Abstract
Arbuscular mycorrhizal fungi (AMF) are efficient for improving crop production and quality in organic farming systems. Our objective was to examine the effects of two AMF inocula, Rhizophagus intraradices and Diversispora spp., against a non-inoculated control on lettuce and green onion grown organically in an intercropping cropping system. At time of harvest, colonization levels were very low, and not different compared to the control that was colonized by the native mycorrhizal community. The yield of inoculated lettuce and green onion was unchanged, as also observed in the root system architecture analysis parameters. In both crops, color was not affected, limiting the possibility for consumers to reject the product. Nitrate accumulation was well below the limits set by European Commission in all treatments. Sugar, chlorophyll, K, Na and P contents were also quantified, showing no particular variations. In AMF-treated lettuce, important phytochemical characteristics, such as phenol content and ascorbic acid, showed a tendency for reduced values, while antioxidant capacity was significantly reduced by both AMF treatments. The study provides a description about the effect of AMF on two important co-cultivated crops. Research should be continued in order to determine best matches between plant material and AMF inocula that could result in enhanced production and nutritional quality.
1. Introduction
Organic production is an integrated farming approach where agro-system health is enhanced and promoted, only natural inputs are used, natural nutrition of crops is promoted, and the natural management of pathogens, weeds, and insects is favored [1,2,3]. This particular production system focuses mainly on soil fertility and rational plant nutrition, the enhancement of biodiversity conservation, eco-friendly control of biotic factors, and the conservation of the ecosystem. Furthermore, in organic farming the health of the plants is favored by applying green or animal manure and composting [4]. The characteristics of organic farming and its products are specific, and they have to comply with several European regulations (i.e., (EC) 834/2007, (EC) 889/2008, (EC) 834/2007, and (EEC) 2092/91) related to rules of implementation, organic production, and labeling [5]. However, in organically produced crops, since only substances derived from nature are permitted, insect/weed control can be difficult to achieve and may result in production loss. Nevertheless, income could be similar despite the possible lower production due to higher prize per production unit.
Lettuce (Lactuca sativa L.) and its various types is considered to be the most popular vegetable of Asteraceae family, which is mainly consumed as fresh salad. Nowadays, there is cultivation of lettuce in every country as an annual vegetable [6]. The Allium genus consists of a plethora of species which mainly are grown for their bulb. However, some of them are cultivated for their fresh above ground parts, such as green onions (Allium cepa L.) [7].
Mycorrhiza fungi form symbiotic associations with plants [8], and they are used widely in organic farming systems to ensure the sustainability of the ecosystem [9]. The fungus and the host’s co-existence physiologically, ecologically, and reproductively affect both individuals. The host is benefited by the nutrient supply through the fungus, while the latter is provided with carbon compounds, a typical mutualistic symbiosis [9,10]. Mycorrhizae are well known for their ability to expand the volume of the soil, which plants can take advantage of, by acting additionally to the rhizosphere. However, there is a growing indication that they may also affect different aspects of plant physiology. The symbiosis of watermelon and the AMF resulted in a positive effect in chilling resistance of the crops [11]. The inoculation of onion with mycorrhizal fungi (Rizophagus intraradices) was found to promote the health of the host, as well as improve onion yield and nutritional quality [12]. Various plants and fungi combinations could produce good results [13]. Moreover, suitable fungal inocula should be selected in order for the relationship between plant and fungi to be successful. Mycorrhizae, in many cases, pre-exist in soils; however, the main concern is to match the genotype of the plant to the mycorrhizal strain. AMF existing in the soil often do not match the crops; therefore, inoculation with a different mycorrhizal strain is required [14]. Environmental conditions are of great importance in order to select the host plant and the strain [14] and, in closed systems, such as greenhouses, inoculation is expected to be beneficial [15]. Hart et al. [15] also mention that the inoculation and establishment of AMF plants is necessary in poor soils before planting non-AMF plants.
Related to this study, R. intraradices is the most common fungus in commercial mycorrhizal inocula, and is a species found very often in agricultural soils. The Diversispora spp. species used was initially isolated from sand dunes, and previous research with lettuce in a saline soil has shown that it may increase root biomass, while host plants had less proline in shoots, relative to plants inoculated with other AMF [16]. Proline accumulation may be a mechanism to overcome saline stress, but also less proline may be an indication of a lack of saline stress. Therefore, the Diversispora species was used in this saline soil to investigate whether it may reduce or increase saline stress and enhance onion growth.
