Early Response of ‘Mexican’ Lime, ‘Fina’ Clementine Mandarin, and ‘Campbell’ Valencia Orange on Selected Rootstocks Grown under Fertigation Practices in an Oxisol in Puerto Rico
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Experimental Area
2.2. Scion–Rootstock Combinations, Disease Testing, and Orchard Management
Experimental Orchard Design
2.3. Soil Fertility, Tree Growth, Leaf Nutrient Analysis, and Yield
HLB Detection Using Conventional PCR
2.4. Statistical Analysis
3. Results
3.1. Soil Chemical Properties
3.2. Leaf Tissue Macronutrient and Trace Element Concentrations
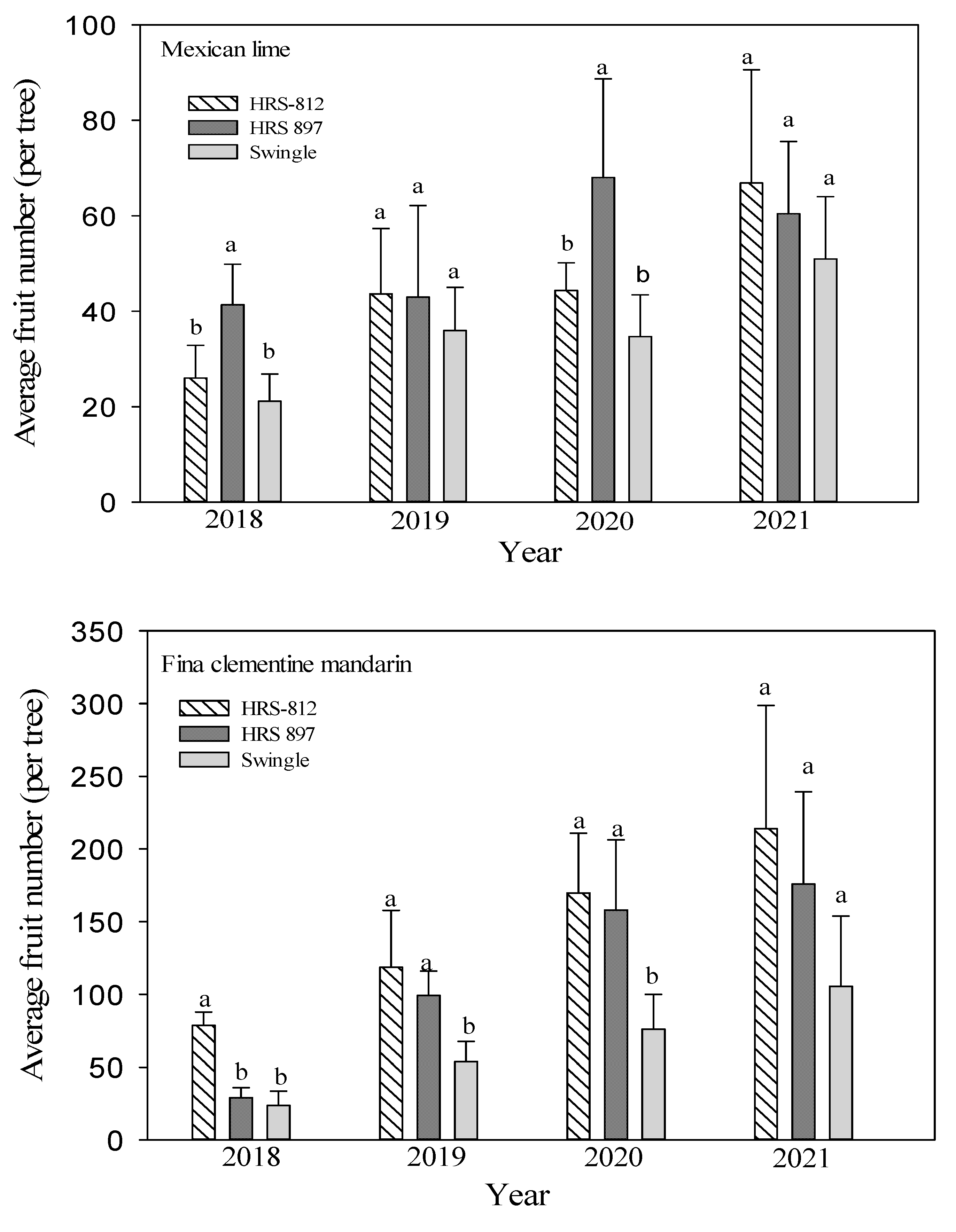
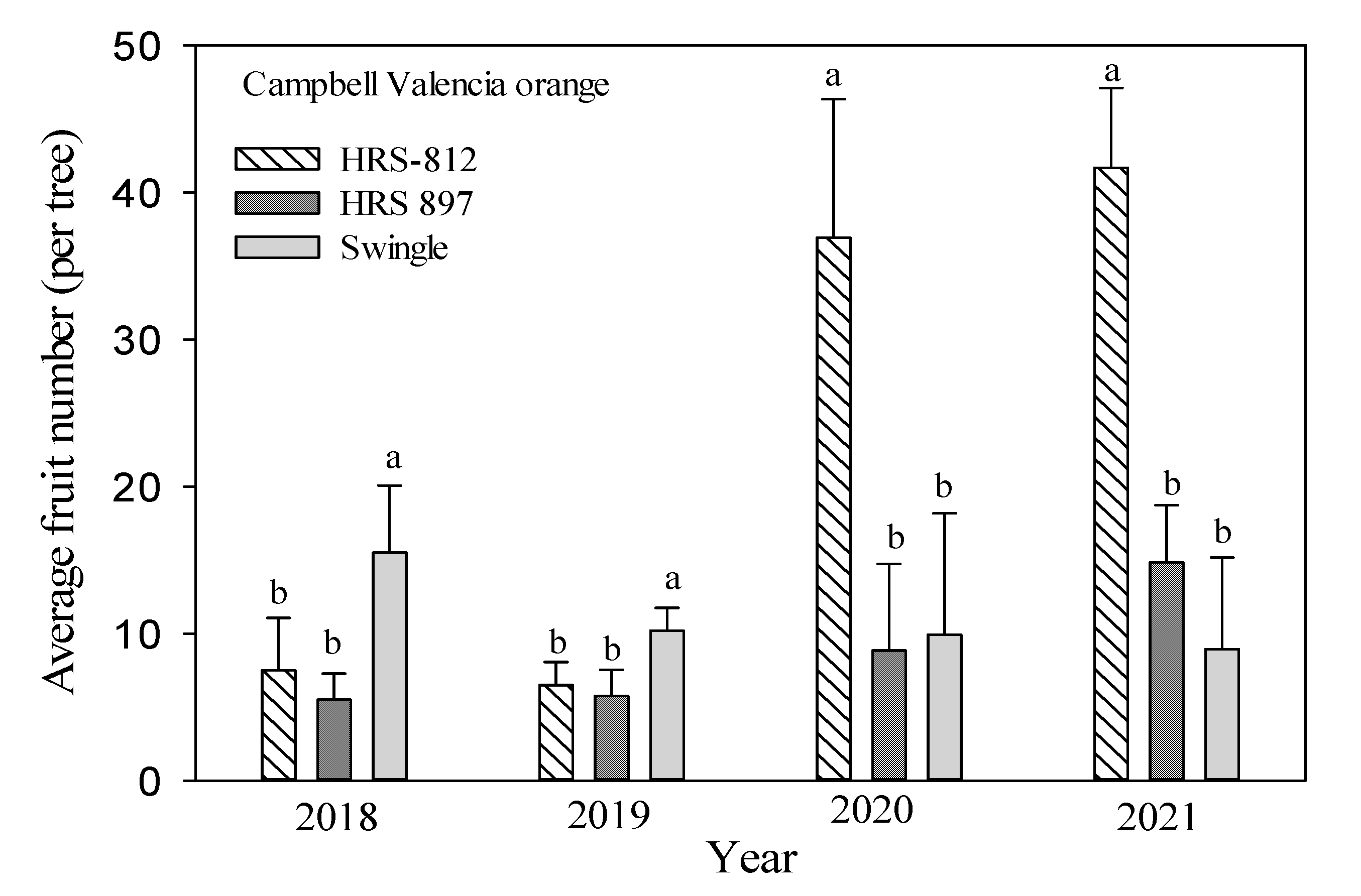
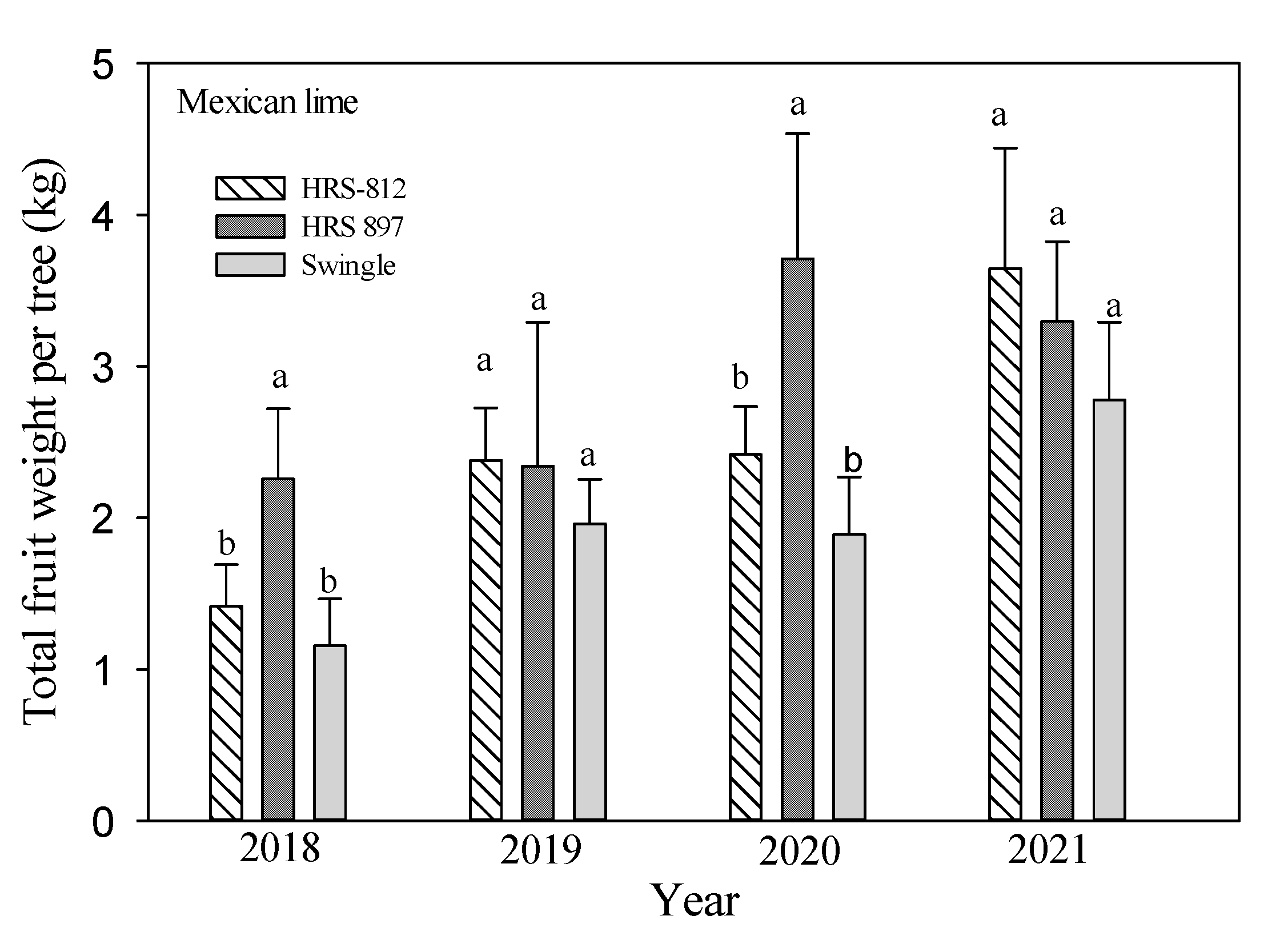
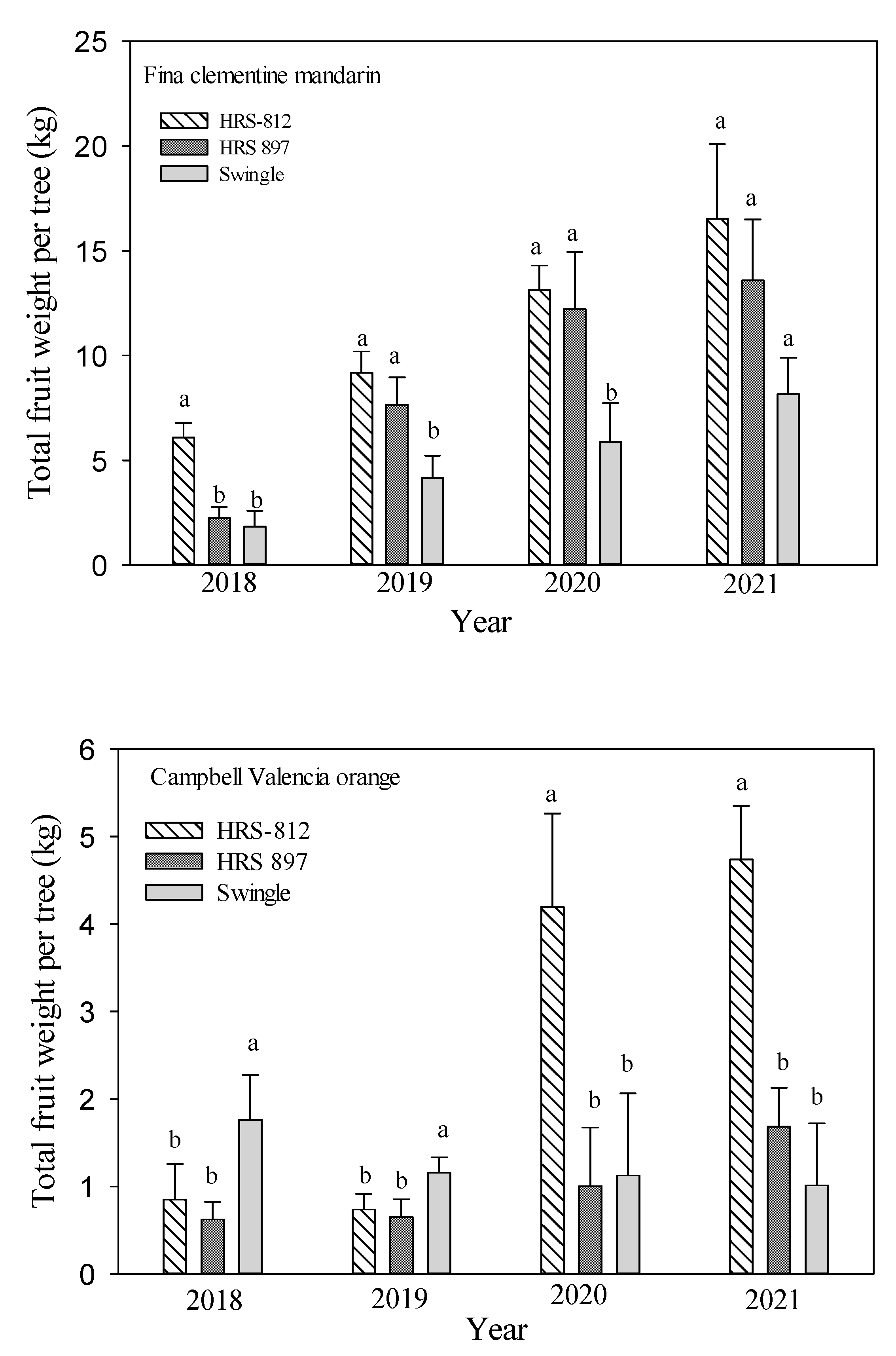
3.3. Tree Growth and Yield
3.4. Number and Percentage of Trees Infected and Dead by HLB
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Departamento de Agricultura, División Estadísticas Agrícolas, Estado Libre Asociado de Puerto Rico. Ingreso Bruto Agrícola; Departamento de Agricultura, División Estadísticas Agrícolas, Estado Libre Asociado de Puerto Rico: San Juan, PR, USA, 2015. [Google Scholar]
- Román-Paoli, E.; Tirado-Corbalá, R.; Román-Pérez, F. Response of ‘Red Rhode Valencia’ orange (Citrus sinensis) to microirrigation in Adjuntas, Puerto Rico. J. Agric. Univ. P. R. 2020, 104, 129–138. [Google Scholar] [CrossRef]
- Tirado-Corbalá, R.; Segarra-Carmona, A.; Matos-Rodríguez, M.; Rivera-Ocasio, D.; Estévez de Jensen, C.; Pagán, J. Assessment of Two Sweet Orange Cultivars Grafted on Selected Rootstocks Grown on an Inceptisol in Puerto Rico. Horticulturae 2020, 6, 30. [Google Scholar] [CrossRef]
