Abstract
Wild relatives of okra represent a good source of variation for breeding programs, particularly for traits related to biotic and abiotic stresses and fruit quality. However, wild species remain largely unexploited for okra breeding. The cultivated okra accession Abelmoschus esculentus cv. ‘Arka Anamika’ was crossed with three wild accessions: Abelmoschus manihot, Abelmoschus ficulens and Abelmoschus moschatus. The crossability was estimated based on fruit set, seed set and germination rate. The results of cross compatibility studies revealed that the crosses are compatible only when cultivated A. esculentus is used as a female, and reciprocal crosses were not successful in any of the three wild species. Higher fruit set (87.2%) and seed germination (92%) were obtained from the crosses ‘Arka Anamika’ × Abelmoschus manihot. The interspecific F1s exhibited normal growth, flowering and fruit formation, but the hybrids were completely sterile on selfing due to abnormal meiosis. Characterization of the parents and interspecific hybrids revealed that the interspecific hybrids were generally more vigorous than the parents and displayed greater alliance towards their wild parent. The sterile F1s were further treated with 0.1% colchicine to restore fertility. The three interspecific crosses showed considerable differences in mean performance and heterosis over mid parent and better parent. The cross ‘A. Anamika’ × A. manihot displayed positive heterosis over mid parent and better parent for the yield traits, viz., fruit yield per plant, number of fruits per plant, number of primary branches per plant and plant height. The information obtained in the study on crossability relationship, phenotypic characterization and heterosis in interspecific hybrids will assist breeders in the development of prebreeding material.
1. Introduction
Okra (Abelmoschus esculentus L. Moench) belongs to the family Malvaceae, has chromosome number 2n = 8x = 72 or 144, and originated in South East Asia []. It is a commonly used vegetable and is widely employed in culinary preparations. It has high nutritional value, particularly due to vitamin C (30 mg/100 g), vitamin A (20 mg/100 g), zinc (6 mg/100 g), β carotene (300 µg/100 g) and is a rich source of folic acid (300 µg/100 g) []. Okra is an important part of the diet due to its high dietary fibre content, low calorie count, and rich supply of minerals including Ca, P, K, and Mg. The fruits are a rich source of iodine that helps cure goiter and the leaves are used as a remedy for dysentery [].
Okra is categorized under the often cross-pollinated group with outcrossing of 4 to 30% and it is an exciting crop for breeders and geneticists for its monadelphous condition of the stamens, large flowers amenable to emasculation and the high level of seed production in one pollination []. Geographical separation, genetic barriers to crossability and different parents of evolution make India the wealthiest source of genetic diversity for okra []. The availability of diverse cultivars is essential to meet the necessity of regional adaptation to various climatic conditions and fulfill farmers needs for varied phenotypic traits in okra [].
The yield and quality of okra is affected by a variety of biotic and abiotic stresses. Viruses, fungal pathogens, insect pests and mites are some of the major biotic bottlenecks. Yellow vein mosaic disease (YVMV) is the most severe constraint responsible for 90 to 100% yield loss. The disease reduces the yield substantially and affects the marketability of the fruits [].
Presently, none of the cultivated okra varieties has stable resistance to this YVMV disease. The evolution of new viral strains seems to be one of the major factors responsible for the breakdown of resistance, as the resistance in the majority of the cases is location specific []. Thus, intervarietal transfer of YVMV resistance is unlikely. The one approach to addressing this challenge involves utilizing genetic resources to enhance the resistance in cultivated okra []. Crop wild relatives, which include crop progenitors and other closely related species, are a source of germplasm with indisputably favorable genes for dealing with biotic and abiotic stresses [].
Dark green fruits, high mucilage, extended bearing, perennation propensity, strong branching, reduced fruit length, drought resistance, high temperature tolerance and YVMV resistance are a few of the favorable characteristics of the wild okra species. Interspecific hybridization in okra is of immense value to exploit species diversity in the genus Abelmoschus for developing male sterile lines and advanced breeding lines that possess resistance to YVMV and okra Enation Leaf Curl viral diseases []. Several disease resistant varieties have succumbed to disease through interspecific hybridization, most likely as a result of novel virus strains or the inadequate contribution of resistance genes transferred from wild species due to the lack of adaptive gene complexes which are prevalent in wild species [].
Many wild and semi-wild species of the genus Abelmoschus have been found growing in variety of habitats in the Indian subcontinent, which is regarded as its center of diversity []. A. esculentus is the only known cultivated species in India. Abelmoschus moschatus Medik occurs as a semi-wild species and is also cultivated for its aromatic seed oil, while the rest are truly wild types. Stable and viable sources of resistance to YVMV in okra have been reported in Abeloschus Angulosus Wall., Abelmoschus Tetraphyllus (Roxb. Ex Hornem), A. moschatus, Abelmoschus caillei (A. Chev.) and Abelmoschus manihot (L.) Medik. [,].
Several wild relatives of A. esculentus have been identified as a potential source of resistance to various biotic stresses, but their utilization in okra breeding is often limited [] because interspecific hybridization is restricted by their natural habitat (geographic isolation), flower structure (i.e., shape or colour), flowering time, or pollen mediator, which are involved in pre-zygotic barriers []. Hybridizing cultivated okra species with wild relatives is essential to widen the primary gene pool.
Heterosis for agronomically essential traits must be manifested prior to obtaining productive hybrids. The genetic distance between the parental lines has a strong correlation with the occurrence of heterosis in okra hybrids []. Interspecific hybridization improves crops by transferring specific traits, such as yield, improved quality, pest, and stress resistance to crops from their wild relatives []. However, in the case of okra, despite being one of the most important vegetable crops and being inter-crossable with many wild relatives, the interspecific hybridisation is limited to the transfer of YVMV resistance.
Though India enjoys the status of being one of the largest okra producers globally, several issues still need to be addressed. Crossability barriers in interspecific hybridization among A. esculentus and wild relatives need to be thoroughly studied. Hence, the objective of this study was to characterize some Abelmoschus species, their hybrids, crossability relationships and fertility restoration through colchiploidy in interspecific F1 hybrids of Abelmoschus spp.
2. Materials and Methods
2.1. Plant Material and Location of the Experimental Site
Three wild species of Abelmoschus, viz., Abelmoschus manihot, Abelmoschus ficulens (L.) Wight & Arn. and Abelmoschus moschatus, were collected from the Western Ghats region of Karnataka, India (Table 1) and were grown and maintained at the College of Agriculture, Shivamogga, KSNUAHS, Karnataka, India. Among the three wild species, A. manihot was identified as resistant to okra YVMV by morphological and molecular screening. The other two species, viz., A. moschatus and A. ficulens, are perennials with profuse branching habit and have an extended pod bearing period. Although, the fruits are smaller and not edible, backcrossing can transfer the smaller fruit size to cultivated okra. Nowadays, smaller fruits are preferred for industrial and culinary purposes. Due to the hairiness of their leaves and fruits, these wild species are less susceptible to insect pests. The cultivated species Abelmoschus esculentus variety ‘Arka Anamika’ has good keeping, cooking qualities and consumer preference, developed by interspecific hybridization of A. esculentus × A. manihot spp. tetraphyllus var. tetraphyllus. As a result of backcrossing, the ‘Arka Anamika’ shares 99.9% of its genome with A. esculentus. Hence, it was chosen as an A. esculentus parent. Interspecific hybridization was attempted between ‘Arka Anamika’ and the three wild genotypes during the post rainy season of 2019–2020 at KSNUAHS, Shivamogga. To maintain homozygosity, both cultivated and wild accessions were selfed by covering healthy flower buds prior to anthesis.