Inoculation with AMF has been applied extensively in the attempt of promoting plant growth and nutrient use efficiency, especially in sustainable agriculture. The present study aimed to examine two different AMF inocula, Rhizophagus intraradices and Diversispora spp., and how they affect organically produced lettuce and green onion, grown in greenhouse soil, concerning their yield and nutritional quality.
2. Materials and Methods
2.1. Experimental Set-Up
The experiment was conducted in a greenhouse, which was certified as organic, in Sapes, Thrace, Greece (25°42′ E, 41°01′ N) in 2020. Certification of the greenhouse was conducted by private bodies approved by the Ministry of Agriculture, under Regulation (EC) No 834/2007 and Regulation No 889/2008. The height of the plastic tunnels was 3.5 m, and they were covered by EVA film—Kritifil 180 m, a 3-layer, long life, thermic plastic (Plastika Kritis, Heraklion, Crete, Greece). The plastic was characterized by the following optical properties: total light transmission 89%, diffusion 45%, and infrared transmission <17%. The irrigation water was characterized by medium hardness, while 4.17 t/ha goat manure was used with 1.92% N; 1.14% P2O5; 2.05% K2O on a fresh weight basis as basal fertilization.
A block design that was completely randomized, with one-way treatment classification, was applied in the greenhouse. The composition of soil was 13.52% organic matter, 8% clay, 20% silt, and 72% sand. The CaCO3 content was 4.1%, the pH was 7.03, and the EC was 7.43 mS/cm. The nutrient content in the soil was as follows: N (total) 266 ppm, P (Olsen) 311 ppm, H3COONH4—exchangeable K 1156 ppm, Mg 1890 ppm, and Ca >2000 ppm were determined according to Sparks (1996) [17]; Fe 17.76 ppm, available Zn 13.16 ppm, Mn 4.61 ppm, and Cu 0.43 ppm were extracted with DTPA (Lindsay and Norvell 1978) [18], and B 18.20 ppm was extracted with hot water (Keren 1996) [19].
2.2. Plant and Fungal Material
Co-cultivation of Romaine lettuce (Lactuca sativa L. cv ‘Paris Island’) with green onion (Allium cepa cv. Sturon) took place in the greenhouse. The lettuce density was 11 plants m−2, and the green onion density was 33 plants m−2. The AMF inocula consisted of substrate (1:1 sand:vermiculite), hyphae, spores, and root fragments of pure pot cultures of the respective fungi, with corn as a host. Application was achieved by mixing the inoculum at 1–2% of final substrate mixed with soil:manure:peat (50:30:20 v/v) and a small part of marble, <2%, dust in the potting mix in the seeding bed for lettuce, or by applying the inoculum (6–7 g in every spot) under the onions at planting.
On 2 February, lettuce seeds were sown in trays with the potting mix described above, and on 31 March the transplant of the seedlings took place in the soil. Green onion sets were planted on 1 April. No plant protection products were used, while weed control was achieved only by hand.
2.3. Harvest-Sample Preparation and Root Scanning
Both species were uprooted using a spade in order to be harvested on 5 May, and the samples were transferred to the lab in the university farm in a few hours. Each replication of lettuce consisted of two plants (a total of three replications), and every treatment (three replications each) of green onion consisted of a 15-plant batch. The samples of both lettuce and green onion were washed to remove any dirt or insects with tap water and blotted, and the roots were cut off and washed with water thoroughly. The root systems of one lettuce plant and two green onion plants per treatment were scanned with specialized software (WinRHIZO Pro, Regent Instruments Inc., Québec, Canada) to determine root length, volume, surface area, and number of tips. Samples of lettuce and green onion roots, control plants, and AMF-treated plants were preserved in alcohol 90% for later determination of AMF root colonization.
The color of lettuce leaves was measured non-destructively on fresh leaves using a chroma meter (Minolta CR-400, Osaka, Japan) at the top of the upper surface of the leaves. For each sample, four measurements out of four different leaves were taken. Regarding green onions, four color measurements were taken along the length of four onions, along the white to the green part of the plant, at 1, 3, 5 and 7 cm. Scales L* (lightness), a*, and b* were used to express color. By using the a* and b* values, the hue angle was calculated, as well as the color C* (chroma) [20]. The L* factor refers to the brightness, the hue angle refers to the color in the form of a sphere where 0° is the red color, 90° is the yellow color, 180° is the green color, and 270° is the blue color, while the factor C* refers to the color intensity [20]. The relative chlorophyll content was also measured non-destructively using a CCM-200 plus chlorophyll meter (Opti-Sciences, Hudson, NH, USA). Part of each sample was frozen at −30 °C for the determination of phytochemical composition, while other parts of the samples were oven-dried at 70 °C for nutrient content quantification.