- Tirado-Corbalá, R.; Rivera-Ocasio, D.; Segarra-Carmona, A.; Román-Paoli, E.; González, A. Performance of Two Citrus Species Grafted to Different Rootstocks in the Presence of Huanglongbing Disease in Puerto Rico. Horticulturae 2018, 4, 38. [Google Scholar] [CrossRef]
- Zamora-Echevarría, J.L. Manejo Nutricional del “Citrus Greening” y su Costo; El Frutal: Mayagüez, PR, USA, 2013; Volume 9, No. 1. [Google Scholar]
- Jenkins, D.A.; Hall, D.G.; Goenaga, R. Diaphorina citri (Hemiptera: Liviidae) abundance in Puerto Rico declines with elevation. J. Econ. Entomol. 2015, 108, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Atta, A.A.; Morgan, K.T.; Mahmoud, K.A. Split application of nutrients improve growth and yield of Huanglongbing-affected citrus trees. Soil Sci. Soc. Am. J. 2021, 85, 2040–2053. [Google Scholar] [CrossRef]
- Srivastava, A.K. Integrated nutrient management in citrus. In Advances in Citrus Nutrition; Srivastava, A.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 369–389. [Google Scholar] [CrossRef]
- Román-Pérez, F.M.; O’Hallorans, J. Crecimiento y desarrollo de árboles jóvenes de cítricas utilizando fertilizantes de liberación controlada. Caribb. Food Crops Soc. 1997, 33, 82–87. [Google Scholar]
- Román-Pérez, F.M.; González-Vélez, A.; Macchiavelli, R. Efecto de cuatro patrones en la producción y calidad de la china “Hamlin” (Citrus sinensis [L.] Osb.) en tres localidades de Puerto Rico. J. Agric. Univ. P. R. 2011, 95, 25–34. [Google Scholar]
- Román-Pérez, F.M.; González-Vélez, A. Liberación de los patrones de cítricas “Swingle Citrumelo”, “Carrizo” y “HRS 812” para Puerto Rico. J. Agric. Univ. P. R. 2013, 97, 101–106. [Google Scholar] [CrossRef]