Table 1.
Abelmoschus species used for hybridization studies.
2.2. Emasculation and Pollination Techniques
Hand emasculation and pollination were followed by hybridization. The emasculation of unopened flower buds was performed by removing the petals and undehisced anthers with a sharp knife between 4:00 and 6:00 p.m. To prevent drying of the stigmatic surface and prevent cross pollination owing to insect activity, the flower buds were then covered with paper bags. The pollen grains were tapped directly over the stigmas of emasculated flowers in the morning between 9:00 and 10:00 a.m. for cross-pollination. The flowers were again covered with paper bags. Pollen grains were collected from a single tagged plant of the wild donor species for each cross. Self-pollination was performed at the same time following similar procedures. One day after pollination, the bags were carefully removed from all fertilized flowers. Thirty-five days after pollination, dried and fully mature crossed fruits were harvested, F1 hybrid seeds were extracted, and the number of seeds set on A. esculentus was recorded. Both direct and reciprocal crosses, viz., A. Anamika × A. manihot, A. Anamika × A. ficulens, A. Anamika × A. moschatus, A. manihot × A. Anamika, A. ficulens × A. Anamika and A. moschatus × A. Anamika, were attempted for all the wild genotypes with ‘Arka Anamika’.
2.3. Evaluation of Hybridization Success
The success of hybridization was determined by examining each flower three days after pollination. Fertilized flowers developed fruit capsules with white seeds between 3 to 4 days after pollination. Depending on the accessions crossed and where fertilization failed, the flowers dropped 2 to 3 days after pollination without developing any fruit capsules.
2.4. Crsossability Indices
Crossability indices such as fruit set (%), reproductive success/average number of seeds per fruit, crossing efficiencies (Rao []) and seed germination (%) were estimated using all the four parental species and their crosses as below according to Patil et al. [].
2.5. In-Vitro Pollen Viability Test
One percent acetocarmine stain was used to estimate the pollen viability of parental species, their F1s and amphidiploids. A few drops of dye were poured onto the cavity slide using a dropper and then pollen grains from freshly opened flowers were spread on the slide. A few minutes later, the slides were observed under a microscope (Model-Olympus-CX21i). A pollen grain was considered viable if it took a dark red stain, otherwise it was judged as non-viable or sterile. Then counts of viable and non-viable pollen grains were used to calculate viable pollen grain percentage as below. Five slide counts for each parental species and their F1 and amphidiploids were recorded.
2.6. In Vitro Pollen Germination
An artificial pollen germination medium was prepared as per the composition suggested by Patil et al. [] for okra. The germination medium consisted of 100 ppm boric acid, 15% sucrose, 10% PEG (polyethyleneglycol-6000 grade) and 1% agar dissolved in boiling water at 5.6 pH. The medium was poured onto the cavity slides and pollen from freshly opened flowers was distributed on the surface of the cooled but still somewhat fluid medium. The cavity slides were then kept at 23–25 °C. One hour later, the slides were observed under a microscope (Model-Olympus-CX21i). Pollen grain was considered germinated when the tube had grown to a length of approximately twice the diameter of the pollen grain. Then counts of germinated pollen grains were used to calculate germinated pollen grain percentage as below. The counts of five slides for each parental species, their F1 and amphidiploid were recorded.
2.7. In Vitro Pollen Diameter
Pollen grains from freshly opened flowers of the parental species, their F1 hybrids and amphidiploids, were spread uniformly on pollen germination medium poured on cavity slides. Then slides were immediately observed under a stereo zoom automontage microscope to assess the diameter of the pollen grains (µm). Counts from five slides for each parental species and their F1 and amphidiploid were recorded. The data on pollen viability, pollen germination and pollen diameter was subjected to analysis of variance using the “agricolae” package of R software (ver. 4.1.3) [] for calculating standard deviation.
2.8. Morphological Characterization of Interspecific Crosses
Twenty-five plants of each of the interspecific hybrids, viz., ‘Arka Anamika’ × A. manihot, ‘Arka Anamika’ × A. ficulens and ‘Arka Anamika’ × A. moschatus, along with their four parental species, were characterized by recording observations on 23 qualitative phenotypic characters including growth, stem, leaf, flower and fruit based on NBPGR Minimal Descriptor [] and okra DUS Guidelines (PPV&FRA2009) []. The quantitative characters, viz., internodal length, number of internodes, plant height and number of primary branches, were assessed after the flowering period had terminated. The data was subjected to analysis of variance using the “agricolae” package of R software (ver. 4.1.3) [] for calculating standard error.
2.9. Estimation of Heterosis (%) in Interspecific Hybrids
Heterosis was estimated among three interspecific hybrids, viz., ‘Arka Anamika’ × A. manihot, ‘Arka Anamika’ × A. ficulens and ‘Arka Anamika’ × A. moschatus, for the ten yield contributing traits. The mean values for each trait from the selected ten plants were used to estimate heterosis. The heterotic effects were measured as the deviation of the F1 mean from the mid parent (relative heterosis) and better parent (heterobeltiosis) as per Arunachalam [] The heterosis analysis was performed using the “gpbstat” package of R software (ver. 4.1.3) (CRAN, Karnataka, India) []. Heterosis was calculated as follows:
where:
F1 = Mean value of F1
MP = Mean value of both parents
BP = Mean value of better parent
2.10. Treatment of Interspecific F1s with Colchicine to Overcome Sterility
Fully mature and properly dried F1 hybrid seeds obtained from the crosses between ‘Arka Anamika’ and three wild species were collected and sown in the germination trays to raise seedlings of F1 hybrids for colchicine treatment during the Kharif season of 2021. Seedlings of interspecific crosses were treated with 0.1% colchicine on the apical meristem using a cotton swab method at the two leaf (pseudo cotyledonary) stage. The colchicine treatment was performed from the 4th to 7th day after germination four times a day at 3 h intervals from 9.00 a.m. to 6.00 p.m. [].
3. Results
3.1. Crossability of A. esculentus cv. ‘Arka Anamika’ with Their Wild Species
Selfing of all the four parental species, A. esculentus, A. manihot, A. ficulens and A. moschatus resulted in more than 90% fruit set and seed germination. Although the seed germination was above 90% in A. esculentus and A. moschatus, there was low seed germination in A. manihot and A. ficulens due to hard seed coats (Table 2). Prabhu and Warade [] recorded low seed germination in A. manihot var. tetraphyllus due to the hard seed coats.