2.4. Quantification of AMF Colonization in Roots: Clearing and Staining Roots
The roots were stained by being placed in 10% KOH for 40 min at 80 °C in a water bath, washing the KOH with several washes with tap water, followed by placing the roots in acidified tap water for 15 min, decanting the acidic solution, and finally adding a 1:1:1 lactic acid:DI water:glycerol solution with 0.05% trypan blue stain, as described by Sylvia [21]. The estimation of the AMF root length colonization percentage was conducted on a microscope at ×100 to ×400 magnification, after at least 10 pieces, 3 cm long, of the roots were cut out with a scalpel and put on a microscope slide. At least 100 intersections were measured.
2.5. Chemical Composition
Upon tissue homogenization of both species, a quantity of the pulp was filtered through filter paper so that soluble solids could be determined in the extract. The measurement was performed by using the Atago PR-1 (Atago Co., Ltd., Tokyo, Japan) electronic refractionmeter in the tissue extract.
2.6. Antioxidant Capacity
Antioxidant activity was determined using a ferric reducing antioxidant potential (FRAP) assay [22], where 80% methanol (MeOH) was used for extraction. A working solution consisting of CH3COONa (pH 3.6), tripyridyl-triazine, and FeCl3 as added to each sample. The absorbance was measured at 593 nm, and the results were expressed as μg of ascorbic acid equivalent per g fresh weight.
2.7. Phenolics
Total soluble phenols in the supernatants for TPC assay were determined using the Folin–Ciocalteu reagent following the method of Scalbert et al. (1989) [23]. For this, 0.5 mL of each sample was transferred in test tubes, followed by addition of 10% Folin–Ciocalteu reagent and 7.5% NaCO3. The absorbance was measured at 760 nm. The results were expressed as mg of gallic acid equivalent per g fresh weight, according to a reference curve modeled on gallic acid.
2.8. Nitrate Content
The content of nitrate was determined using a photo meter, according to Cataldo et al. [24]. In brief, 2.5 g of frozen homogenized tissue was diluted in 25 mL deionized water, and 0.2 mL of each sample extract was added to two tubes. Then, 0.8 mL of 5% salicylic acid diluted in H2SO4 was added in one tube of each sample. The second tube of each sample was filled with 0.8 mL of pure H2SO4. Next, 19 mL of 2N NaOH was added to all tubes 20 min after the last addition. The absorbance was measured at 410 nm. A reference curve modeled on ΚΝO3 (5–100 mg/L) was constructed for the results to be expressed as μg of nitrate per g fresh weight.
2.9. Vitamin C
The determination of vitamin C (ascorbic acid) was conducted as mentioned by Wevar Oller et al. [25]. Plant tissue and 950 μL sodium phosphate buffer (pH 6.3) were mixed together, and a wavelength of 265 nm was used to measure the absorbance. The procedure was repeated by adding 1 U ascorbate oxidase (AO). The concentration of vitamin C was considered to be the difference in absorbance at 265 nm before and after adding AO against a standard curve constructed using L-Ascorbic acid, and the results were expressed as μmol/g fresh weight.
2.10. Nutrient Content
The determination of P, K, and Na elements in plant tissues requires the organic matter to be destroyed and the inorganic substances to be liquified. In this experiment, plant samples were ashed at 500 °C for 6 h, and then the ash was dissolved in 2M HCl, filtered, and analyzed for P through the molybdenum blue-ascorbic acid method (Olsen and Sommers, 1982) [26]. Phosphorus (P) was determined spectrophotometrically, while the determination of K and Na was conducted on a flame photometer [27].
2.11. Statistical Analysis
Analysis of variance (one single factor with three levels, Diversispora spp., Rhizophagus intraradices, and non-treated (control)) was applied to the data of both species, and a mean separation was conducted by LSD at a significance level of 0.05 using Microsoft Excel.