- Castle, W.S.; Bowman, K.D.; Grosser, J.D.; Ferrarezi, R.S.; Futch, S.H.; Rogers, S. Florida Citrus Rootstocks Selection Guide, 4th ed.; Horticultural Sciences Department, UF/IFAS Extension: Gainesville, FL, USA, 2019; Last revision 2019. [Google Scholar]
- Muñoz, M.; Lugo, W.I.; Santiago, C.; Matos, M.; Ríos, S.; Lugo, J. Taxonomic Classification of the Soils of Puerto Rico, 2017; Bulletin 313; University of Puerto Rico, Mayagüez Campus: Mayagüez, Puerto Rico; College of Agricultural Sciences Agricultural Experiment Station: San Juan, Puerto Rico, 2018; p. 20. [Google Scholar]
- Li, W.; Hartung, J.; Levy, L. Quantitative real-time PCR for detection and identification of ‘Candidatus Liberibacter species associated with citrus huanglongbing. J. Microbiol. Methods 2006, 66, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Morgan, K.T.; Kadyampakeni, D.M. Nutrition of Florida Citrus Trees, 3rd ed.; IFAS Extension Document SL 253; University of Florida: Gainesville, FL, USA, 2020. [Google Scholar]
- Mills, H.A.; Jones, J.B., Jr. Plant Analysis Handbook II; Micro-Macro Pub.: Athens, GA, USA, 1996. [Google Scholar]
- Thomas, G.W. Soil pH and soil acidity. In Methods of Soil Analysis, Part 3—Chemical Methods; Sparks, D.L., Ed.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 475–490. [Google Scholar]
- Warncke, D.; Brown, J.R. Potassium and other basic cations. In Recommended Chemical Soil Test Procedures for North Central Region; NCR Publication No. 221; Missouri Agricultural Station: Columbia, MO, USA, 1998; pp. 31–33. [Google Scholar]