Table 2.
Crossability indices between A. esculentus cv. ‘Arka Anamika’ with A. manihot, A. ficulens and A. moschatus.
A. esculentus and A. manihot were crossed directly (A. esculentus × A. manihot) and reciprocally (A. manihot × A. esculentus). Successful fruit set and seed set were seen only in direct crosses. In contrast, the reciprocal cross failed to produce any fruit and seed, leaving all crossability indices at zero. The direct cross recorded 87.2% fruit set, an average of 34.53 seeds per fruit and 82.4% crossing efficiency with 92% F1 seed germination. Crossability estimates were higher when A. esculentus was used as a seed parent with A. ficulens than in the reciprocal crosses. The direct crosses exhibited 77.4% fruit set with an average of 36.23 per fruit. A higher crossability index of 81.5% with 12.6% seed germination was observed in F1. In comparison, the reciprocal cross showed 27% fruit set and an average of 9.26 seeds per fruit with 28.5% crossing efficiency. However, F1 seeds of the reciprocal cross failed to germinate (Table 2).
In the crosses of A. esculentus with A. moschatus, the direct cross yielded a fruit set of 70.7% and a crossability index of 74.0% with an average of 30.21 viable seeds per fruit with shrivelled and unfilled seeds due to embryo abortion. In the case of reciprocal crosses, fruits fell off from the plant from two to several days after pollination (Figure 1d).

Figure 1.
Fruits of A. ficulens (a), A. moschatus (b), A. esculentus × A. ficulens F1 (c), immature fruit drop in the cross A. moschatus × A. esculentus (d), fruits of A. esculentus cv. ‘Arka Anamika’, F1 between A. esculentus × A. manihot and A. manihot, respectively (e), sterile fruits with no seed set in F1 of the cross A. esculents × A. manihot (f), fertile and sterile pollen in F1 of the cross A. esculentus × A. moschatus (g), malformation of leaves in colchicine treated interspecific F1 (h), partial seed set in colchicine treated F1 of cross A. esculentus × A. manihot (i).
Further selfing of these F1 plants exhibiting sterility (Figure 1f), met with failure to set fruits and sometimes empty fruits with shrivelled seeds and no viable seeds due to complete seed sterility exhibited by hybrids.
3.2. Pollen Viability and Germination Studies
Results of the pollen studies in interspecific hybrids indicated the considerable variability among parents and crosses. Pollen viability for all the parental species using the acetocarmine test and germination on an artificial medium confirmed very high (>95%) pollen fertility in all the parental species. The pollen studies revealed the lowest pollen viability and pollen germination of F1 pollen grains compared with their parental species (Figure 1g). Pollen grains of all four species were relatively uniform with a narrow diameter range. The A. esculentus (0.177 μm) and A. manihot (0.173 μm) had larger pollen diameters than A. ficulens (0.136 μm) and A. moschatus (0.142 μm). The pollen diameter of the interspecific hybrids varied greatly from 0.172 μm to 0.254 μm (A. esculentus × A. manihot), 0.123 μm to 0.186 μm (A. esculentus × A. ficulens) and 0.132 μm to 0.196 μm (A. esculentus × A. moschatus) (Figure 2). Pollen viability experiments confirmed very high pollen fertility in all the parental species (Table 3).
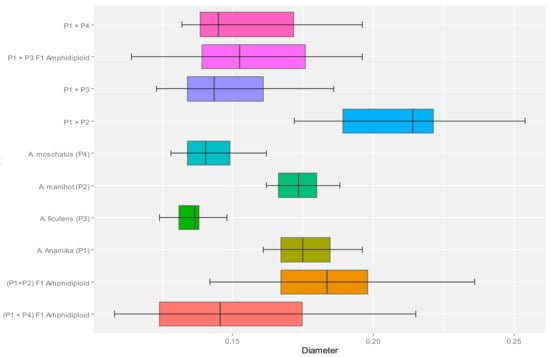
Figure 2.
Box plot depicting the variation in pollen diameter (µm) among parents and crosses. The dashed line shows the overall mean (standard deviation = 0.022).

Table 3.
Pollen viability and pollen germination in A. esculentus cv. ’Arka Anamika’, A. manihot, A. ficulens and A. moschatus, their F1 and amphidiploid F1.
3.3. Morphological Characterization of Parents and Interspecific Hybrids Based on Qualitative Phenotypic Characters
All the four parental species (‘Arka Anamika’, A. manihot, A. ficulens and A. moschatus) showed good early plant vigour, and their F1 hybrids were more vigorous than their parents. Regarding plant growth habit, except for A. moschatus (which was medium), the other three species, including their three F1 hybrids, were found to be erect growing. The branching habit was low in ‘Arka Anamika’, A. manihot and A. ficulens, whereas profuse in A. moschatus and A. Anamika × A. manihot (F1). The number of branches was few (<2) in ‘A. Anamika’, medium (3–5) in A. manihot and A. ficulens but many (>5) in A. moschatus and ‘Arka Anamika’ × A. manihot (F1). Interspecific F1 hybrids were highly vigorous, erect growing and profuse branching compared with either parental species with greater alliance towards the wild parent (Table 4).

Table 4.
Morphological Characterization of qualitative traits of interspecific F1 hybrids of Abelmoschus species with their parents.
3.4. Stem Characters
For stem related traits, stem colour and stem pubescence were recorded. The stem colour was light green in all the parents and their F1 hybrids except red in A. moschatus. Stem pubescence was seen only in A. moschatus.
3.5. Leaf Characters
The depth of leaf lobbing was medium in ‘Arka Anamika’, A. ficulens, A. moschatus, ‘Arka Anamika’ × A. ficulens and ‘Arka Anamika’ × A. moschatus; deep in A. manihot and ‘Arka Anamika’ × A. manihot. The serration of leaf margins was strong in all the parents and hybrids. Vein colour was observed to be light green in ‘Arka Anamika’, ‘Arka Anamika’ × A. ficulens and ‘Arka Anamika’ × A. moschatus; dark green in A. manihot, A. ficulens and ‘Arka Anamika’ × A. manihot; and red in A. moschatus.
3.6. Floral Characters
In the context of floral characters, flower colour was yellow in most of the parents and their hybrids but white in A. ficulens and yellowish white in its hybrid with ‘Arka Anamika’. Epicalyx segments varied from eight to ten in ‘Arka Anamika’; seven to nine in ‘Arka Anamika’ × A. ficulens and ‘Arka Anamika’ × A. moschatus; and five to seven in A. manihot, A. ficulens, A. moschatus and ‘Arka Anamika’ × A. manihot. The shape of epicalyx segments was linear in most of the parents and their F1s but lanceolate in A. manihot and its hybrid with ‘Arka Anamika’. Interspecific hybrid flowers were more prominent and intermediate than in their wild parents. At the same time, epicalyx segments resembled their wild parents in hybrids.
