Characterization of the Berry Quality Traits and Metabolites of ‘Beimei’ Interspecific Hybrid Wine Grapes during Berry Development and Winemaking
Abstract
:1. Introduction
2. Materials and Methods
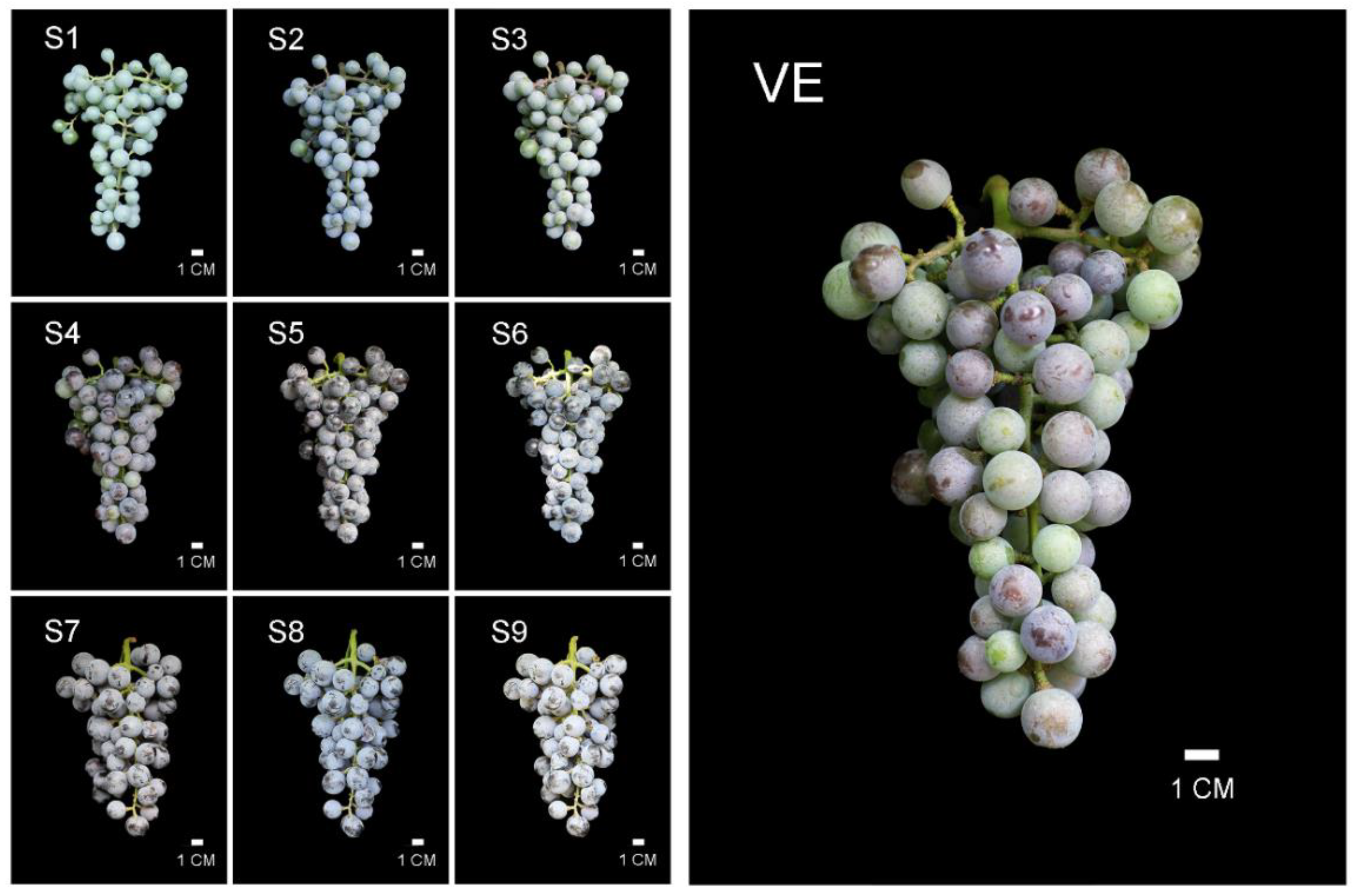
2.1. Plants and Growth Conditions
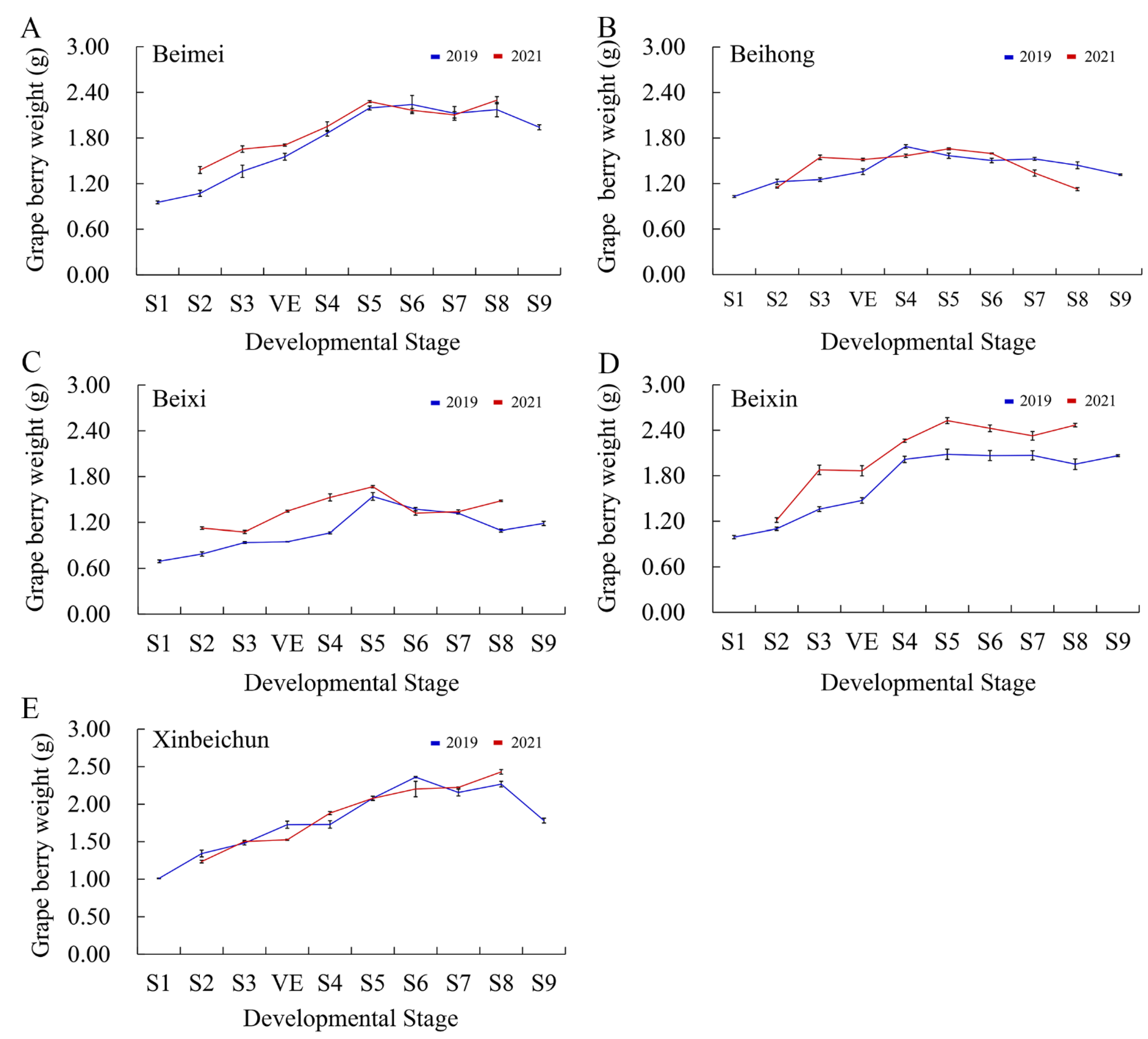
2.2. Determination of Grape Berry Weight
2.3. Determination of Water Content
2.4. Determination of Soluble Solids
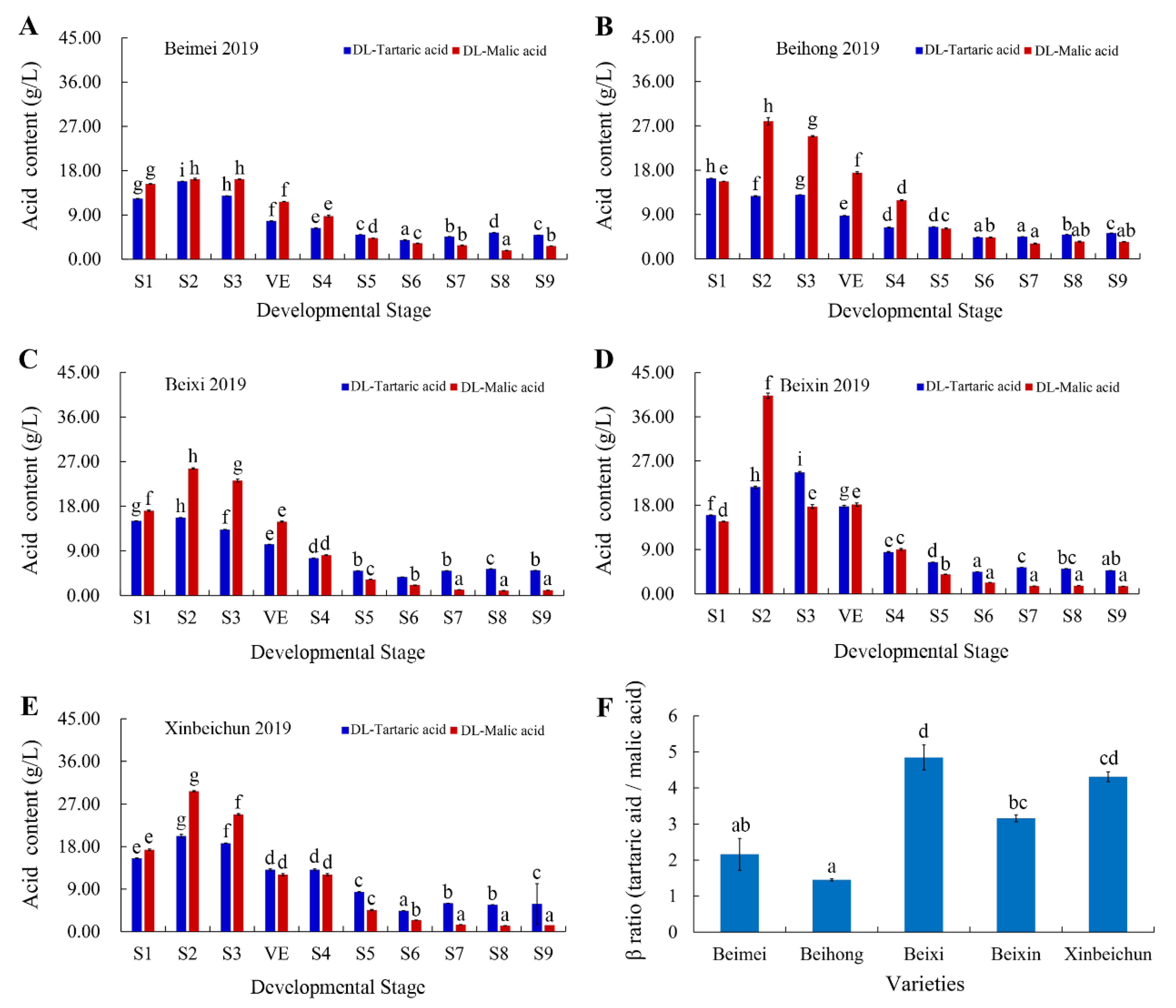
2.5. Extraction and Determination of Sugar and Acid
2.6. Winemaking and Sampling of Beimei Dry Red Wine
2.7. Analysis of the General Parameters of Beimei Red Wine
2.8. Determination of Resveratrol
2.9. Determination of Procyanidins
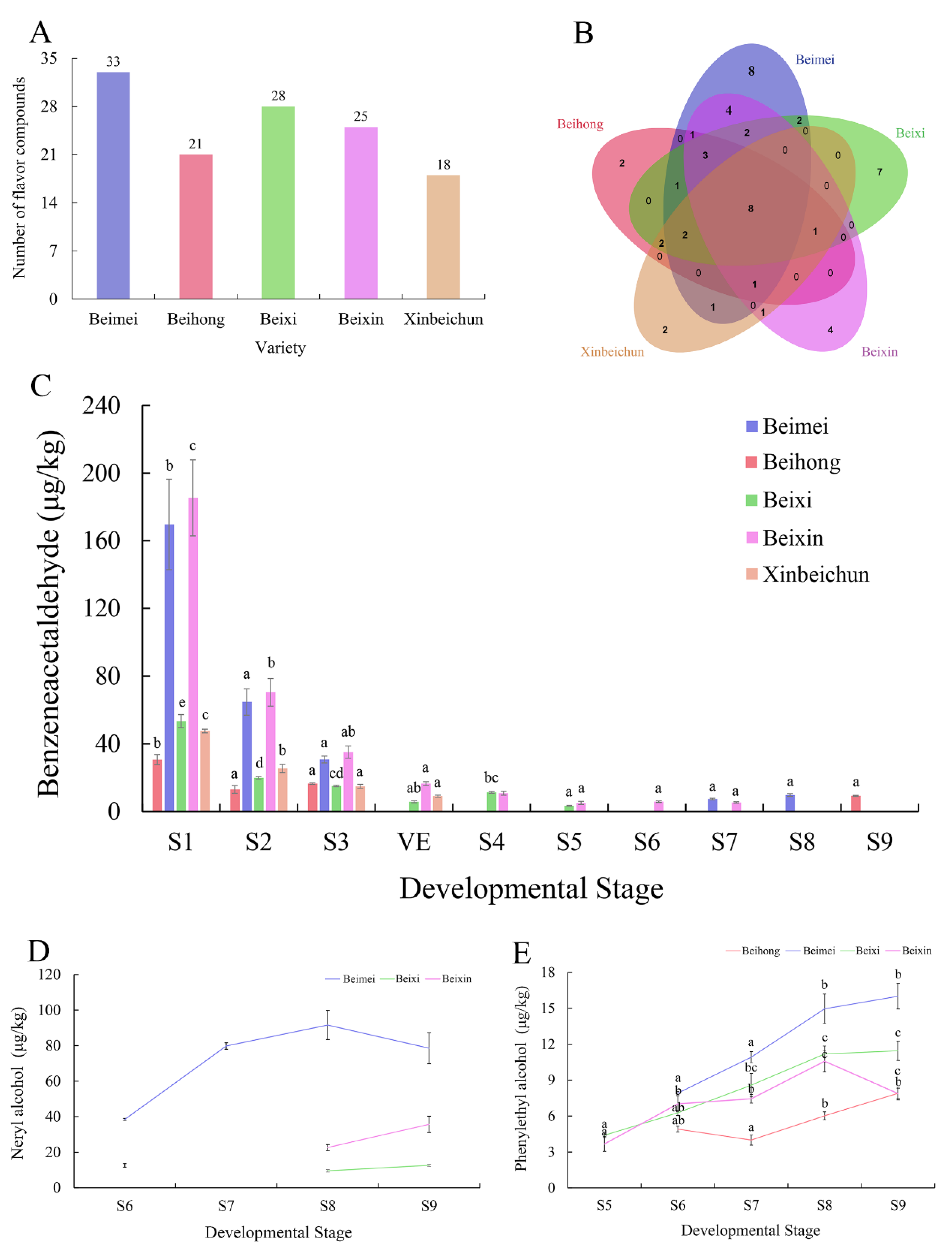
2.10. Extraction and Determination of Flavor
3. Results
3.1. Weight Changes of “Bei” Varieties’ Berries
3.2. Water Content Changes of “Bei” Varieties
3.3. Detection of Soluble Solids of “Bei” Varieties
3.4. Analysis of Sugars in “Bei” Varieties
3.5. Analysis of Acids in “Bei” Varieties
3.6. Analysis of “Bei” Varieties for the Flavor Compounds
3.7. Evaluation of Beimei Red Wine
3.8. Changes of Metabolites during Beimei Red Winemaking
3.9. Flavor Compounds of Beimei Red Wine
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A




| Variety | Compounds | CAS | Flavour | S1 (μg/kg) | S2 (μg/kg) | S3 (μg/kg) | VE (μg/kg) | S4 (μg/kg) | S5 (μg/kg) | S6 (μg/kg) | S7 (μg/kg) | S8 (μg/kg) | S9 (μg/kg) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beimei | 3-Hexen-1-ol, acetate, (Z)- | 003681-71-8 | Grass | 19.21 ± 2.75 a | 33.14 ± 4.59 a | 17.12 ± 0.84 a | ND | ND | ND | ND | ND | ND | ND |
| 2-Hexenal | 000505-57-7 | Grass | 124.96 ± 13.61 a | 139.82 ± 15.98 a | 124.72 ± 8.38 a | 543.70 ± 196.96 b | 616.65 ± 9.79 b | 1326.71 ± 11.00 d | 803.89 ± 9.35 bc | 1251.12 ± 31.86 d | 1055.48 ± 52.50 cd | 599.81 ± 60.18 b | |
| 3-Hexen-1-ol | 000544-12-7 | Grass | 82.06 ± 8.88 a | 109.50 ± 14.98 a | 115.12 ± 5.62 a | ND | ND | ND | ND | ND | ND | ND | |
| Methyl salicylate | 000119-36-8 | Holly Leaf | ND | ND | ND | ND | ND | ND | 3.18 ± 0.07 a | 5.44 ± 0.29 a | 4.72 ± 0.46 a | 14.57 ± 1.55 b | |
| 2-Hexen-1-ol, (Z)- | 000928-95-0 | Apple | ND | 33.03 ± 3.85 a | 61.46 ± 3.32 a | 67.75 ± 11.57 ab | 48.67 ± 0.70 a | 35.23 ± 3.78 a | 150.51 ± 12.43 c | 78.49 ± 4.39 ab | 124.71 ± 34.02 bc | 148.97 ± 13.93 c | |
| 2-Octenal, (E)- | 002548-87-0 | Cucumber | ND | 4.48 ± 0.86 | ND | ND | ND | ND | ND | ND | ND | ND | |
| Geranial | 005392-40-5 | Lemon | ND | ND | ND | ND | ND | ND | 3.27 ± 0.08 a | 4.38 ± 0.21 ab | 5.86 ± 0.79 b | 9.40 ± 0.37 c | |
| Cis-3-Hexenyl hexanoate | 031501-11-8 | Pear | 1.65 ± 0.17 | 2.82 ± 0.37 | ND | ND | ND | ND | ND | ND | ND | ND | |
| Myrcene | 000123-35-3 | Orange | ND | ND | ND | ND | ND | ND | ND | 11.41 ± 0.39 a | 11.96 ± 0.98 a | 19.50 ± 2.08 b | |
| Nonanal | 000124-19-6 | Sweet Orange | 5.23 ± 0.67 ab | 5.72 ± 0.63 ab | 5.14 ± 0.19 ab | 4.75 ± 0.42 a | 6.98 ± 0.16 bc | 5.34 ± 0.19 ab | 8.10 ± 0.23 c | 7.04 ± 0.10 bc | 8.51 ± 0.62 cd | 10.30 ± 0.82 d | |
| Hexanoic acid, ethyl ester | 000123-66-0 | Fruit | 7.93 ± 1.87 | ND | ND | ND | ND | ND | 7.90 ± 0.10 | ND | ND | ND | |
| 1-Hexanol, 2-ethyl- | 000104-76-7 | Special Smell | 7.06 ± 0.79 | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| Benzene acetaldehyde | 000122-78-1 | Hosta Flower | 169.62 ± 26.80 b | 64.80 ± 7.83 a | 30.78 ± 2.02 a | ND | ND | ND | ND | 7.35 ± 0.40 a | 9.75 ± 0.83 a | ND | |
| Caryophyllene | 000087-44-5 | Clove | 4.24 ± 0.75 ab | 3.30 ± 0.12 a | 6.12 ± 0.86 ab | 10.36 ± 2.63 b | 6.41 ± 0.49 ab | ND | ND | ND | ND | ND | |
| Phenylethyl Alcohol | 000060-12-8 | Rose | ND | ND | ND | ND | ND | ND | 7.91 ± 0.14 a | 10.92 ± 0.46 a | 14.96 ± 1.23 b | 16.01 ± 1.07 b | |
| Citronellol | 000106-22-9 | Rose | ND | ND | ND | ND | ND | ND | ND | 5.92 ± 0.10 | ND | 12.96 ± 0.78 | |
| Rose oxide | 016409-43-1 | Rose | 4.69 ± 0.87 | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| Geraniol | 000106-24-1 | Rose | ND | ND | ND | ND | ND | ND | ND | ND | ND | 147.79 ± 13.20 | |
| Neryl alcohol | 000106-25-2 | Rose | ND | ND | ND | ND | ND | ND | 38.48 ± 0.50 a | 79.79 ± 1.87 b | 91.63 ± 8.22 b | 78.56 ± 8.66 b | |
| 2-Hexen-1-ol, acetate, (E)- | 002497-18-9 | Fragrant | ND | 8.67 ± 1.00 | 7.34 ± 0.59 | ND | ND | ND | ND | ND | ND | ND | |
| Benzaldehyde | 000100-52-7 | Fragrant | 12.17 ± 2.26 a | 7.93 ± 4.89 a | 3.81 ± 2.06 a | ND | ND | ND | ND | ND | ND | ND | |