- Hossner, L.R. Dissolution for total elemental analysis. In Methods of Soil Analysis, Part 3—Chemical Methods; Sparks, D.L., Ed.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 49–64. [Google Scholar]
- Fallahi, E.; Mousavi, Z. Performance of Orlando tangelo trees on ten rootstocks in Arizona. J. Am. Soc. Hortic. Sci. 1991, 116, 2–5. [Google Scholar] [CrossRef]
- USDA, APHIS, PPQ, CPHST. Work Instructions Plant Sample Extraction for Use in Citrus Greening or HLB (Huanglongbing) Molecular Diagnosis Assays; National Plant Germplasm and Quarantine Laboratory: Beltsville, MD, USA, 2009; p. 7. [Google Scholar]
- Spann, T.M.; Atwood, R.A.; Yates, J.D.; Rogers, M.E.; Brlansky, R.H. Dooryard Citrus Production: Citrus Greening Disease; EDIS HS1131; University of Florida, Institute of Food and Agricultural Sciences: Gainesville, FL, USA, 2010. [Google Scholar]
- Zekri, M. The Critical Importance of Citrus Tree Nutrition; Ag Net Media, Inc.: Newberry, FL, USA, 2016. [Google Scholar]
- Obreza, T.A.; Zekri, M.; Futch, S.H. General soil fertility and citrus tree nutrition. In Nutrition of Florida Citrus Trees, 2nd ed.; Obreza, T.A., Morgan, K.T., Eds.; IFAS Extension Document SL 253; University of Florida: Gainesville, FL, USA, 2008; p. 100. [Google Scholar]
- Bowman, K.D.; Rouse, R.E. US-812 Citrus Rootstock. HortScience 2006, 41, 832–836. [Google Scholar] [CrossRef]
- Wutscher, H.K.; Bowman, K.D. Performance of ‘Valencia orange on 21 rootstocks. HortScience 1999, 34, 622–624. [Google Scholar] [CrossRef]
- Albrecht, U.; Bowman, K.D. Tolerance of trifoliate citrus hybrids to Candidatus Liberibacter asiaticus. Sci. Hortic. 2012, 147, 71–80. [Google Scholar] [CrossRef]