3.7. Fruit Characters
The fruit colour was light green in ‘Arka Anamika’, A. moschatus, ‘Arka Anamika’ × Abelmoschus ficulens and ’Arka Anamika’ × Abelmoschus moschatus. In contrast, it was dark green in A. manihot, A. ficulens and ‘Arka Anamika’ × A. manihot. The fruits were observed to be long in cultivated ‘Arka Anamika’, small in all the wild species, and intermediate (medium) in all the hybrids (Figure 1b,c,e). The fruit surface between ridges was flat in all the parental and F1s except that A. moschatus and ‘Arka Anamika’ × A. ficulens had a concave surface. Fruit pubescence was weak in cultivated ‘Arka Anamika’ but strong in all the wild species and their hybrids except ‘Arka Anamika’ × A. ficulens, which had medium pubescence. Constriction at the basal part of the fruit was absent in all the parents and hybrids. The fruit apex shape was blunt in all the wild species but narrow acute in cultivated ‘Arka Anamika’. All the hybrids with ‘Arka Anamika’ had an acute shape at the apex. Without exception, five locules per fruit were observed in all the parental species and their hybrids.
3.8. Stigma and Pollen Characters
The number of stigmatic lobes was five in all parents and hybrids. The pollen was fertile with uniform diameter in parental species, and there was sterile pollen of varied diameter observed in the interspecific crosses.
3.9. Mean Performance and Heterosis (%)
The mean performance of all the seven genotypes, including the four parents and three interspecific hybrids is presented in Table 5. The three interspecific crosses showed considerable differences in mean performance and heterosis over mid parent and better parent. The heterosis values were positive for most vigour related traits, whereas the fruit length (−17.69 to −50.1%) and fruit diameter (−22.18 to −61.29%) showed highly negative heterosis for the three crosses compared with the mid parent and better parent (Figure 3). The number of fruits per plant, fruit weight, fruit length and fruit yield had the more significant variability among the quantitative traits studied.

Table 5.
Mean performance of quantitative traits of interspecific F1 hybrids of Abelmoschus species with their parents.
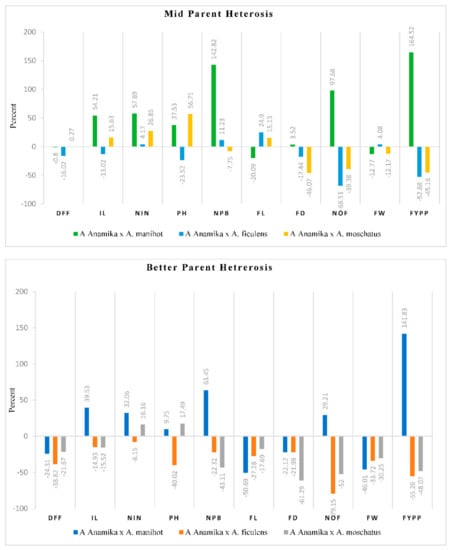
Figure 3.
Range of heterosis over mid parent and better parent in the three interspecific crosses. DFF = Days to 50 per cent flowering, IL = Internodal length (cm), NIN = Number of internodes, PH = Plant height (cm), NPB = Number of primary branches, FL = Fruit length (cm), FD = Fruit diameter, NOF = Number of fruits per plant, FW = fruit weight (g) and FYPP = Fruit yield per plant (g).
Days to 50 per cent flowering is one of the crucial traits in crop improvement. For traits like Days to flowering, earliness and heterosis in the negative direction is desirable. In okra, early flowering is always desirable as it increases the duration of the fruit bearing period. The cultivated species ‘Arka Anamika’ was the earliest to flower in 43.21 days, whereas the wild genotypes, viz., A. manihot (82.15 days), A. ficulens (94.52 days) and A. moschatus (76.83 days), were late to flower. The interspecific F1 hybrids flowered early compared to their wild parents. The magnitude of heterosis ranged from 0.3% (A. Anamika × A. moschatus) to −16.0% (A. Anamika × A. ficulens) compared with the mid parent and −21.7% (A. Anamika × A. moscahtus) to −38.8% (A. Anamika × A. ficulens) compared with the better parent.
Plant height is one of the essential traits of okra. Plant height is directly correlated with the number of internodes and internodal length. Plant height, the number of internodes and intermodal length varied widely among the parents and crosses. The parents, A. manihot (210.05 cm), A. ficulens (220.18 cm) and the cross A. Anamika × A. manihot (230.52 cm) were found to be the tallest. In contrast, A. moschatus (62.53 cm) recorded the lowest plant height. Positive heterosis of smaller magnitude for plant height over better parent was observed for the crosses A. Anamika × A. manihot (9.7%) and A. Anamika × A. moschatus (17.5%). The cross A. Anamika × A. ficulens displayed negative heterosis over both mid parent and better parent.
The average fruit weight among the parents ranged from 4.53 g (A. manihot) to 19.04 g (A. Anamika). In contrast, among the crosses it varied from 10.28 g (A. Anamika × A. manihot) to 13.28 g (A. Anamika × A. moschatus). The crosses exhibited heterosis in a negative direction over the better and mid parent, except the cross A. Anamika × A. ficulens recorded positive heterosis of 4.1%. The heterosis over the better parent for fruit weight was highly negative with values ranging between −30.2% (A. Anamika × A. moschatus) and −40.0% (A. Anamika × A. manihot).
The numbers of breeding lines used for alien introgression are vast. The results presented here reveal the possibility of interspecific hybrids between amphidiploid A. esculentus possessing good agronomic characteristics and diploid wild species. A. manihot, A. ficulens and A. moschatus recorded lower fruit yield due to flower and fruit drop. Among the three crosses, the crosses A. Anamika × A. ficulens and A. Anamika × A. moschatus exhibited negative heterosis over the mid and better parent. The cross A. Anamika × A. manihot recorded 141.8% and 164.5% positive heterosis over the better parent and mid parent, respectively, for fruit yield. The interspecific hybrid between A. Anamika × A. manihot recorded maximum mean values and displayed positive heterosis for important yield related traits, viz., fruit yield per plant, number of fruits per plant, number of primary branches, over the mid parent and better parent.
3.10. Hybrid Sterility Associated with Interspecific Hybridization and Colchiploidy
Colchicine treatment was successfully carried out at the two-leaf stage to restore the fertility in the interspecific hybrids, which is a prerequisite for advancing the interspecific hybrids to future generations. The seedlings exhibited scorching symptoms on the apical region after the colchicine treatment, but mortality of seedlings was not observed. The interspecific F1s exhibited vigorous sideward growth with a normal fruit set (100%) and partial seed set (65%) (Figure 1h,i). Improvement in the fruit yield per plant is the ultimate aim of okra breeding. Considerably more variation for fruit yield per plant was observed among the crosses than among the parents. Among the crosses, A. Anamika × A. manihot recorded the maximum fruit yield of 920.66 g per plant. The fruit yields for the other two crosses were 170.25 g for A. Anamika × A. ficulens and 221.05 g for A. Anamika × A. moschatus.