3. Results and Discussion
3.1. Colonization
Plants grew normally without physiological disorders, and showed balanced development in both crops. Colonization of either with mycorrhizal inocula did not differ statistically on the day of harvest (Table 1). Colonization was very low for both control (non-inoculated, but with local soil community), R. intraradices, and Diversispora spp., with a trend for the latter for lower colonization in lettuce and higher in onion (Table 1). Colonization failure has been mentioned before [28], due to various practices of management (high concentration of P in the soil) in conventional soils, or even organic ones, that do not favor AMF colonization [29,30]. There is little information indicating the best match for AMF and plant species, and there is always the risk of the native AMF competing with the inocula, even if the plant–inocula combination is suitable [31]. This could be because native strains are better adjusted than the inocula. This may also lead to colonization failure. Another parameter that should be considered is the time of inoculation with AMF in order to benefit the crop [31]. Crop response to inoculation with mycorrhiza can be hardly predicted [30], considering that an AMF strain could reduce yield when it is outcompeted by native strains [32]. In our experiment, colonization was measured only at harvest. In further research, colonization should be measured immediately after inoculation to be sure of successful inoculation, immediately after transplant to be sure the inocula has established the field, during cultivation, and at harvest in order to avoid misleading results.

Table 1.
Mycorrhizal colonization and root system parameters of lettuce and green onion on the day of harvest after application of two AMF (R. intraradices or Diversispora spp.) or without (control). Different letters within row (average values ± SE) indicate significant differences (p < 0.05).
3.2. Root Architecture
The root system is the position of entrance for mycorrhizal fungi in order to colonize the host. In lettuce, both inocula tended to favor the root by growing in length and surface area, compared to the control plants (Table 1); however, there were no significant differences. Root volume and the formation of root tips of lettuce also tended to increase with the application of both inocula (Table 1). In green onions, differences between treatments were observed, with a negative trend which was not significant.
Zuccarini [33] found that inoculation with R. intraradices led to increased dry root biomass. Berta et al. [34] also concluded that Prunus ceracifera plants inoculated with Glomus mosseae or Glomus intraradices developed a greater root weight. Furthermore, they noticed that the intensity of root branching was higher in inoculated plants.
3.3. Color Parameters
Color is the first, simplest, and most easily observable quality factor that consumers take into consideration during vegetable selection. A deep-green leafy vegetable is considered more attractive than a yellowish one. Inoculation of lettuce with either inocula did not affect the color parameter L* (Table 2). However, inoculating green onion with R. intraradices led to a significant reduction in L* on the first and fifth cm, but Diversispora spp. significantly reduced L* by 9% on the third cm (Table 2). Inoculating either lettuce or green onion with R. intraradices or Diversispora spp. did not affect C*. Neither the lettuce’s nor the green onion’s hue angle were altered, whether inoculation occurred or not (Table 2).

Table 2.
Color parameters of lettuce and green onion leaves after application of two arbuscular mycorrhizal inocula (R. intraradices or Diversispora spp.) or without (control). Different letters within a column (average values ± SE) indicate significant differences (p < 0.05).
3.4. Yield
The yield of lettuce significantly reduced when plants were treated with Diverspora spp. (Figure 1). Fresh weight tended to increase when both species were treated with R. intraradices in this research (Figure 1), agreeing with the reported results of Baslam et al. [35]. Similar results were also observed by Charoonnart [36], who infected butterhead lettuce with another AMF, Funneloformis mosseae. Moreover, Rozpadek [12] positively correlated the yield of green onion and Rhizophagus irregularis. AMF treatment on green onion in this research tended to increase yield, as also observed by Charron [37], whose results were similar when inoculating onion (Allium cepa L. cv. Improved Autumn Spice) with root segments of G. intraradices or spores of G. versiformae, using the technique of pre-inoculation.
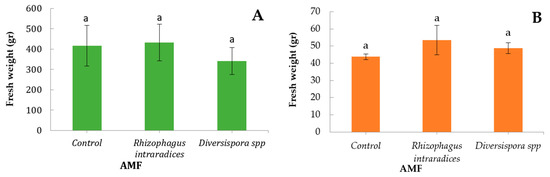
Figure 1.
Yield of lettuce (A) and green onion (B) after application of two arbuscular mycorrhizal inocula (R. intraradices or Diversispora spp.) or without (control). Bars (average values ± SE) followed by different letters indicate significant differences (p < 0.05).
3.5. Chemical Composition
Concerning the nitrate content in both species, there was an 8 and 14% increase, respectively, when inoculated with Diversispora spp., but not a significant one. Inoculation with R. intraradices led to a tendency to increasing nitrate content in green onion by 15% (Table 3). However, it should be mentioned that the upper limits of nitrates decreed by the Regulation (EU) No. 1258/2011 of the European committee were far above the nitrate content found in the leaves. In this case, 4000 mg/g f.w is the critical limit of nitrates content in lettuce leaves at harvest between 1 April and 30 September cultivated in greenhouse, and in this cultivation the maximum value taken was 414 mg/g f.w.