| Beihong | Styrene | 000100-42-5 | Fragrant | ND | ND | ND | ND | ND | ND | ND | ND | 23.81 ± 3.49 | ND |
| Benzyl Alcohol | 000100-51-6 | Fragrant | ND | ND | ND | ND | ND | 4.83 ± 0.24 a | 5.26 ± 0.04 a | 11.19 ± 0.84 b | 16.46 ± 1.34 c | 22.88 ± 1.48 d | |
| 2,4-Hexadienal, (E,E)- | 000142-83-6 | OU | ND | ND | ND | ND | ND | 5.08 ± 0.22 | ND | ND | ND | 10.94 ± 1.05 | |
| 3,7-dimethyl-, (Z)-1,3,6-Octatriene, | 003338-55-4 | OU | ND | ND | ND | ND | ND | ND | ND | 8.14 ± 0.18 | 13.43 ± 1.39 | ND | |
| Geranic acid | 000459-80-3 | OU | ND | ND | ND | ND | ND | ND | 7.04 ± 0.29 a | 9.62 ± 0.30 ab | 11.95 ± 1.15 b | ND | |
| (1S)-(+)-3-Carene | 000498-15-7 | OU | ND | ND | ND | ND | ND | ND | ND | 32.48 ± 0.75 | ND | ND | |
| Cis-citral | 000106-26-3 | OU | ND | ND | ND | ND | ND | ND | ND | ND | ND | 5.13 ± 0.18 | |
| (E)-2-Hexenyl butyrate | 053398-83-7 | OU | ND | 9.83 ± 1.09 | 5.74 ± 0.11 | ND | ND | ND | ND | ND | ND | ND | |
| cis-Linaloloxide | 1000121-97-4 | OU | ND | 5.75 ± 0.74 | ND | ND | ND | ND | ND | ND | ND | ND | |
| Cis-3-Hexenyl butyrate | 016491-36-4 | OU | 5.62 ± 0.77 a | 13.93 ± 1.40 b | 4.74 ± 0.37 a | ND | ND | ND | ND | ND | ND | ND | |
| 1-Hexanol | 000111-27-3 | OU | ND | ND | ND | 127.30 ± 2.26 b | 153.21 ± 2.47 bc | 71.80 ± 2.94 a | 158.30 ± 11.20 bc | 112.37 ± 4.41 ab | 141.38 ± 21.72 b | 191.91 ± 11.54 c | |
| Hexanal | 000066-25-1 | OU | 95.52 ± 11.92 a | 115.75 ± 15.51 a | 112.57 ± 5.32 a | 258.98 ± 65.16 b | 411.64 ± 5.98 c | 743.46 ± 20.36 e | 557.73 ± 8.27 d | 777.44 ± 20.44 e | 698.36 ± 30.15 e | 508.36 ± 45.94 cd | |
| Eucalyptol | 000470-82-6 | Cool | 9.82 ± 2.28 | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| 3-Hexen-1-ol | 000928-96-1 | Grass | 111.35 ± 17.31 ab | 192.66 ± 33.45 c | 175.37 ± 12.56 bc | 131.18 ± 4.49 abc | 153.21 ± 2.47 bc | 71.80 ± 2.94 a | ND | ND | ND | ND | |
| 2-Hexenal | 000505-57-7 | Grass | 177.26 ± 18.19 ab | 94.45 ± 17.18 a | 164.16 ± 3.88 ab | 439.26 ± 164.55 bc | 608.06 ± 1.21 c | 1326.71 ± 11.00 d | 1122.80 ± 159.39 d | 594.25 ± 11.62 c | 598.54 ± 31.83 c | 341.46 ± 17.87 abc | |
| Cis-3-Hexenyl hexanoate | 031501-11-8 | Pear | 17.49 ± 12.50 | 11.05 ± 1.84 | ND | ND | ND | ND | ND | ND | ND | ND | |
| Butanoic acid, hexyl ester | 002639-63-6 | Sweet Fruit | ND | 10.02 ± 1.73 | 4.76 ± 0.29 | ND | ND | ND | ND | ND | ND | ND | |
| Hexanoic acid, ethyl ester | 000123-66-0 | Fruit | ND | ND | ND | ND | ND | ND | ND | ND | ND | 18.30 ± 2.35 | |
| Nonanal | 000124-19-6 | Sweet Orange | 4.80 ± 0.47 ab | 5.07 ± 0.75 b | 6.04 ± 0.33 bc | 4.69 ± 0.36 ab | 6.98 ± 0.16 c | 5.34 ± 0.19 b | 5.284 ± 0.24 b | 3.36 ± 0.08 a | 4.40 ± 0.24 ab | ND | |
| 2-Hexen-1-ol, (E)- | 000928-95-0 | Apple | ND | ND | 31.42 ± 1.89 a | 57.91 ± 1.76 abc | 48.67 ± 0.70 ab | 35.23 ± 3.78 a | 73.55 ± 25.94 abc | 101.41 ± 11.68 c | 83.74 ± 4.63 bc | 43.18 ± 4.26 ab | |
| Benzene acetaldehyde | 000122-78-1 | Hosta flower | 30.64 ± 3.00 b | 13.03 ± 2.27 a | 16.48 ± 0.42 a | ND | ND | ND | ND | ND | ND | 9.21 ± 0.36 | |
| Caryophyllene | 000087-44-5 | Clove | 17.39 ± 7.27 a | 49.96 ± 10.69 b | 35.77 ± 2.82 ab | 18.70 ± 5.75 a | 6.41 ± 0.49 a | ND | ND | ND | ND | ND | |
| Geraniol | 000106-24-1 | Rose | ND | ND | ND | ND | ND | ND | ND | ND | ND | 2.54 ± 0.04 | |
| Benzaldehyde | 000100-52-7 | Fragrant | ND | ND | ND | ND | ND | ND | ND | ND | ND | 4.92 ± 0.23 | |
| Phenylethyl Alcohol | 000060-12-8 | Rose | ND | ND | ND | ND | ND | ND | 4.91 ± 0.25 ab | 3.99 ± 0.42 a | 6.02 ± 0.32 b | 7.90 ± 0.43 b | |
| Benzyl Alcohol | 000100-51-6 | Fragrant | ND | ND | ND | ND | ND | 4.83 ± 0.24 a | 13.04 ± 4.47 ab | 19.12 ± 2.47 b | 18.65 ± 0.88 b | 23.68 ± 1.44 b | |
| Beixi | Cis-3-Hexenyl butyrate | 016491-36-4 | OU | 12.66 ± 5.08 a | 42.65 ± 7.18 b | 20.54 ± 1.28 ab | ND | ND | ND | ND | ND | ND | ND |
| (E)-2-Hexenyl butyrate | 053398-83-7 | OU | ND | 5.07 ± 0.82 | 4.57 ± 0.22 | ND | ND | ND | ND | ND | ND | ND | |
| 2,4-Hexadienal, (E,E)- | 000142-83-6 | OU | ND | ND | ND | ND | ND | 5.08 ± 0.22 | ND | ND | ND | ND | |
| Hexanoic acid | 000142-62-1 | OU | ND | ND | ND | ND | ND | ND | ND | ND | 4.54 ± 0.68 | ND | |
| 1-Octanol | 000111-87-5 | OU | ND | ND | ND | ND | ND | ND | ND | ND | ND | 3.08 ± 0.14 | |
| 1-Hexanol | 000111-27-3 | OU | ND | ND | ND | ND | ND | ND | 121.18 ± 27.60 a | 193.26 ± 29.19 a | 165.36 ± 9.90 a | 203.44 ± 25.72 a | |
| Hexanal | 000066-25-1 | OU | 158.98 ± 20.33 a | 118.24 ± 23.87 a | 166.29 ± 12.43 a | 266.18 ± 58.03 ab | 411.64 ± 5.98 b | 743.46 ± 20.36 c | 716.71 ± 7.36 c | 664.96 ± 34.61 c | 741.64 ± 32.69 c | 751.46 ± 83.79 c | |