4. Discussion
Successful interspecific hybridization and backcrossing is necessary for utilizing wild species as germplasm. Successful interspecific hybridization in the species A. esculentus has helped to generate considerable variability []. However, barriers in the interspecific hybrids of okra have impeded the transmission of desired genes from wild species to the cultivated ones [,].
Fertile progenies need to be produced by crosses between the wild Abelmoschus species and okra to use the wild relatives of Okra resistant to the tested diseases in the crop improvement programme. Although F1 plants were obtained in some instances, these plants were highly sterile, and it was challenging to produce subsequent generations or even to carry out backcrosses []. Fortunately, most of the well adapted cultivated okra genotypes are crossable with the wild species.
Undomesticated related species have a large gene pool that can provide protection against major diseases, pests, and unfavorable environmental factors []. Different okra species have different growth habits due to selection or as a natural adaptation mechanism. The genetic distance and polyploidy nature of A. esculentus and other wild relatives are barriers to success in a hybridization programme to transfer desirable resistant genes []. However, the desired gene transfer from the wild to cultivated species has been hampered by barriers in the interspecific hybrids of okra [,]. Resistance to YVMV is not stable in developed varieties, and a frequent breakdown of resistance has been observed in cultivated species. There is a need to incorporate wild relatives in developing prebreeding lines resistant to biotic stresses [,]. Luckily, most genotypes of the cultivated okra can be crossed with wild species. This suggests that there are many different breeding lines that can be used to introduce aliens into a population.
Results from crossability studies will assist the breeders in deciding the appropriate breeding methods to use when transferring genes for desirable traits found in one genotype into the genetic background of another genotype to develop novel varieties []. In the present study, when the cultivated type was used as a female parent, the progeny plants were produced without incompatibility difficulties. The direct crosses between A. esculentus and A. manihot recorded maximum fruit set, number of seeds per fruit, and the highest crossability index and germination percentage. Due to the variation in the chromosome number and polyploidy nature of Abelmoschus species, there is an incongruity between and within different Abelmoschus species. Therefore, before transferring any traits, the crossability relationship between the two species needs to be studied [,].
The crossability studies indicate that accessions of cultivated A. esculentus are crossable with the wild Abelmoschus spp. in one direction. Similar results for cross compatibility between A. manihot and A. esculentus were reported by Reddy []. In contrast, Prabu and Warade [] found that both the direct and reciprocal crosses were compatible between A. esculentus and A. manihot. The success of the direct cross and failure of the reciprocal cross in the present investigation might be attributed to the lower ploidy level of the seed parent in the former and the higher ploidy level of the seed parent in the latter []. This is because in interspecific crosses, the embryo and endosperm developed better when the ovulate parent had higher ploidy [].
Various factors, including unilateral incompatibility, interspecific incompatibility, unilateral hybridization, unilateral inhibition, and unidirectional crossability, have been associated with this phenomenon of successful hybridization in only one direction []. The species included in the crossability studies affects the success of interspecific hybridization between domesticated okra and their wild counterparts. Differences in the ploidy level among the okra species used in this study might have contributed to the success or failure of hybridization [,]. Colchicine treatment of the seedlings was effective in overcoming the sterility in the interspecific F1 hybrid plants, resulting in partially fertile amphidiploids.
The putative interspecific hybrids displayed morphological traits, such as flower corolla colour, leaf blade lobbing, and fruit shape, which were intermediate between the two parents. These traits are qualitative and considered as the essential characteristics in identifying a particular plant variety. Qualitative characters are mostly genetically controlled; thus, they are less independent of the environmental response [,].
The interspecific F1s identical to each other exhibited normal growth, flowering, and fruit formation. In addition, the interspecific hybrids exhibited high vigour and manifested traits from both parents. Interspecific hybrid flowers were larger and closely resembled their wild parents with intermediate epicalyx segments. Fruits were reduced in length, with strong hairiness and concave fruit surfaces between ridges due to seedlessness. Pollen sterility, seed sterility and varied pollen diameter confirmed the hybridity of the interspecific hybrids []. The variation in fruit size, flower colour, flower size, leaf shape, fruit hairiness, petiole colour, inside petal base colour and number of primary branches were easily recognizable with a visual appraisal. Most previous authors also relied on morphological characteristics to confirm the hybridity of interspecific crosses of Abelmoschus species [,].
An erect plant type is advantageous to okra since it allows maximum and uniform exposure or distribution of all leaves and other vegetative parts for better interception of sunlight and also results in increased yields []. Larger diversity in fruit size, shape, and length was observed among parents and crosses. Adeniji and Aremu [] reported greater viability for these traits.
Among the three interspecific hybrids, A. Anamika × A. manihot was highly vigorous in terms of the plant height, number of primary branches and fruit yield per plant. A vigorous hybrid between A. esculentus and A. manihot was obtained by Sureshbabu []. Even though the hybrid plants had normal flowering and visible fruit set, there were abortive seeds (rudimentary ovules) when the mature, dried pods were unfurled. The interspecific hybrid plants were fully sterile. The sterility in interspecific F1 hybrids may be due to irregular meiosis []. A common postzygotic reproductive barrier between species or subspecies is hybrid sterility. This effect provides the initial force of genetic differentiation and speciation, and it represents a major barrier to the effective use of inter-subspecific genetic resources in many crops, including okra.
Chromosome homology, which is primarily evaluated by the frequency of bivalent formation, determines the reproductive behaviour in F1s. Meshram and Dhapake [] reported that meiosis was abnormal in F1 between A. esculentus and A. tetraphyllus and it showed an average of 37 bivalent and 55 univalents at metaphase I. Sterility in the interspecific hybrid can be attributed to this abnormal meiotic behaviour. Reddy [] obtained a fertile plant from a colchicine treated sterile F1 hybrid between A. esculentus and A. manihot subsp. tetraphyllus. Similarly, Patel et al. [] obtained partially fertile colchiploids of the cross A. esculentus × A. tetraphyllus.
Pollen viability is normally estimated by in vitro germination because the in vivo technique is laborious and time consuming. The true indicator of pollen viability commonly used is in vitro pollen germination []. Pollen grains of the parental species were highly fertile with uniform diameter when compared to F1s with partial sterility and varied pollen diameter []. It is relatively normal for well-established plant species to have high pollen fertility due to normal meiosis [,]. Plants treated with colchicine exhibited reduced height and altered leaf shape. A similar effect of colchicine was recorded by Wright [] and Kerr [], who stated that induced amphidiploids seemed to grow more slowly and growth abnormalities were the first indication of successful colchicine treatment.