- Albrecht, U.; Bowman, K.D. Tolerance of trifoliate citrus hybrid US-897 (Citrus reticulata Blanco x Poncirus trifoliata L. Raf.) to Huanglongbing. HortScience 2011, 46, 16–22. [Google Scholar] [CrossRef]

| Scion | Rootstocks | OM z | pH | Ca | K | Mg | Na | P | S | NO3-N |
|---|---|---|---|---|---|---|---|---|---|---|
| % | 1:1 | mg·kg−1 | ppm | |||||||
| ‘Mexican’ lime | ‘Swingle’ | 3.68 b,y | 6.55 a | 930 a | 95.5 b | 91.1 b | 12.0 b | 11.0 b | 10.5 a | 2.5 a |
| ‘HRS 812’ | 3.75 b | 6.38 a | 909 a | 95.0 b | 90.3 b | 12.2 b | 11.5 b | 9.75 a | 2.5 a | |
| ‘HRS 897’ | 4.38 a | 6.38 a | 846 a | 129 a | 103 a | 14.4 a | 14.8 a | 10.8 a | 3.0 a | |
| ‘Fina’ clementine mandarin | ‘Swingle’ | 4.92 a | 7.38 a | 1595 a | 88.6 a | 73.7 a | 12.2 a | 13.8 a | 7.75 a | 2.5 a |
| ‘HRS 812’ | 4.63 a | 6.98 a | 1422 a | 84.0 a | 76.9 a | 13.0 a | 11.3 b | 8.25 a | 2.5 a | |
| ‘HRS 897’ | 4.78 a | 7.38 a | 1832 a | 75.2 a | 67.1 a | 11.9 a | 14.0 a | 7.25 a | 2.75 a | |
| ‘Campbell’ Valencia orange | ‘Swingle’ | 5.43 a | 7.73 a | 1838 a | 69.6 b | 55.5 a | 9.93 b | 8.75 b | 6.75 b | 2.25 b |
| ‘HRS 812’ | 5.28 a | 7.58 a | 1930 a | 115 a | 54.8 a | 12.3 a | 11.8 a | 7.85 a | 3.0 a | |
| ‘HRS 897’ | 5.44 a | 7.40 a | 1698 a | 96.3 a | 65.7 a | 12.4 a | 12.0 a | 9.0 a | 3.5 a | |
| Scion | Rootstocks | N z | Ca | K | Mg | Na | P | S |
|---|---|---|---|---|---|---|---|---|
| % | mg·kg−1 | |||||||
| ‘Mexican’ lime | ‘Swingle’ | 2.09 b | 3.52 a | 1.15 b | 0.263 a | 0.108 b | 0.245 a | 0.290 a |
| ‘HRS 812’ | 2.31 a | 3.58 a | 1.62 a | 0.243 a | 0.135 a | 0.183 b | 0.250 a | |
| ‘HRS 897’ | 2.30 a | 2.92 b | 1.50 a | 0.225 a | 0.145 a | 0.173 b | 0.200 b | |
| ‘Fina’ clementine mandarin | ‘Swingle’ | 1.97 a | 4.05 a | 1.19 b | 0.263 a | 0.045 b | 0.318 a | 0.250 a |