| 3-Hexen-1-ol, (Z)- | 000928-96-1 | Grass | 75.58 ± 3.76 | 126.97 ± 7.91 | ND | ND | ND | ND | ND | ND | ND | ND | |
| 2-Hexenal, (E)- | 000505-57-7 | Grass | 136.88 ± 8.14 a | 194.57 ± 7.98 a | 83.70 ± 0.90 a | 339.99 ± 23.46 b | 481.48 ± 21.66 c | 1265.05 ± 21.57 e | 806.21 ± 43.63 d | 822.22 ± 66.70 d | 190.93 ± 20.29 a | 428.64 ± 29.85 bc | |
| Eucalyptol | 000470-82-6 | Cool | 34.70 ± 2.15 | 13.52 ± 1.25 | ND | ND | ND | ND | ND | ND | ND | ND | |
| Methyl salicylate | 000119-36-8 | Holly Leaf | ND | ND | ND | ND | ND | 4.14 ± 0.21 | ND | ND | ND | ND | |
| 2-Hexen-1-ol, (E)- | 000928-95-0 | Apple | ND | ND | ND | 115.37 ± 6.39 ab | 80.88 ± 3.30 ab | 39.22 ± 0.94 a | 50.76 ± 5.87 a | 62.70 ± 18.51 a | 677.90 ± 50.50 c | 170.28 ± 10.21 b | |
| g-Terpinene | 000099-85-4 | Orange | 8.23 ± 0.74 | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| Nonanal | 000124-19-6 | Sweet Orange | 6.76 ± 0.12 cd | 7.09 ± 0.52 d | 5.61 ± 0.34 bcd | 6.27 ± 0.29 bcd | 3.87 ± 0.09 a | 5.02 ± 0.25 ab | 5.95 ± 0.52 bcd | 5.66 ± 0.42 bcd | 5.22 ± 0.44 abc | 5.65 ± 0.20 bcd | |
| Ethyl hexanoate | 000123-66-0 | Fruit | ND | ND | ND | ND | 10.58 ± 0.50 | ND | ND | ND | ND | ND | |
| Hexyl butyrate | 002639-63-6 | Sweet Fruit | ND | 5.88 ± 0.37 | ND | 3.47 ± 0.31 | ND | ND | ND | ND | ND | ND | |
| Benzaldehyde | 000100-52-7 | Bitter Almond | 6.37 ± 2.72 | 2.74 ± 0.12 | ND | ND | ND | ND | ND | ND | ND | ND | |
| Phenylacetaldehyde | 000122-78-1 | Hosta Flower | 53.40 ± 3.88 e | 19.86 ± 0.74 d | 15.07 ± 0.44 cd | 5.71 ± 0.59 ab | 11.28 ± 0.48 bc | 3.36 ± 0.18 a | ND | ND | ND | ND | |
| Phenylethyl Alcohol | 000060-12-8 | Rose | ND | ND | ND | ND | ND | 4.39 ± 0.19 a | 6.27 ± 0.23 ab | 8.58 ± 0.98 bc | 11.18 ± 0.67 c | 11.45 ± 0.80 c | |
| Citronellol | 000106-22-9 | Rose | 2.60 ± 0.11 a | 3.24 ± 0.08 ab | 4.17 ± 0.14 ab | 3.94 ± 0.25 ab | 4.68 ± 0.17 b | 9.39 ± 0.50 d | 10.26 ± 0.97 de | 11.64 ± 0.43 e | 6.93 ± 0.51 c | 11.84 ± 0.37 e | |
| Neryl alcohol | 000106-25-2 | Rose | ND | ND | ND | ND | ND | ND | ND | ND | 9.52 ± 0.69 | 12.58 ± 0.72 | |
| Geraniol | 000106-24-1 | Rose | 2.39 ± 0.19 a | ND | 2.44 ± 0.16 a | ND | 2.38 ± 0.10 a | ND | 21.66 ± 1.64 bc | 26.98 ± 3.34 cd | 19.41 ± 1.44 b | 33.37 ± 1.80 d | |
| o-Xylene | 000095-47-6 | Fragrant | ND | ND | 40.57 ± 3.10 | ND | ND | ND | ND | ND | ND | ND | |
| Benzyl Alcohol | 000100-51-6 | Fragrant | ND | ND | ND | ND | ND | 2.69 ± 0.18 a | 5.44 ± 0.05 a | 8.56 ± 2.22 a | 7.47 ± 0.53 a | 16.43 ± 1.53 b | |
| Methyl geranate | 002349-14-6 | OU | ND | ND | ND | ND | ND | ND | 2.21 ± 0.22 a | 3.40 ± 0.15 a | 6.48 ± 0.44 b | 6.82 ± 0.27 b | |
| 3-Hexenyl hexanoate | 084434-19-5 | OU | ND | 2.45 ± 0.07 | ND | ND | ND | ND | ND | ND | ND | ND | |
| Beixin | Geranic acid | 000459-80-3 | OU | ND | ND | ND | ND | ND | ND | 4.44 ± 0.51 | 5.37 ± 0.15 | ND | ND |
| Octylbutyrate | 000110-39-4 | OU | ND | ND | 2.90 ± 0.19 | ND | ND | ND | ND | ND | ND | ND | |
| Cis-3-Hexenyl butyrate | 016491-36-4 | OU | 9.59 ± 0.20 a | 18.97 ± 1.28 b | 11.10 ± 0.14 a | ND | ND | ND | ND | ND | ND | ND | |
| Trans-2-Hexenyl butyrate | 053398-83-7 | OU | ND | 10.87 ± 0.62 b | 5.96 ± 0.17 a | 10.52 ± 0.25 b | ND | ND | ND | ND | ND | ND | |
| 2,4-Hexadienal, (E,E)- | 000142-83-6 | OU | ND | ND | ND | ND | ND | 6.19 ± 0.18 | 5.07 ± 0.40 | ND | ND | ND | |
| Hexyl acetate | 000142-92-7 | OU | ND | ND | ND | ND | 4.91 ± 0.13 | ND | ND | ND | ND | ND | |
| m-isopropyltoluene | 000535-77-3 | OU | 33.51 ± 1.55 d | 26.51 ± 1.17 c | 13.32 ± 0.71 b | 11.11 ± 0.88 ab | 8.24 ± 0.44 a | ND | ND | ND | ND | ND | |
| 1-Hexanol | 000111-27-3 | OU | ND | ND | ND | 136.19 ± 7.84 ab | 98.32 ± 3.33 a | 57.76 ± 1.41 a | 72.29 ± 2.88 a | 104.84 ± 6.76 a | 693.83 ± 68.67 c | 238.38 ± 13.27 b | |
| Hexanal | 000066-25-1 | OU | 121.82 ± 8.00 a | 128.73 ± 4.97 a | 88.64 ± 4.78 a | 263.35 ± 25.62 b | 292.85 ± 10.59 b | 645.10 ± 23.66 de | 538.18 ± 43.80 c | 718.77 ± 39.32 e | 139.99 ± 14.14 a | 559.42 ± 35.05 cd | |
| 2-Hexenal | 000505-57-7 | Grass | 102.82 ± 15.66 a | 118.90 ± 5.88 a | 190.52 ± 22.79 a | 178.68 ± 15.60 a | 713.47 ± 93.30 b | 1024.10 ± 245.40 bc | 1025.57 ± 112.34 bc | 1305.57 ± 92.54 | 949.66 ± 92.21 bc | 853.69 ± 27.18 b | |
| 3-Hexen-1-ol | 000544-12-7 | Grass | 72.94 ± 11.47 ab | 118.31 ± 11.44 cd | 112.75 ± 10.70 cd | 88.86 ± 7.59 bc | ND | 45.29 ± 8.35 a | 61.87 ± 6.53 ab | ND | 132.57 ± 4.84 d | 53.45 ± 2.41 ab | |
| Leaf acetate | 003681-71-8 | Grass | ND | 50.83 ± 0.89 | ND | ND | ND | ND | ND | ND | ND | ND | |