Table 3.
Nitrate content, total soluble solids, potassium, sodium, and phosphorus concentrations, fresh weight, and relative chlorophyll content of lettuce and green onion at harvest after application of two arbuscular mycorrhizal inocula (R. intraradices or Diversispora spp.) or without (control). Different letters within row (average values ± SE) indicate significant differences (p < 0.05).
Inoculation of green onion with AMF appears to favor soluble solids content [38], in contradiction to our results (Table 3). Infection with AMF did not alter Na, K, and P content in lettuce leaves. Tigka and Ipsilantis [16] found the same in their research, using the same inocula on lettuce. The content of soluble solids, Na, K, and P in green onion were not affected by either inocula. Similarly, Lone et al. [39] reported minimum differences in these parameters when green onion was inoculated with AMF. However, the work by Golubkina et al. [39] resulted in enhanced soluble solids and chlorophyll content of green onion by using R. intraradices in combination with humic substances and CO2.
Chlorophyll content in both lettuce and green onion was not affected significantly, whether the plants were treated or not. On the other hand, Zuccarini [26] found that inoculation of lettuce with R. intraradices resulted in an increased chlorophyll content. Moreover, an increase in chlorophyll content of 30% has been noted before in A. cepa when inoculated with a Glomus species [38].
Both Diversispora spp. and R. intraradices did not significantly affect the total phenolic content of lettuce compared to the control. Moreover, inoculating green onion did not have a significant difference on the phenolic content with either Diversispora spp. or R. intraradices. However, the above-mentioned differences were not significant (Figure 2A,B).
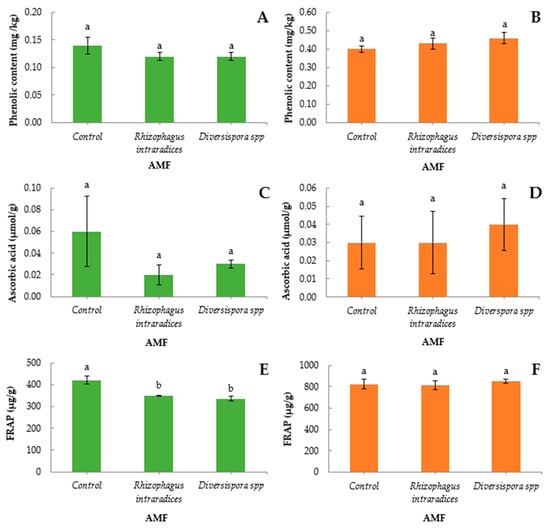
Figure 2.
Phenolic content, ascorbic acid content, and antioxidant capacity (FRAP) of lettuce (A,C,E) and green onion (B,D,F) after application of two arbuscular mycorrhizal inocula (R. intraradices or Diversispora spp.) or without (control). Bars (average values ± SE) followed by different letters indicate significant differences (p < 0.05).
Both inocula negatively but not significantly affected the content of ascorbic acid in lettuce (Figure 2C). Ascorbic acid in green onion was slightly positively, but not significantly affected when inoculated with Diversispora spp., (Figure 2D). Golubkina et al. [38] found a significant elevation of ascorbic acid content in green onion when treated with R. intraradices, whereas, similar to our results, Albrechtova et al. [40] found no effect on ascorbic acid content in green onion when treated with AMF.
Both inocula significantly reduced the antioxidant content in lettuce by 20% (Figure 2E). The above-mentioned tendency for reduced phenolic and ascorbic acid contents, although non-significant, acted cumulative or even synergistically towards a reduced antioxidant potential, as portrayed by the FRAP method. Contrary to our findings, Baslam et al. [35] positively correlated inoculation with AMF and total phenolic content in various lettuce cultivars inoculated with a Glomus spp. In addition, Baum et al. [14] also reported enhanced accumulation of antioxidant compounds in lettuce treated with mycorrhizal fungi. In several recent studies involving lettuce, Charoonnart et al. [36] examined the effect of Funneliformis mosseae, Santander et al. [41] used the Claroideoglomus claroideum inoculum, and Avio et al. [42] inoculated lettuce with Rhizoglomus irregulare and Funneliformis mosseae. The three studies concluded in the increase in antioxidant activity, whether enzymic or not, of AMF-inoculated plants. In our case, a reduction in antioxidant capacity of lettuce with AMF inoculation may have occurred due to the different strains of AMF used in these experiments, or even the different lettuce cultivars combined with different AMF strains. Lower antioxidant compound content may indicate less stressed plants. It is possible that inoculation with the two tested fungi in the present study provided a more favorable environment for the inoculated plants; however, if this was the case, it did not also lead to higher yield or nutrient content. In any case, the physiological mechanisms underlying the effect of AMF on antioxidant compound accumulation has not yet been identified [43]. No significant differences were found in the antioxidant capacity of green onion, although Diversispora spp. tended to increase the antioxidant content of each parameter (Figure 2F).