Heterosis is a phenomenon where the phenotype of F1 hybrids is superior to that of their parents []. The development of high-yielding and stable okra genotypes based on interspecific hybridization requires knowledge of heterotic effects occurring in the F1 generation. A higher magnitude of heterosis was observed in all the three interspecific crosses for most yield traits in both positive and negative directions []. The degree of heterosis would depend upon the genetic diversity of the parents. It is assumed that if two parents are more genetically diverse, the higher the extent of heterosis []. An increase in the genetic distance between the parents increases the heterosis potential by decreasing the genetic and phenotypic stability []. Although differences were observed among the interspecific crosses of different wild species, hybrids were generally vigorous, displaying greater heterosis in positive and negative directions for most of the traits [,]. Fruit length was shorter in all the interspecific hybrids than in the cultivated Abelmoschus, exhibiting negative heterosis over the mid and better parent. For canning and pickling, smaller fruits are preferred nowadays [].
Additionally, the results showed that pre-fertilization barriers such as the non-germination of pollen grains could hinder the hybridization of okra species, which would result in the failure of fruit set as observed []. One potential mechanism for plant diversification is interspecific hybridization. Hybridization, including wild and cultivated species, has been used to transfer genetic material to crops []. In India, interspecific hybridization had been followed in the development of varieties, viz., Parbhani Kranti [], Punjab-7 [] and ‘Arka Anamika’ []. Despite the existence of crossability barriers, it is possible to transfer desirable genes from wild species of Abelmoschus such as A. manihot, A. ficulens and A. moschatus. Successful interspecific hybridization in the species A. esculentus will contribute significant variability to the primary gene pool of the species []. The information obtained from this study will contribute to the production of F1 hybrids and subsequent plant generations in developing new cultivars with enhanced quality traits.
5. Conclusions
Knowledge about species variability, crossability relationships and conservation of wild species will assist in developing a breeding strategy for the successful introgression of essential genes into the cultigen pool. The crossability studies of the three wild species, viz., A. manihot, A. ficulens and A. moschatus, with A. esculentus cv. ‘Arka Anamika’ were successful only when the cultivated A. esculentus was used as a seed parent. The germination rates in the two wild species A. ficulens and A. moschatus were low due to hard seed coats. Similarly, in the interspecific F1s the crosses, viz., A. Anamika × A. ficulens and A. Anamika × A. moschatus, lower seed germination was recorded. In contrast, the cross combination A. Anamika × A. manihot recorded a higher seed germination rate of 92%. The higher germination rate in the wild species was achieved by scarification. Morphological characterization revealed that the interspecific hybrids were highly vigorous, erect growing and had profuse branching compared with the parental species with greater alliance towards the wild parent.
The interspecific hybrids presented lower pollen viability and germination with varied pollen diameters than their parents. The sterility in F1 hybrids suggests the existence of barriers to the introgression of genes from wild species. The sterility in the interspecific hybrids was successfully overcome by treating the seedlings with colchicine, resulting in fertile amphidiploids. The A. Anamika × A. manihot interspecific hybrid recorded positive heterosis over better parent and mid parent for the important yield traits, viz., fruit yield per plant, number of fruits per plant and number of primary branches per plant. Given the adaptation of wild species to different biotic and abiotic stresses. The information obtained in the study on crossability relationships, phenotypic characterization and heterosis will help the breeders in the development of prebreeding material. The wild species A. manihot used in this study can be used as a source to establish prebreeding material in okra with resistance to okra yellow vein mosaic virus. Wild relatives of okra from India are yet to be explored. Despite the fact that substantial cytogenetic evidence for the evolutionary history of the okra has been accumulated, several hybrid combinations are still to be attempted and evaluated for breeding needs or genomic relationships. In this regard, advanced molecular biological tools and techniques, viz., RNA-Sequencing, TILLING, and reverse genetics, will allow for more in-depth study of wild okra cultivars to overcome diverse biotic challenges.
Author Contributions
Conceptualization, B.M.D., N.S. and S.S.; methodology, B.M.D., L.D.; K.M.S. and N.S.; software, N.S. and S.S.; formal analysis, N.S., B.M.D., A.A., H.O.E., A.M.E.-S.; investigation, N.S.; data curation, N.S.; writing—original draft preparation, N.S., B.M.D., A.M.E.-S., M.M.A., S.A., H.S., A.A., H.O.E.; writing—review and editing, S.S., A.M.E.-S., M.M.A., S.A., H.S., A.A., H.O.E.; visualization, N.S.; supervision, B.M.D. and S.S.; project. All authors have read and agreed to the published version of the manuscript.
Funding
The current work was funded by Taif University Researchers Supporting Project number (TURSP-2020/75), Taif University, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors are thankful to the Director of Research, KSNUAHS Shivamogga for providing research facilities. The first author is also thankful to ICAR for providing a Junior/Senior Research Fellowship for the present studies.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sutar, S.P.; Patil, P.; Aitawade, M.; John, J.; Malik, S.; Rao, S.; Yadav, S.; Bhat, K.V. A new species of Abelmoschus medik. (Malvaceae) from Chhattisgarh, India. Genet. Resour. Crop Evol. 2013, 60, 1953–1958. [Google Scholar] [CrossRef]
- Liu, Y.; Qi, J.; Luo, J.; Qin, W.; Luo, Q.; Zhang, Q.; Wu, D.; Lin, D.; Li, S.; Dong, H.; et al. Okra in Food Field: Nutritional Value, Health Benefits and Effects of Processing Methods on Quality. Food Rev. Int. 2021, 37, 67–90. [Google Scholar] [CrossRef]
- Kumar, S.; Dagnoko, S.; Haougui, A.; Ratnadass, A.; Pasternak, D.; Kouame, C. Okra (Abelmoschus spp.) in West and Central Africa: Potential and progress on its improvement. Afr. J. Agric. Res. 2010, 5, 3590–3598. Available online: http://oar.icrisat.org/id/eprint/168 (accessed on 14 May 2022).