| 2-Hexen-1-ol, (E)- | 000928-95-0 | Apple | ND | ND | 61.08 ± 6.87 a | 125.42 ± 10.91 b | 116.27 ± 14.19 b | 41.93 ± 8.27 a | 51.25 ± 4.78 a | 31.73 ± 1.81 a | 119.66 ± 12.65 b | ND | |
| Nonanal | 000124-19-6 | Sweet Orange | ND | ND | ND | ND | ND | 3.31 ± 0.49 a | 5.52 ± 0.78 ab | 4.96 ± 0.18 ab | 6.39 ± 0.49 b | ND | |
| Geranial | 005392-40-5 | Lemon | ND | ND | ND | ND | ND | ND | ND | ND | 2.28 ± 0.13 | ND | |
| Cis-3-Hexenyl hexanoate | 031501-11-8 | Pear | ND | 4.66 ± 0.23 | ND | ND | ND | ND | ND | ND | ND | ND | |
| Heptanal | 000111-71-7 | Fruit | ND | 4.72 ± 0.15 | ND | ND | ND | ND | ND | ND | ND | ND | |
| Hexanoic acid, ethyl ester | 000123-66-0 | Fruit | 7.92 ± 1.94 | ND | ND | ND | ND | ND | 5.19 ± 0.87 | ND | ND | ND | |
| Butanoic acid, hexyl ester | 002639-63-6 | Sweet Fruit | ND | 2.85 ± 0.12 | ND | ND | ND | ND | ND | ND | ND | ND | |
| Caryophyllene | 000087-44-5 | Clove | 5.32 ± 0.55 ab | 15.10 ± 2.02 d | 13.57 ± 0.23 cd | 9.30 ± 0.77 bc | 4.10 ± 0.16 a | ND | ND | ND | ND | ND | |
| Benzene acetaldehyde | 000122-78-1 | Hosta Flower | 185.32 ± 22.43 c | 70.52 ± 8.18 b | 35.13 ± 3.68 ab | 16.47 ± 1.12 a | 10.77 ± 1.17 a | 5.03 ± 0.90 a | 5.78 ± 0.44 a | 5.30 ± 0.34 a | ND | ND | |
| Phenylethyl Alcohol | 000060-12-8 | Rose | ND | ND | ND | ND | ND | 3.66 ± 0.61 a | 7.01 ± 0.67 b | 7.44 ± 0.36 b | 10.58 ± 0.89 c | 7.88 ± 0.53 bc | |
| Citronellol | 000106-22-9 | Rose | ND | ND | ND | ND | ND | ND | ND | ND | 3.60 ± 0.46 | ND | |
| Neryl alcohol | 000106-25-2 | Rose | ND | ND | ND | ND | ND | 12.51 ± 1.04 a | ND | 22.63 ± 1.70 ab | 35.70 ± 4.61 b | ND | |
| Benzyl Alcohol | 000100-51-6 | Rose | ND | ND | ND | ND | ND | ND | 4.35 ± 0.47 a | 5.75 ± 0.76 a | 11.53 ± 0.64 b | 5.32 ± 0.68 a | |
| Styrene | 000100-42-5 | Fragrant | ND | ND | ND | ND | ND | ND | ND | ND | 11.81 ± 1.52 | ND | |
| Benzaldehyde | 000100-52-7 | Fragrant | 1.87 ± 0.55 | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| Xinbeichun | Butanoic acid, 2-hexenyl ester, (E)- | 053398-83-7 | OU | ND | 11.77 ± 0.28 | ND | ND | ND | ND | ND | ND | ND | ND |
| Cyclopentene, 1-methyl- | 000693-89-0 | OU | 27.09 ± 3.21 | ND | ND | 13.07 ± 0.24 | ND | ND | ND | ND | ND | ND | |
| Cyclohexene oxide | 000286-20-4 | OU | ND | ND | ND | ND | ND | ND | ND | 20.77 ± 0.03 | ND | ND | |
| Cis-3-Hexenyl 2-methylbutanoate | 053398-85-9 | OU | ND | 4.34 ± 0.21 | ND | ND | ND | ND | ND | ND | ND | ND | |
| Butanoic acid, 3-hexenyl ester, (Z)- | 016491-36-4 | OU | ND | 19.69 ± 1.43 b | 4.93 ± 0.08 a | 2.86 ± 0.13 a | ND | ND | ND | ND | ND | ND | |
| 3-Carene | 013466-78-9 | OU | ND | ND | ND | ND | ND | 5.18 ± 0.17 a | 12.98 ± 1.03 b | 13.59 ± 0.82 b | 23.77 ± 1.65 c | 9.82 ± 0.28 b | |
| Hexanal | 000066-25-1 | OU | 130.67 ± 18.82 a | 159.03 ± 8.79 a | 146.58 ± 18.57 a | 144.25 ± 16.18 a | 346.93 ± 46.91 ab | 455.18 ± 118.00 bc | 640.12 ± 72.28 cd | 829.96 ± 67.64 d | 615.34 ± 64.76 cd | 605.88 ± 38.50 cd | |
| 2-Hexenal | 000505-57-7 | Grass | 85.37 ± 1.14 a | 46.71 ± 4.26 a | 93.25 ± 10.77 a | 69.11 ± 1.71 a | 69.11 ± 1.71 a | 1195.75 ± 45.83 c | 634.12 ± 53.83 b | 744.92 ± 73.67 b | 795.91 ± 158.28 b | ND | |
| 3-Hexen-1-ol, (Z)- | 000928-96-1 | Grass | 121.29 ± 1.51 a | 128.76 ± 8.39 a | 131.02 ± 14.49 a | ND | ND | ND | ND | ND | ND | ND | |
| Eucalyptol | 000470-82-6 | Cool | 59.14 ± 2.52 c | 26.82 ± 2.32 b | 9.31 ± 1.00 a | 2.37 ± 0.12 a | 2.37 ± 0.12 a | ND | ND | ND | ND | ND | |
| Hexanoic acid, 3-hexenyl ester, (Z)- | 031501-11-8 | Pear | 6.57 ± 0.04 | 6.40 ± 0.55 | ND | ND | ND | ND | ND | ND | ND | ND | |
| 2-Hexen-1-ol, (E)- | 000928-95-0 | Apple | ND | 42.14 ± 2.97 b | 48.63 ± 5.24 b | 96.14 ± 2.71 d | 96.14 ± 2.71 d | 63.15 ± 0.62 c | 16.94 ± 0.63 a | 23.39 ± 2.91 a | 27.63 ± 3.77 a | ND | |
| Nonanal | 000124-19-6 | Sweet Orange | 6.75 ± 0.21 a | 8.80 ± 0.58 ab | 8.18 ± 0.51 ab | 10.01 ± 0.28 b | 10.01 ± 0.28 b | 14.07 ± 0.35 c | 10.47 ± 0.92 b | 6.24 ± 0.62 a | 7.89 ± 1.18 ab | ND | |
| Hexanoic acid, ethyl ester | 000123-66-0 | Fruit | ND | ND | ND | 3.65 ± 0.38 | 3.65 ± 0.38 | ND | ND | ND | ND | ND | |
| Butanoic acid, hexyl ester | 002639-63-6 | Sweet Fruit | ND | 7.35 ± 0.71 | ND | ND | ND | ND | ND | ND | ND | ND | |
| Benzene acetaldehyde | 000122-78-1 | Hosta Flower | 47.60 ± 1.06 c | 25.41 ± 2.39 b | 14.88 ± 1.20 a | 9.02 ± 0.56 a | 9.02 ± 0.56 a | ND | ND | ND | ND | ND | |