4. Conclusions
Lettuce and green onion are popular commodities throughout the world, often grown in intercropping systems. The establishment of external AMF is currently an important technique for improving crop production and quality, especially in organic farming, where biological factors are among the few that can be introduced in the production process. By inoculating lettuce and green onion with two AMF, R. intraradices or Diversispora spp., the yield and the root system architecture remained unchanged, compared to the control. The first and most efficient quality factor during vegetable selection by consumers, color, was largely unaffected by both fungi, thereby limiting the possibility for rejection by the market. Nitrate content was within the limits set by the European Commission, while soluble sugar content, K, Na and P contents, and relative chlorophyll content were not significantly affected by the two AMF. In lettuce, important phytochemical characteristics, such as phenolics and ascorbic acid, showed a tendency for reduction with both AMF. This was also displayed by the analysis of antioxidant potential, which was lower in both AMF compared to the control, possibly due to less stressful conditions, although this is not yet clearly identified. The manuscript provides an initial description about the effect of AMF on two important co-cultivated crops. Further research must be conducted in order to determine the best match between plant material and mycorrhizal inocula which leads to the enhancement of production and nutritional quality.
Author Contributions
Conceptualization and methodology: A.K., N.K. and I.I.; data analysis: A.K. and I.I.; experimental measurements: E.P., F.B., N.K. and I.I.; writing—original draft preparation: E.P. and F.B.; writing—review and editing: E.P., F.B., I.I. and A.K.; supervision: A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hydbom, S.; Ernfors, M.; Birgander, J.; Hollander, J.; Jensen, E.S.; Olsson, P.A. Reduced tillage stimulated symbiotic fungi and microbial saprotrophs, but did not lead to shift in the saprotrophic microorganism community structure. Appl. Soil Ecol. 2017, 119, 104–114. [Google Scholar] [CrossRef]
- Mathew, R.P.; Feng, Y.; Githinji, L.; Ankumah, R.; Balkcom, K.S. Impact of No-Tillage and Conventional Systems on Soil Microbial Communities. Appl. Environ. Soil Sci. 2012, 2012, 548620. [Google Scholar] [CrossRef] [Green Version]
- Kladivko, E.J. Tillage systems and soil ecology. Soil Till. Res. 2001, 61, 61–66. [Google Scholar] [CrossRef]
- Rigby, D.; Cáceres, D. Organic farming and the sustainability of agricultural systems. Agric. Syst 2001, 68, 21–40. [Google Scholar] [CrossRef]
- Sanders, J. (Ed.) Evaluation of the EU Legislation on Organic Farming; Thünen-Institute: Braunschweig, Germany, 2013. [Google Scholar]
- De Vries, I.M. Origin and domestication of Lactuca sativa L. Gen. Res. Crop. Evol. 1997, 44, 165–174. [Google Scholar] [CrossRef]
- Pareek, S.; Sagar, N.A.; Shama, A.; Kumar, V. Onion (Allium Cepa L.). In Fruit and Vegetable Phytochemicals: Chemistry and Human Health, 2nd ed.; Elhadi, M.Y., Ed.; John Wiley and Sons Ltd.: Hoboken, NJ, USA, 2018; pp. 1145–1161. [Google Scholar]
- Trivedi, P.; Wallenstein, M.D.; Delgado-Baquerizo, M.; Singh, B.K. Microbial modulators and mechanisms of soil carbon storage. In Soil Carbon Storage, Modulators, Mechanisms and Modeling; Brajesh, K.S., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 73–115. [Google Scholar]
- The significance of mycorrhizal fungi for crop productivity and ecosystem sustainability in organic farming systems. In Proceedings of the 16th IFOAM Organic World Congress, Modena, Italy, 16–20 June 2008.