| ‘HRS 812’ | 2.09 a | 4.12 a | 1.45 a | 0.263 a | 0.078 a | 0.290 b | 0.238 a | |
| ‘HRS 897’ | 2.16 a | 3.37 b | 1.35 a | 0.285 a | 0.085 a | 0.292 b | 0.200 b | |
| ‘Campbell’ Valencia orange | ‘Swingle’ | 2.11 b | 3.66 a | 1.51 a | 0.233 a | 0.168 a | 0.315 a | 0.175 a |
| ‘HRS 812’ | 2.30 a | 3.40 a | 1.27 b | 0.223 a | 0.183 a | 0.270 b | 0.189 a | |
| ‘HRS 897’ | 2.33 a | 3.43 a | 1.58 a | 0.232 a | 0.163 a | 0.280 b | 0.200 a | |
| Scion | Rootstocks | Al z | B | Cu | Fe | Mn | Zn |
|---|---|---|---|---|---|---|---|
| % | mg·kg−1 | ||||||
| ‘Mexican’ lime | ‘Swingle’ | 37.3 b | 58.8 b | 4.50 a | 121 b | 59.3 a | 11.0 a |
| ‘HRS 812’ | 44.0 a | 86.3 a | 4.75 a | 190 a | 48.5 a | 18.8 a | |
| ‘HRS 897’ | 36.0 b | 79.3 a | 4.25 a | 140 b | 50.0 a | 14.3 a | |
| ‘Fina’ clementine mandarin | ‘Swingle’ | 36.5 b | 94.3 a | 7.00 a | 141 a | 53.5 a | 13.8 a |
| ‘HRS 812’ | 49.0 a | 70.3 b | 5.50 a | 119 a | 53.0 a | 12.5 a | |
| ‘HRS 897’ | 37.8 b | 89.0 a | 5.50 a | 104 a | 42.0 a | 12.3 a | |
| ‘Campbell’ Valencia orange | ‘Swingle’ | 37.8 b | 98.8 a | 4.50 a | 77.3 a | 36.0 a | 9.50 a |
| ‘HRS 812’ | 43.8 a | 71.8 b | 3.50 a | 66.0 a | 40.5 a | 8.75 a | |
| ‘HRS 897’ | 37.8 b | 94.8 a | 4.00 a | 80.0 a | 38.0 a | 8.50 a | |
| Scion | Rootstocks | Tree Height (m) | Root: Shoot Ratio | Canopy Volume (m3) | Tree Efficiency (Fruits m−3) | Total Fruit | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2019 | 2020 | 2021 | 2019 | 2020 | 2021 | 2019 | 2020 | 2021 | 2019 | 2020 | 2021 | (#) | ||
| ‘Mexican’ lime | ‘Swingle’ | 1.73 a | 1.80 a | 2.28 a,y | 50.0 a | 75.6 a | 90.8 a | 6.40 b | 6.66 b | 8.43 a | 5.61 c | 5.21 c | 9.78 a | 143 c |