| Benzene, 1,3-dimethyl- | 000108-38-3 | Fragrant | 47.14 ± 3.02 | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| Cis-3-Hexenyl 2-methylbutanoate | 053398-85-9 | OU | ND | 7.40 ± 0.84 | ND | ND | ND | ND | ND | ND | ND | ND | |
| 2-Hexen-1-ol, acetate | 002497-18-9 | OU | ND | 8.53 ± 0.70 b | ND | 4.58 ± 0.12 a | 4.58 ± 0.12 a | ND | ND | ND | ND | ND | |
| Butanoic acid, 3-hexenyl ester, (Z)- | 016491-36-4 | OU | 15.36 ± 0.66 b | 30.96 ± 3.04 c | 7.89 ± 0.84 a | 2.68 ± 0.09 a | 2.68 ± 0.09 a | ND | ND | ND | ND | ND | |
| Butanoic acid, 2-hexenyl ester, (E)- | 053398-83-7 | OU | ND | 14.99 ± 1.43 b | 7.21 ± 0.93 a | 5.74 ± 0.01 a | 5.74 ± 0.01 a | ND | ND | ND | ND | ND | |
| 1-Hexanol | 000111-27-3 | OU | ND | ND | ND | ND | ND | 69.21 ± 2.71 b | 47.89 ± 2.56 a | 62.28 ± 6.48 ab | 51.09 ± 1.14 ab | ND | |
| 2,4-Hexadienal, (E,E)- | 000142-83-6 | OU | ND | ND | ND | ND | ND | ND | ND | ND | 3.55 ± 0.63 | ND | |
| Octanal | 000124-13-0 | OU | ND | ND | ND | ND | ND | 4.27 ± 0.19 | 10.40 ± 0.96 | ND | ND | ND | |
| Hexanal | 000066-25-1 | OU | 112.88 ± 5.74 a | 82.85 ± 8.11 a | 162.87 ± 20.37 a | 169.06 ± 7.98 a | 169.06 ± 7.98 a | 461.04 ± 21.82 b | 429.89 ± 36.03 b | 453.90 ± 39.32 b | 552.82 ± 88.47 b | ND |
References
- Komarek, M.; Cadkova, E.; Chrastny, V.; Bordas, F.; Bollinger, J.C. Contamination of vineyard soils with fungicides: A review of environmental and toxicological aspects. Environ. Int. 2010, 36, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.W.; Tang, R.K.; Zhang, Y.Y.; Liu, X.J.; Gao, Y.Y.; Dai, Z.W.; Li, S.H.; Wu, B.H.; Wang, L.J. Genome-wide identification of B-box proteins and VvBBX44 involved in light-induced anthocyanin biosynthesis in grape (Vitis vinifera L.). Planta 2021, 253, 114. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.H.; Cao, Y.G.; Guan, L.; Xin, H.P.; Li, J.H.; Li, S.H. Genome-wide transcriptional profiles of the berry skin of two red grape cultivars (Vitis vinifera) in which anthocyanin synthesis is sunlight-dependent or -independent. PLoS ONE 2014, 9, e105959. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.I.; Lombard, P.B. Environmental and management practices affecting grape composition and wine quality—A review. Am. J. Enol. Vitic. 1993, 44, 409–430. [Google Scholar]
- Reynolds, A.G.; Price, S.F.; Wardle, D.A.; Watson, B.T. Fruit environment and crop level effects on Pinot noir. I. Vine performance and fruit composition in British Columbia. Am. J. Enol. Vitic. 1994, 45, 452–459. [Google Scholar]
- Reynolds, A.G.; Wardle, D.A.; Naylor, A.P. Impact of training system, vine spacing, and basal leaf removal on Riesling. vine performance, berry composition, canopy microclimate, and vineyard labor requirements. Am. J. Enol. Vitic. 1996, 47, 63–76. [Google Scholar]
- Zarco-Tejada, P.J.; Berjón, A.; López-Lozano, R.; Miller, J.R.; Martin, P.; Cachorro, V.; Gonzalez, M.R.; Frutos, A. Assessing vineyard condition with hyperspectral indices: Leaf and canopy reflectance simulation in a row-structured discontinuous canopy. Remote Sens. Environ. 2005, 99, 271–287. [Google Scholar] [CrossRef]
- Ollat, N.; Gaudillere, J.P. The effect of limiting leaf area during stage I of berry growth on development and composition of berries of Vitis vinifera L. cv. Cabernet Sauvignon. Am. J. Enol. Vitic. 1998, 49, 251–258. [Google Scholar]
- Yang, B.H.; Yao, H.; Zhang, J.X.; Li, Y.Q.; Ju, Y.L.; Zhao, X.F.; Sun, X.Y.; Fang, Y.L. Effect of regulated deficit irrigation on the content of soluble sugars, organic acids and endogenous hormones in Cabernet Sauvignon in the Ningxia region of China. Food Chem. 2020, 312, 126020. [Google Scholar] [CrossRef]
- Ju, Y.L.; Min, L.; Tu, T.Y.; Zhao, X.F.; Yue, X.F.; Zhang, J.X.; Fang, Y.L.; Meng, J.F. Effect of regulated deficit irrigation on fatty acids and their derived volatiles in ‘Cabernet Sauvignon’ grapes and wines of Ningxia, China. Food Chem. 2018, 245, 667–675. [Google Scholar] [CrossRef]
- Centeno, A.; Baeza, P.; Lissarrague, J.R. Relationship between Soil and Plant Water Status in Wine Grapes under Various Water Deficit Regimes. Horttechnology 2010, 20, 585–593. [Google Scholar] [CrossRef] [Green Version]
- Xu, G.Z.; Wang, Y.; Ren, C.; Fan, P.G.; Kuang, Y.F.; Wang, Y.; Liang, Z.C. Genome wide analysis of GH gene family reveals VvGH9 positively regulates sugar accumulation under low sugar content in grape. Sci. Hortic. 2021, 7, 453. [Google Scholar] [CrossRef]
- Shiraishi, M. Three descriptors for sugars to evaluate grape germplasm. Euphytica 1993, 71, 99–106. [Google Scholar] [CrossRef]