- Harley, J.L. The Significance of mycorrhizal. Mycol. Res. 1989, 92, 129–139. [Google Scholar] [CrossRef]
- Bidabadi, S.S.; Mehralian, M. Arbiscular Mycorrhizal Fungi Inoculation to Enhance Chilling Stress Tolerance of Waterlemon. Gesunde Pflanz. 2020, 72, 171–179. [Google Scholar] [CrossRef]
- Rozpądek, P.; Rąpała-Kozik, M.; Wężowicz, K.; Grandin, A.; Karlsson, S.; Wanżny, R.; Anielska, T.; Turnau, K. Arbuscular mycorrhiza improves yield and nutritional properties of onion (Allium cepa). Plant. Physiol. Biochem. 2016, 107, 264–272. [Google Scholar] [CrossRef]
- Klironomos, J.N. Variation in plant response to native and exotic arbuscular mycorrhizal fungi. Ecology 2003, 84, 2292–2301. [Google Scholar] [CrossRef]
- Baum, C.; El-Tohamy, W.; Gruda, N. Increasing the productivity and product quality of vegetable crops using arbuscular mycorrhizal fungi: A review. Sci. Hortic. 2015, 187, 131–141. [Google Scholar] [CrossRef]
- Hart, M.M.; Antunes, P.M.; Chaudhary, V.B.; Abbott, L.K. Fungal inoculants in the field: Is the reward greater than the risk? Fun. Ecol. 2017, 32, 126–135. [Google Scholar] [CrossRef] [Green Version]
- Tigka, T.; Ipsilantis, I. Effects of sand dune, desert and field arbuscular mycorrhizae on lettuce (Lactuca sativa L.) growth in a natural saline soil. Sci. Hortic. 2020, 264, 109191. [Google Scholar] [CrossRef]
- Sparks, D.L. Methods of Soil Analysis: Part 3, Chemical methods 5.3; American Society of Agronomy Inc.: Madison, WI, USA, 1996. [Google Scholar]
- Lindsay, W.L.; Norvell, W. Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Boron, K.R. Methods of Soil Analysis, Part. 3: Chemical Methods, 3rd ed.; Sparks, D.L., Page, E.L., Helmke, P.A., Loeppert, R.H., Eds.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 603–626. [Google Scholar]
- McGuire, R.G. Reporting of Objective Color Measurements. Hortscience 1992, 72, 1254–1255. [Google Scholar] [CrossRef] [Green Version]
- Sylvia, D.M. Vesicular-Arbuscular Mycorrhizal Fungi. In Methods of Soil Analysis: Part. 2. Microbiological and Biochemical Properties, 3rd ed.; Peter, J., Bootomley, P.J., Scott Angle, J., Weaver, R.W., Eds.; Soil Science Society of America: Madison, WI, USA, 2020; pp. 351–360. [Google Scholar]
- Jones, A.; Pravadali-Cekic, S.; Dennis, G.R.; Bashir, R.; Mahon, P.J.; Shalliker, R. A Ferric reducing antioxidant potential (FRAP) of antioxidants using reaction flow chromatography. Anal. Chim. Acta. 2017, 967, 93–101. [Google Scholar] [CrossRef]
- Scalbert, A.; Monties, B.; Janin, G. Tannins in Wood: Comparison of Different Estimation Methods. J. Agric. 1989, 37, 1324–1329. [Google Scholar] [CrossRef]
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil. Sci. Plant. Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Oller, A.L.W.; Agostini, E.; Milrad, S.R.; Medina, M.I. In situ and de novo biosynthesis of vitamin C in wild type and transgenic tomato hairy roots: A precursor feeding study. Plant Sci. 2009, 177, 28–34. [Google Scholar] [CrossRef]
- Olsen, S.R.; Sommers, L.E. Phosphorus. In Methods of Soil Analysis: Part. 2, 2nd ed.; Sparks, D.L., Page, E.L., Helmke, P.A., Loeppert, R.H., Eds.; American Society of Agronomy, Inc.: Madison, WI, USA, 1982; pp. 403–430. [Google Scholar]
- Jones, J.B., Jr.; Case, V.W. Sampling, Handling and Analyzing Plant Tissue Samples. In SSSA Book Series, Soil Testing and Plant. Analysis, 3rd ed.; Westerman, R.L., Ed.; Soil Science Society of America, Inc.: Madison, WI, USA, 1990; pp. 389–420. [Google Scholar]