- Verma, A.; Sood, S.; Singh, Y. Combining ability studies for yield and contributing traits in okra (Abelmoschus esculentus L. Moench). J. Appl. Nat. Sci. 2016, 8, 1594–1598. [Google Scholar] [CrossRef]
- Kumar, A.; Verma, R.B.; Solankey, S.S.; Adarsh, A. Appraisal of okra (Abelmoschus esculentus L. Moench) genotypes for yield and yellow vein mosaic virus incidence. Indian Phytopathol. 2015, 68, 201–206. [Google Scholar]
- Reddy, M.T.; Pandravada, S.R.; Sivaraj, N.; Sunil, N.; Dikshit, N.; Kamala, V. Backyard farming of okra (Abelmoschus esculentus L. Moench) in traditional agricultural landscapes of Adilabad district, Telangana. India J. Glob. Agric. Ecol. 2016, 6, 147–161. [Google Scholar]
- Sanwal, K.; Singh, M.; Singh, B.; Naik, P.S. Resistance to Yellow Vein Mosaic Virus and Okra Enation Leaf Curl Virus: Challenges and future strategies. Curr. Sci. 2014, 106, 1470–1471. [Google Scholar]
- Chakraborty, S.; Pandey, P.K.; Singh, B. Okra enation leaf curl disease-a threat to cultivation of Okra (Abelmoschus esculentus (L.) Moench). Veg. Sci. 1997, 24, 52–54. [Google Scholar]
- Senevirathna, H.M.S.I.; Wasala, S.K.; Senanayake, D.M.J.B.; Weerasekara, D.; Wickamasinghe, H.A.M.; Deepa, P.K.G.A. Characterization and Detection of Yellow Vein Disease of Okra(Abelmoschus esculentus (L.) Moench) in Sri Lanka. Trop. Agric. Res. 2016, 27, 360–369. [Google Scholar] [CrossRef][Green Version]
- Hajjar, R.; Hodgkin, T. The use of wild relatives in crop improvement: A survey of developments over the last 20 years. Euphytica 2007, 156, 1–13. [Google Scholar] [CrossRef]
- Kumari, M.; Naresh, P.; Singh, H.S. Utilization of wild species for biotic stress breeding in okra: A review. Adv. Plants Agric. Res. 2019, 9, 337–340. [Google Scholar] [CrossRef]
- Prabu, T.; Warade, S.D. Crossability studies in genus Abelmoschus. Veg. Sci. 2013, 40, 11–16. [Google Scholar]
- Vredebregt, J.H. Taxonomic and ecological observations on species of Abelmoschus Medik. Report of an International Workshop on Okra Genetic Resources. NBPGR Int. Crop Netw. Ser. 1991, 5, 69–76. [Google Scholar]
- Thakur, M.R.; Arora, S.K. Okra. In Vegetable Crops in India; Bose, T.K., Somm, M.G., Eds.; Naya Prokash: Calcutta, India, 1986; pp. 606–622. [Google Scholar]
- Singh, B.; Rai, M.; Kalloo, G.; Satpathy, S.; Pandey, K.K. Wild taxa of okra (Abelmoschus species): Reservoir of genes for resistance to biotic stresses. Acta Hortic. 2007, 752, 323–332. [Google Scholar] [CrossRef]
- Lowry, D.B.; Modliszewski, J.L.; Wright, K.M.; Wu, C.A.; Willis, J.H. Review. The strength and genetic basis of reproductive isolation barriers in flowering plants. Biol. Sci. 2008, 363, 3009–3021. [Google Scholar] [CrossRef]
- Kumar, S.; Reddy, M.T. Heterotic potential of Single cross hybrids in okra (Abelmoschus esculentus (L.) Moench). J. Glob. Agric. Ecol. 2016, 4, 45–66. [Google Scholar]
- Bowley, S.R.; Taylor, N.L. Introgressive hybridization. In CRC Handbook of Plant Science in Agriculture; Christie, B.R., Ed.; CRC Press: Boca Raton, FL, USA, 1987; Volume 1, pp. 23–59. Available online: https://www.jstor.org/stable/24084997 (accessed on 18 May 2022).
- Rao, N.N. Barriers to hybridization between Solanum melongena and some other species of Solanum. In The Biology and Taxonomy of the Solanaceae; Linnean Society Symposium Series; Hawker, J.G., Lester, R.N., Skelding, A.D., Eds.; FAO: Rome, Italy, 1979; pp. 605–615. [Google Scholar]
- Patil, P.; Malik, S.K.; Negi, K.S.; John, J.; Yadav, S.; Chaudhari, G.; Bhat, K.V. Pollen germination characteristics, pollen-pistil interaction and reproductive behaviour in interspecific crosses among Abelmoschus esculentus Moench and its wild relatives. Grana 2013, 52, 1–14. [Google Scholar] [CrossRef]
- Mendiburu, F.; Yaseen, M. Agricolae: Statistical Procedures for Agricultural Research; R Package Version 1.4.0; CRAN: Lima, Peru, 2020. [Google Scholar]
- Mahajan, R.; Sapra, R.; Umesh, S.; Singh, M.; Sharma, G. Minimal Descriptors (for Characterization and Evaluation) of Agri-Horticultural Crops (Part I); National Bureau of Plant Genetic Resources: New Delhi, India, 2000; p. 230. [Google Scholar]
- Kaul, G.L.; Swamy, K.M.R.; Singh, D.P.; Dhankar, B.S.; Pandey, S.K.; Rai, M.; Chakrabaty, S.K. Protection of Plant Varieties and Farmers’ Rights Authority. In Guidelines for the Conduct of Test for Distinctiveness, Uniformity and Stability on Okra/Lady’s Finger (Abelmoschus moschatus (L.) Moench; Chandu Press: Delhi, India, 2009. [Google Scholar]
- Arunachalam, V. The fallacy behind the use of modified line x tester design. Indian J. Genet. 1974, 34, 200–207. [Google Scholar]
- Patil, N.; Gangavathi, L.R. Gpbstat: Comprehensive Statistical Analysis of Plant Breeding Experiment; R Package Version 0.3.5; CRAN: Karnataka, India, 2021; Available online: https://cran.r-project.org/package=gpbStat (accessed on 18 May 2022).