- Kliewer, W. Concentration of tartrates, malic acids, glucose and fructose in the fruits of genus Vitis. Am. J. Enol. Vitic. 1967, 18, 87–96. [Google Scholar]
- Shiraishi, M. Proposed descriptors for organic acids to evaluate grape germplasm. Euphytica 1995, 81, 13–20. [Google Scholar] [CrossRef]
- Lott, R.V.; Barrett, H.C. Dextrose, levulose, sucrose, and acid content of the juice from 39 grape clones. Vitis 1967, 6, 257–268. [Google Scholar]
- Khakimov, B.; Bakhytkyzy, I.; Fauhl-Hassek, C.; Engelsen, S.B. Non-volatile molecular composition and discrimination of single grape white of chardonnay, riesling, sauvignon blanc and silvaner using untargeted GC-MS analysis. Food Chem. 2022, 369, 130878. [Google Scholar] [CrossRef]
- Uzhel, A.S.; Borodina, A.N.; Gorbovskaya, A.V.; Shpigun, O.A.; Zatirakha, A.V. Determination of full organic acid profiles in fruit juices and alcoholic beverages using novel chemically derivatized hyperbranched anion exchanger. J. Food Compos. Anal. 2021, 95, 103674. [Google Scholar] [CrossRef]
- Guidoni, S.; Allara, P.; Schubert, A. Effect of cluster thinning on berry skin anthocyanin composition of Vitis vinifera cv. Nebbiolo. Am. J. Enol. Vitic. 2002, 53, 224–226. [Google Scholar]
- Boulton, R. The copigmentation of anthocyanins and its role in the color of red wine: A critical review. Am. J. Enol. Vitic. 2001, 52, 67–87. [Google Scholar]
- Goldy, R.G.; Maness, E.P.; Stiles, H.D.; Clark, J.R.; Wilson, M.A. Pigment quantity and quality characteristics of some native Vitis rotundifolia Michx. Am. J. Enol. Vitic. 1989, 40, 253–258. [Google Scholar]
- Wulf, L.W.; Nagel, C.W. High pressure liquid chromatographic separation of anthocyanins of Vitis vinifera. Am. J. Enol. Vitic. 1978, 29, 42–49. [Google Scholar]
- Koeppen, B.H.; Basson, D.S. The anthocyanin pigments of barlinka grapes. Phytochemistry 1966, 5, 183–187. [Google Scholar] [CrossRef]
- Kong, J.H.; Wu, J.; Guan, L.; Hilbert, G.; Delrot, S.; Fan, P.G.; Liang, Z.C.; Wu, B.H.; Matus, J.T.; Gomes, E.; et al. Metabolite analysis reveals distinct spatio-temporal accumulation of anthocyanins in two teinturier variants of cv. ‘Gamay’ grapevines (Vitis vinifera L.). Planta 2021, 253, 84. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.C.; Wu, B.H.; Fan, P.G.; Yang, C.X.; Duan, W.; Zheng, X.B.; Liu, C.Y.; Li, S.H. Anthocyanin composition and content in grape berry skin in Vitis germplasm. Food Chem. 2008, 111, 837–844. [Google Scholar] [CrossRef]
- Kupe, M.; Ercisli, S.; Karatas, N.; Skrovankova, S.; Mlcek, J.; Ondrasova, M.; Snopek, L. Some Important Food Quality Traits of Autochthonous Grape Cultivars. J. Food Qual. 2021, 2021, 9918529. [Google Scholar] [CrossRef]
- Farias, C.A.A.; Moraes, D.P.; Lazzaretti, M.; Ferreira, D.F.; Daniele, F.; Zabot, G.L.; Barin, J.S.; Ballus, C.A.; Barcia, M.T. Microwave hydrodiffusion and gravity as pretreatment for grape dehydration with simultaneous obtaining of high phenolic grape extract. Food Chem. 2020, 337, 127723. [Google Scholar] [CrossRef]
- Alves, E.G.; Silva, L.M.A.; Lima, T.O.; Ribeiro, P.R.V.; Vidal, C.S.; Carvalho, E.S.S.; Druzian, J.I.; Marques, A.T.B.; Canuto, K.M. 1H NMR and UPLC-HRMS-based metabolomic approach for evaluation of the grape maturity and maceration time of Touriga Nacional wines and their correlation with the chemical stability. Food Chem. 2022, 382, 132359. [Google Scholar] [CrossRef]
- Ferreira, V.; Lopez, R.; Cacho, J.F. Quantitative determination of the odorants of young red wines from different grape varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Liu, C.Y.; Wang, L.J.; Wang, J.F.; Wu, B.H.; Liu, W.; Fan, P.G.; Liang, Z.C.; Li, S.H. Resveratrols in Vitis berry skins and leaves: Their extraction and analysis by HPLC. Food Chem. 2013, 136, 643–649. [Google Scholar] [CrossRef]
- Wang, J.F.; Ma, L.; Xi, H.F.; Wang, L.J.; Li, S.H. Resveratrol synthesis under natural conditions and after UV-C irradiation in berry skin is associated with berry development stages in ‘Beihong’ (V. vinifera × V. amurensis). Food Chem. 2015, 168, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Hajdu, E. Climatic resistance in some interspecific wine grape hybrid families. Vitis 1990, 29, 358–365. [Google Scholar]
- Amélie, S.; Paul, A.; Étienne, O.; Tamara, T.; Karine, P. Volatile Compounds from Grape Skin, Juice and Wine from Five Interspecific Hybrid Grape Cultivars Grown in Qébec (Canada) for Wine Production. Molecules 2015, 20, 10980–11016. [Google Scholar]
- Ambers, C.P. A historical hypothesis on the origin of the Norton grape. J. Wine Res. 2013, 24, 85–95. [Google Scholar] [CrossRef]
