.; Zhang, Y.; Xu, D.; Wu, M.; Xie, W.; Su, Q.; Wang, S. Pesticide Resistance and Related Mutation Frequencies of Tetranychus urticae in Hainan, China. Horticulturae 2022, 8, 590. https://doi.org/10.3390/horticulturae8070590
Tian T, Wu M, Zhang Y, Xu D, Wu M, Xie W, Su Q, Wang S. Pesticide Resistance and Related Mutation Frequencies of Tetranychus urticae in Hainan, China. Horticulturae. 2022; 8(7):590. https://doi.org/10.3390/horticulturae8070590
Chicago/Turabian StyleTian, Tian, Mingmei Wu, Yan Zhang, Dandan Xu, Mingyue Wu, Wen Xie, Qi Su, and Shaoli Wang. 2022. "Pesticide Resistance and Related Mutation Frequencies of Tetranychus urticae in Hainan, China" Horticulturae 8, no. 7: 590. https://doi.org/10.3390/horticulturae8070590
APA StyleTian, T., Wu, M., Zhang, Y., Xu, D., Wu, M., Xie, W., Su, Q., & Wang, S. (2022). Pesticide Resistance and Related Mutation Frequencies of Tetranychus urticae in Hainan, China. Horticulturae, 8(7), 590. https://doi.org/10.3390/horticulturae8070590