- Reddy, M.T. Crsossability behaviour and fertility restoration through colchiploidy in interspecific hybrids of Abelmoschus esculentus × Abelmoschus manihot subsp. tetraphyllus. Int. J. Plant Sci. 2015, 4, 172–181. [Google Scholar]
- Reddy, M.T. Genetic Diversity, Heterosis Combining Ability and Stability in Okra (Abelmoschus esculentus (L.) Moench). Ph.D. Thesis, Ranga Agricultural University, Rajendranagar, Hyderabad, Andhra Pradesh, India, 2010. [Google Scholar]
- Rajamony, L.; Chandran, M.; Rajmohan, K. In vitro embryo rescue of interspecific crosses for transferring virus resistance in Okra (Abelmoschus esculentus (L.) Moench). Acta Hortic. 2006, 725, 235–240. [Google Scholar] [CrossRef]
- Jatkar, M.A.; Prabu, T.; Warade, S.D. Induction of colchiploidy in sterile interspecific okra F1 hybrids. Crop Res. 2007, 34, 133–136. [Google Scholar]
- Martin, J.; Rubiales, D.; Sillero, J.C.; Prats, E. Identification and characterization of sources of resistance in Avena sativa, A. byzantinea and A. strigosa germplasm against a pathotype of Puccinia coronate f.sp. avenae with virulence against the Pc94 resistance gene. Plant Pathol. 2012, 61, 315–322. [Google Scholar] [CrossRef]
- Shetty, A.A.; Singh, J.P.; Singh, D. Resistance to yellow vein mosaic virus in okra: A review. Biol. Agric. Hortic. Int. J. Sustain. Prod. Syst. 2013, 29, 159–164. [Google Scholar] [CrossRef]
- Amiteye, S.; Amitaaba, T.; Akama, C.; Amoatey, H.M. Hybridization Studies of Okra (Abelmoschus spp. (L.) Moench) Accessions. Int. J. Biotechnol. Trends Technol. 2019, 9, 42–47. [Google Scholar] [CrossRef]
- Kohler, C.; Mittelsten, S.O.; Erilova, A. The impact of the triploid block on the origin and evolution of polyploid plants. Trends Genet. 2010, 26, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.A.; Nijs, T.D.P.; Peloquin, S.J.; Hanneman, R.E. The significance of genic balance to endosperm development in interspecific crosses. Theor. Appl. Genet. 1980, 57, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Afful, N.T.; Nyadanu, D.; Akromah, R.; Amoatey, H.M.; Annor, C.; Diawouh, R.G. Evaluation of crossability studies between selected eggplant accessions with wild relatives S. torvum, S. anguivi and S. aethopicum (Shum group). J. Plant. Breed Crop Sci. 2018, 10, 1–12. [Google Scholar] [CrossRef]
- Safdar, M. Impacts of genome size and ploidy level on the morphological traits of Okra. J. Plant Physiol. Pathol. 2018, 6, 30. [Google Scholar] [CrossRef]
- Salameh, M.N. Flow cytometric analysis of nuclear DNA between okra landraces (Abelmoschus esculentus L.). Am. J. Agric. Biol. Sci. 2014, 9, 245–250. [Google Scholar] [CrossRef]
- Sinha, A.K.; Mishra, P.K. Agro-morphological characterization and morphology based genetic diversity analysis of rice variety (Oryza sativa) of Bankura district of West Bengal. Int. J. Curr. Res. 2013, 5, 2764–2769. [Google Scholar]
- Bashar, A.; Jahan, N.; Fakhuruddin, A.A.; Hossain, M.K.; Alam, N. Morphological and phytochemical variation in eggplant (Solanum melongena L.). Pharma. Sci. Monit. 2015, 6, 1–11. [Google Scholar]
- Akhond, M.A.Y.; Molla, M.A.H.; Islam, M.O.; Ali, M. Cross compatibility between Abelmoschus esculentus and A. moschatus. Euphytica 2000, 114, 175–180. [Google Scholar] [CrossRef]
- Sekyere, D.O.; Akromah, R.; Nyamah, E.; Brenya, E.; Yeboah, S. Characterization of okra (Abelmoschus spp. L.) germplasm based on morphological characters in Ghana. J. Plant Breed Crop Sci. 2011, 3, 367–378. [Google Scholar] [CrossRef]
- Adeniji, O.T.; Aremu, C.O. Interrelationships among Characters and Path Analysis for Pod Yield Components in West African Okra (Abelmoschus caillei (A. Chev) Stevels. J. Agron. 2007, 6, 162–166. [Google Scholar] [CrossRef]
- Sureshbabu, K.V. Cytogenetic Studies in Okra (Abelmoschus esculentus (L.) Moench). Ph.D. Thesis, University of Agricultural Sciences, Bangalore, India, 1987. [Google Scholar]
- Lukhtanov, V.A.; Dinca, V.; Friberg, M.; Vila, R.; Wiklund, C. Incomplete Sterility of Chromosomal Hybrids: Implications for Karyotype Evolution and Homoploid Hybrid Speciation. Front. Genet. 2020, 11, 583827. [Google Scholar] [CrossRef]
- Meshram, L.D.; Dhapake, D.K. Cytogenetical studies on an interspecific hybrid between A. esculentus (L.) Moench × A. tetraphyllus. In Proceedings of the Fourth International SABRAO Congress, Kulalampur, Indonesia, 4–8 May 1981. [Google Scholar]
- Patel, B.N.; Hegde, G.K.; Manu, T.G. Interspecific hybridization as a way of resistance transfer against viruses in okra: Hindrances and way forward. Plant Genet. Resour. 2021, 19, 357–362. [Google Scholar] [CrossRef]
- Jayaprakash, P. Pollen Germination in vitro. In Pollination in Plants; Mokwala, P.W., Ed.; IntechOpen: Vienna, Austria, 2018; Volume 75, pp. 36–50. [Google Scholar]
- Tyagi, A.P.; Dass, C.R.; Nathan, S.; Racule, T.; Lakhan, S. Pollen fertility status in some exotic flora of Fiji. South Pac. J. Nat. Sci. 1995, 14, 211–222. [Google Scholar]
- Frizo, P.; Brammer, S.P.; Deuner, C.C.; Chechi, A.; Lima, M.I.P.; Scheeren, P.L. Genetic stability in interspecific hybridizations of wheat populations determined by meiotic index and pollen viability. Biotemas 2021, 34, 1–9. [Google Scholar] [CrossRef]
- Wright, J.W. Introduction to Forest Genetics; Academic Press: New York, NY, USA, 1976. [Google Scholar]
- Kerr, A. Tetraploidy Conversions: An Easy and Effective Method of Colchicine Method. 2001. Available online: http://members.tripods.com/~h_syriacus/tetraploidy (accessed on 18 May 2022).
- Shull, G.H. What Is “Heterosis”? Genetics 1948, 33, 439–446. [Google Scholar] [CrossRef]
- Dhillon, T.S.; Sharma, B.R. Interspecific hybridization in okra (Abelmoschus species). Genet. Agr. 1982, 36, 247–255. [Google Scholar]
- Labroo, M.R.; Studer, A.J.; Rutkoski, J.E. Heterosis and Hybrid Crop Breeding: A Multidisciplinary Review. Front. Genet. 2021, 12, 643761. [Google Scholar] [CrossRef]
- Tomkowiak, A.; Bocianowski, J.; Kwiatek, M.; Kowalczewski, L.P. Dependence of the heterosis effect on genetic distance, determined using various molecular markers. Open Life Sci. 2020, 15, 1–11. [Google Scholar] [CrossRef]
- Olivera, D.F.; Mugridge, A.; Chaves, A.R.; Mascheroni, R.H.; Vina, S.Z. Quality Attributes of Okra (Abelmoschus esculentus L. Moench) Pods as Affected by Cultivar and Fruit Size. J. Food Res. 2012, 1, 224–235. [Google Scholar] [CrossRef]
- Jambhale, N.D.; Nerkar, Y.S. Parbhani Kranti, a yellow vein mosaic-resistant Okra. Hort. Sci. 1986, 21, 1470–1471. [Google Scholar]
- Thakur, M.R.; Arora, S.K. Punjab-7, a virus resistant variety of Okra. Prog. Farming 1988, 24, 13. [Google Scholar]
- Dutta, O.P. Breeding of Okra for resistance to yellow vein mosaic virus and okra leaf curl virus. Ann. Rep. 1984, 84, 43. [Google Scholar]
- Yamuna, M.; Sureshbabu, K.V.; George, T.E.; Prasanna, K.P.; Mathew, S.K.; Krishnan, S. Evaluation of promising interspecific hybrid derivatives of okra (Abelmoschus esculentus (L.) Moench). Veg. Sci. 2013, 40, 99–101. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).