- Chai, F.M.; Zhu, W.; Xiang, Y.; Xin, H.P.; Li, S.H. Optimized method for detecting the cold hardiness of grape dormant bud by low temperature exotherms (LTE) analysis and its utilization. Acta Hortic. Sin. 2015, 42, 140–148. [Google Scholar]
- Zhang, Z.; Zou, L.M.; Ren, C.; Ren, F.R.; Wang, Y.; Fan, P.G.; Li, S.H.; Liang, Z.C. VvSWEET10 Mediates Sugar Accumulation in Grapes. Genes 2019, 10, 255. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.F.; Wu, B.H.; Fan, P.G.; Xu, H.Y.; Li, S.H. Inheritance of sugars and acids in berries of grape (Vitis vinifera L.). Euphytica 2007, 153, 99–107. [Google Scholar] [CrossRef]
- Wang, H.; Chen, X.Y.; Zhang, J.X. Characteristic analysis of young red wine from the eastern foot of Helan Mountain based on CIELAB color space parameters. Food Sci. 2014, 35, 20–23. [Google Scholar]
- Zhang, H.H.; Fan, P.G.; Liu, C.X.; Wu, B.H.; Li, S.H.; Liang, Z.C. Sunlight exclusion from Muscat grape alters volatile profiles during berry development. Food Chem. 2014, 164, 242–250. [Google Scholar] [CrossRef]
- Kliewer, W.M. Changes in concentration of glucose, fructose, and total soluble solids in flowers and berries of Vitis vinifera. Am. J. Enol. Vitic. 1965, 16, 101–110. [Google Scholar]
- Taillandier, P.; Portugal, F.R.; Andre, F.; Pierre, S. Effect of ammonium concentration on alcoholic fermentation kinetics by wine yeasts for high sugar content. Food Microbiol. 2007, 24, 95–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez, A.V.; Manca de Nadra, M.C. Sugar and organic acid metabolism in mixed cultures of Pediococcus pentosaceus and Leuconostoc oenos isolated from wine. J. Appl. Microbiol. 2010, 77, 61–66. [Google Scholar] [CrossRef]
- Ju, L.L.; Yang, L.; Yue, X.F.; Li, Y.K.; He, R.; Deng, S.L.; Yang, X.; Fang, L. Anthocyanin profiles and color properties of red wines made from Vitis davidii and Vitis vinifera grapes. Food Sci. Hum. Wellness 2021, 10, 335–344. [Google Scholar] [CrossRef]
- Escudero, A.; Campo, E.; Farina, L.; Cacho, J.; Ferreira, V. Analytical Characterization of the Aroma of Five Premium Red Wines. Insights into the Role of Odor Families and the Concept of Fruitiness of Wines. J. Agric. Food Chem. 2007, 55, 4501. [Google Scholar]
- Ribereau-Gayon, P.; Boidron, J.N.; Terrier, A. Aroma of Muscat grape varieties. J. Agric. Food Chem. 1975, 23, 1042–1047. [Google Scholar] [CrossRef]
- Mele, M.A.; Kang, H.M.; Lee, Y.T.; Islam, M.Z. Grape terpenoids: Flavor importance, genetic regulation, and future potential. Crit. Rev. Food Sci. Nutr. 2020, 61, 1429–1447. [Google Scholar] [CrossRef]
- Arcari, S.; Caliari, V.; Souza, E.; Godoy, H. Aroma profile and phenolic content of merlot red wines produced in high-altitude regions in Brazil. Química Nova 2021, 44, 616–624. [Google Scholar] [CrossRef]
- Wang, X.J.; Liu, Z.Z.; Zhang, R.Y.; Lu, P.C.; Meng, L. Influence of fermentation temperature on resveratrol content of Carbernet Sauvignon and Merlot dry red wine in manas region. Mod. Food 2021, 7, 149–159. [Google Scholar]
- Zhang, P.; Ni, H.M.; Gu, D.C. Determination of resveratrol content in wine based on HPLC. Food Ind. 2018, 39, 314–316. [Google Scholar]





| Variety | Year | S1 | S2 | S3 | VE | S4 | S5 | S6 | S7 | S8 | S9 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Beimei | 2019 | 6.19 | 7.03 | 7.17 | 7.22 | 7.31 | 8.14 | 8.28 | 9.11 | 9.25 | 10.09 |
| 2021 | - | 7.08 | 7.22 | 7.26 | 8.05 | 8.20 | 9.02 | 9.16 | 9.30 | - | |
| Beihong | 2019 | 6.19 | 7.03 | 7.17 | 7.22 | 7.31 | 8.14 | 8.28 | 9.11 | 9.25 | 10.09 |
| 2021 | - | 7.08 | 7.22 | 7.26 | 8.05 | 8.20 | 9.02 | 9.16 | 9.30 | - | |
| Beixi | 2019 | 6.19 | 7.03 | 7.17 | 7.28 | 7.31 | 8.14 | 8.28 | 9.11 | 9.25 | 10.09 |
| 2021 | - | 7.08 | 7.22 | 8.02 | 8.05 | 8.20 | 9.02 | 9.16 | 9.30 | - | |
| Beixin | 2019 | 6.19 | 7.03 | 7.17 | 7.19 | 7.31 | 8.14 | 8.28 | 9.11 | 9.25 | 10.09 |
| 2021 | - | 7.08 | 7.22 | 7.25 | 8.05 | 8.20 | 9.02 | 9.16 | 9.30 | - | |
| Xinbeichun | 2019 | 6.19 | 7.03 | 7.17 | 7.31 | 7.31 | 8.14 | 8.28 | 9.11 | 9.25 | 10.09 |
| 2021 | - | 7.08 | 7.22 | 7.26 | 8.05 | 8.20 | 9.02 | 9.16 | 9.30 | - |
| Alcohol | Total Acid | Residual Sugar | Volatile Acid | Total Sulfur | Dry Extract |
|---|---|---|---|---|---|
| (%vol) | (g/L) | (g/L) | (g/L) | (mg/L) | (g/L) |
| 14.2 ± 0.00 | 10.9 ± 0.10 | 5.9 ± 0.07 | 0.4 ± 0.01 | 56.3 ± 7.31 | 29.5 ± 0.10 |
| Compounds | Flavour | A (μg/L) | B (μg/L) | C (μg/L) | D (μg/L) | E (μg/L) | F (μg/L) |
|---|---|---|---|---|---|---|---|