| ‘HRS 812’ | 1.79 a | 1.91 a | 2.06 b | 54.2 a | 63.9 a | 110 a | 6.50 b | 6.94 b | 7.48 b | 6.71 a | 6.39 b | 8.93 b | 181 b | |
| ‘HRS 897’ | 1.72 a | 1.85 a | 1.93 c | 54.0 a | 84.7 a | 96.4 a | 6.90 a | 7.42 a | 7.74 b | 6.22 b | 9.16 a | 7.80 c | 212 a | |
| ‘Fina’ clementine mandarin | ‘Swingle’ | 2.09 a | 2.12 a | 2.40 a | 47.6 a | 85.5 a | 91.4 a | 7.69 b | 7.80 b | 8.83 a | 6.99 c | 9.74 b | 12.0 c | 259 c |
| ‘HRS 812’ | 2.00 a | 2.05 a | 2.18 b | 47.3 a | 82.2 a | 98.0 a | 7.63 b | 7.82 b | 8.32 a | 13.0 b | 21.7 a | 25.7 a | 580 a | |
| ‘HRS 897’ | 1.93 a | 1.98 a | 2.02 c | 47.1 a | 80.6 a | 85.5 a | 8.30 a | 8.52 a | 8.69 a | 14.3 a | 18.6 a | 20.2 b | 462 b | |
| ‘Campbell’ Valencia orange | ‘Swingle’ | 1.28 c | 1.35 c | 1.89 a | 54.8 a | 77.9 a | 113 a | 4.12 c | 4.35 c | 6.08 b | 2.48 a | 7.98 a | 1.47 b | 44.5 b |
| ‘HRS 812’ | 1.72 b | 1.74 b | 1.80 a | 51.5 b | 98.4 a | 135 a | 6.12 b | 6.19 b | 6.40 b | 1.06 b | 7.16 a | 6.51 a | 95.6 a | |
| ‘HRS 897’ | 1.84 a | 1.90 a | 1.95 a | 54.6 a | 86.2 a | 87.3 b | 7.67 a | 7.92 a | 8.13 a | 0.75 b | 8.59 a | 1.82 b | 34.9 b | |
| Scion | Rootstocks | Number of HLB Infected Trees | Percentage of HLB Infected Trees ^ | Number of Dead Trees | Percentage of Dead Trees ^ |
|---|---|---|---|---|---|
| (#) | (%) | (#) | (%) | ||
| ‘Mexican’ lime | ‘Swingle’ | 5 b,y | 41.7 b | 2 a | 16.7 a |
| ‘HRS 812’ | 9 a | 75.0 a | 0 b | 0 b | |
| ‘HRS 897’ | 3 c | 25.0 c | 0 b | 0 b | |
| ‘Fina’ clementine mandarin | ‘Swingle’ | 5 a | 41.7 a | 0 a | 0 a |
| ‘HRS 812’ | 6 a | 50.0 a | 0 a | 0 a | |
| ‘HRS 897’ | 5 a | 41.7 a | 0 a | 0 a | |