| Beta-Damascenone | rose | ND | ND | ND | ND | 22.81 ± 7.33 a | 36.35 ± 19.85 a |
| Neryl alcohol | rose | ND | ND | ND | ND | 97.74 ± 31.88 a | 177.08 ± 100.61 a |
| Acetic acid, 2-phenylethyl ester | rose | ND | ND | 82.01 ± 1.66 a | 86.75 ± 4.39 a | 164.93 ± 52.99 a | 248.15 ± 133.76 a |
| Phenylethyl Alcohol | rose | ND | ND | 277.09 ± 4.05 a | 555.89 ± 26.48 a | 1570.81 ± 465.91 a | 2648.54 ± 1489.78 a |
| Tetradecanoic acid, ethyl ester | violet, iris | ND | ND | 66.00 ± 0.60 a | ND | 138.79 ± 47.39 a | 191.95 ± 106.14 a |
| Dodecanoic acid, ethyl ester | petal aroma | ND | ND | 970.26 ± 30.25 a | 964.64 ± 42.76 a | 1857.22 ± 616.60 a | 2394.99 ± 1322.48 a |
| Styrene | fragrant | ND | ND | 15.61 ± 1.12 a | 39.95 ± 7.49 a | 110.38 ± 41.23 a | 222.67 ± 28.74 a |
| Hexanoic acid | fruit | ND | ND | 28.73 ± 3.03 | 34.41 ± 2.26 | ND | ND |
| 1-Hexanol | fruit | 43.05 ± 15.64 a | 73.98 ± 0 a | 60.40 ± 4.93 a | 100.28 ± 29.63 a | 278.09 ± 80.28 ab | 347.40 ± 47.45 b |
| Acetic acid, octyl ester | fruit | ND | ND | 32.33 ± 0.70 b | 15.33 ± 2.20 a | 20.35 ± 7.36 ab | ND |
| Decanoic acid, ethyl ester | fruit, brandy | ND | ND | 5356.76 ± 104.94 a | 2587.12 ± 220.32 a | 5257.99 ± 1853.51 a | 7514.63 ± 4214.19 a |
| Octanoic acid, 3-methylbutyl ester | fruit, brandy | ND | ND | 154.65 ± 4.68 b | 67.99 ± 6.93 a | 83.83 ± 29.39 a | 33.15 ± 17.80 a |
| Butanoic acid, ethyl ester | pineapple, banana | ND | ND | 17.13 ± 0.77 a | 39.79 ± 2.61 a | 101.27 ± 31.20 a | 172.86 ± 93.88 a |
| Isoamyl acetate | banana | ND | ND | 1182.75 ± 75.94 a | 1772.34 ± 151.97 a | 3010.50 ± 957.49 a | 4852.33 ± 2704.97 a |
| Octanoic acid, methyl ester | sweet orange | ND | ND | 37.16 ± 4.12 a | 36.11 ± 3.22 a | 129.36 ± 45.92 a | 235.90 ± 133.36 a |
| Octanoic acid, ethyl ester | pineapple, apple | ND | ND | 5596.42 ± 139.47 a | 5122.37 ± 553.93 a | 12,921.28 ± 4565.12 a | 22,822.73 ± 12,941.19 a |
| Hexanoic acid, ethyl ester | pineapple | ND | ND | 1412.64 ± 92.31 a | 1974.88 ± 174.86 a | 3857.39 ± 1283.73 a | 6668.49 ± 3767.05 a |
| Acetic acid, hexyl ester | pear, apple | ND | ND | 584.48 ± 36.43 a | 331.95 ± 27.93 a | 498.78 ± 164.81 a | 790.87 ± 446.95 a |
| Hexadecanoic acid, ethyl ester | cream | ND | ND | 14.65 ± 0.84 a | ND | 300.22 ± 94.96 ab | 470.10 ± 238.06 b |
| Octanoic Acid | Sweat smell | ND | ND | ND | 137.99 ± 9.18 a | 372.41 ± 119.37 a | 587.64 ± 308.71 a |
| Butanedioic acid, diethyl ester | spcial smell | ND | ND | ND | ND | 58.44 ± 18.20 a | 132.14 ± 70.95 a |
| Methyl salicylate | holly leaf | ND | 3.04 ± 0.02 a | 30.78 ± 0.30 ab | 44.51 ± 2.42 ab | 121.28 ± 38.98 ab | 193.53 ± 105.82 b |
| Decanoic acid, methyl ester | OU | ND | ND | 59.42 ± 1.05 a | 15.60 ± 1.52 a | 36.57 ± 12.93 a | 59.34 ± 33.17 a |
| Pentadecanoic acid, 3-methylbutyl ester | OU | ND | ND | 40.98 ± 1.90 a | 72.61 ± 7.45 a | 95.30 ± 32.00 a | 72.82 ± 40.25 a |
| neryl propanoate | OU | ND | ND | 18.89 ± 0.49 a | 20.89 ± 0.94 a | 22.12 ± 7.48 a | ND |
| Dodecane | OU | ND | ND | 10.03 ± 1.06 a | 17.63 ± 2.77 a | 57.78 ± 16.70 a | 87.68 ± 48.99 a |
| 2,3-Butanediol | OU | ND | ND | ND | 41.61 ± 10.46 a | ND | 100.23 ± 54.19 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuang, Y.; Ren, C.; Wang, Y.; Kirabi, G.E.; Wang, Y.; Wang, L.; Fan, P.; Liang, Z. Characterization of the Berry Quality Traits and Metabolites of ‘Beimei’ Interspecific Hybrid Wine Grapes during Berry Development and Winemaking. Horticulturae 2022, 8, 516. https://doi.org/10.3390/horticulturae8060516
Kuang Y, Ren C, Wang Y, Kirabi GE, Wang Y, Wang L, Fan P, Liang Z. Characterization of the Berry Quality Traits and Metabolites of ‘Beimei’ Interspecific Hybrid Wine Grapes during Berry Development and Winemaking. Horticulturae. 2022; 8(6):516. https://doi.org/10.3390/horticulturae8060516
Chicago/Turabian StyleKuang, Yangfu, Chong Ren, Yi Wang, Gathunga Elias Kirabi, Yongjian Wang, Lijun Wang, Peige Fan, and Zhenchang Liang. 2022. "Characterization of the Berry Quality Traits and Metabolites of ‘Beimei’ Interspecific Hybrid Wine Grapes during Berry Development and Winemaking" Horticulturae 8, no. 6: 516. https://doi.org/10.3390/horticulturae8060516
APA StyleKuang, Y., Ren, C., Wang, Y., Kirabi, G. E., Wang, Y., Wang, L., Fan, P., & Liang, Z. (2022). Characterization of the Berry Quality Traits and Metabolites of ‘Beimei’ Interspecific Hybrid Wine Grapes during Berry Development and Winemaking. Horticulturae, 8(6), 516. https://doi.org/10.3390/horticulturae8060516