| ‘Campbell’ Valencia orange | ‘Swingle’ | 7 a | 58.3 a | 3 a | 25.0 a |
| ‘HRS 812’ | 8 a | 66.7 a | 0 b | 0 b | |
| ‘HRS 897’ | 5 a | 41.7 a | 2 a | 16.7 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tirado-Corbalá, R.; Román-Paoli, E.; Segarra-Carmona, A.E.; Estévez de Jensen, C.; Rivera-Ocasio, D. Early Response of ‘Mexican’ Lime, ‘Fina’ Clementine Mandarin, and ‘Campbell’ Valencia Orange on Selected Rootstocks Grown under Fertigation Practices in an Oxisol in Puerto Rico. Horticulturae 2022, 8, 513. https://doi.org/10.3390/horticulturae8060513
Tirado-Corbalá R, Román-Paoli E, Segarra-Carmona AE, Estévez de Jensen C, Rivera-Ocasio D. Early Response of ‘Mexican’ Lime, ‘Fina’ Clementine Mandarin, and ‘Campbell’ Valencia Orange on Selected Rootstocks Grown under Fertigation Practices in an Oxisol in Puerto Rico. Horticulturae. 2022; 8(6):513. https://doi.org/10.3390/horticulturae8060513
Chicago/Turabian StyleTirado-Corbalá, Rebecca, Elvin Román-Paoli, Alejandro E. Segarra-Carmona, Consuelo Estévez de Jensen, and Dania Rivera-Ocasio. 2022. "Early Response of ‘Mexican’ Lime, ‘Fina’ Clementine Mandarin, and ‘Campbell’ Valencia Orange on Selected Rootstocks Grown under Fertigation Practices in an Oxisol in Puerto Rico" Horticulturae 8, no. 6: 513. https://doi.org/10.3390/horticulturae8060513
APA StyleTirado-Corbalá, R., Román-Paoli, E., Segarra-Carmona, A. E., Estévez de Jensen, C., & Rivera-Ocasio, D. (2022). Early Response of ‘Mexican’ Lime, ‘Fina’ Clementine Mandarin, and ‘Campbell’ Valencia Orange on Selected Rootstocks Grown under Fertigation Practices in an Oxisol in Puerto Rico. Horticulturae, 8(6), 513. https://doi.org/10.3390/horticulturae8060513