- Scullion, J.; Eason, W.R.; Scott, E.P. The effectivity of arbuscular mycorrhizal fungi from high input conventional and organic grassland and grass–arable rotations. Plant. Soil 1998, 204, 243–254. [Google Scholar] [CrossRef]
- Dekkers, T.B.M.; van der Werff, P.A. Mutualistic functioning of indigenous arbuscular mycorrhizae in spring barley and winter wheat after cessation of long-term phosphate fertilization. Mycorrhiza 2001, 10, 195–201. [Google Scholar] [CrossRef]
- Charron, G.; Furlan, V.; Bernier-Cardou, M.; Doyon, G. Response of onion plants to arbuscular mycorrhizae. 1. Effects of inoculation method and phosphorus fertilization on biomass and bulb firmness. Mycorrhiza 2001, 11, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Gosling, P.; Hodge, A.; Goodlass, G.; Bending, G.D. Arbuscular mycorrhizal fungi and organic farming. Sci. Direct 2006, 113, 17–35. [Google Scholar] [CrossRef]
- Kahiluoto, H.; Vestberg, M. The effect of arbuscular mycorrhiza on biomass production and phosphorus uptake from sparingly soluble sources by leek (Allium porrum L.) in Finnish field soils. Biol. Agric. Hortic. 1998, 16, 65–85. [Google Scholar] [CrossRef]
- Zuccarini, P. Mycorrhizal infection ameliorates chlorophyll content and nutrient uptake of lettuce exposed to saline irrigation. Pant Soil Environ. 2007, 53, 283–289. [Google Scholar] [CrossRef] [Green Version]
- Berta, G.; Trotta, A.; Fusconi, A.; Hooker, J.E.; Munro, M.; Atkinson, D.; Giovannetti, M.; Morini, S.; Fortuna, P.; Tisserant, B.; et al. Arbuscular mycorrhizal induced changes to plant growth and root system morphology in Prunus cerasifera. Tree Physiol. 1995, 15, 281–293. [Google Scholar] [CrossRef]
- Balsam, M.; Garmendia, I.; Goicoechea, N. Arbuscular Mycorrhizal Fungi (AMF) Improved Growth and Nutritional Quality of Greenhouse-Grown Lettuce. J. Agric. Food Chem. 2011, 59, 5504–5515. [Google Scholar]
- Charoonnart, P.; Seraypheap, K.; Chadchawan, S.; Wangsomboondee, T. Arbuscular mycorrhizal fungus improves the yield and quality of Lactuca sativa in an organic farming system. Sci. Asia 2016, 42, 315–322. [Google Scholar] [CrossRef] [Green Version]
- Charron, G.; Furlan, V.; Bernier-Cardou, M.; Doyon, G. Response of onion plants to arbuscular mycorrhizae. 2 Effects of nitrogen fertilization on biomass and bulb firmness. Mycorrhiza 2001, 11, 145–150. [Google Scholar] [CrossRef]
- Golubkina, N.; Krivenkov, L.; Sekara, A.; Vasileva, V.; Tallarita, A.; Caruso, G. Prospects of Arbuscular Mycorrhizal Fungi Utilization in Prodyction of Allium Plants. Plants 2020, 9, 279. [Google Scholar] [CrossRef] [Green Version]
- Lone, R.; Shuab, R.; Wani, K.A.; Ganaie, M.A.; Tiwari, A.K.; Koul, K.K. Mycorrhizal influence on metabolites, indigestible oligosaccharides, mineral nutrition and phytochemical constituents in onion (Allium cepa L.) plant. Sci. Hortic. 2015, 193, 55–61. [Google Scholar] [CrossRef]
- Albrechtova, J.; Latr, A.; Nedorost, L.; Pokluda, R.; Posta, K.; Vosatka, M. Dual inoculation with mycorrhizal and saprotrophic fungi applicable in sustainable cultivation improves the yield and nutritive value of onion. Sci. World J. 2012, 2012, 374091. [Google Scholar] [CrossRef] [Green Version]
- Santander, C.; Ruiz, A.; García, S.; Aroca, R.; Cumming, J.; Cornejo, P. Efficiency of two arbuscular mycorrhizal fungal inocula to improve saline stress tolerance in lettuce plants by changes of antioxidant defense mechanisms. J. Sci. Food Agric. 2019, 100, 1577–1587. [Google Scholar] [CrossRef] [PubMed]
- Avio, L.; Sbrana, C.; Giovannetti, M.; Frassinetti, S. Arbuscular mycorrhizal fungi affect total phenolics content and antioxidant activity in leaves of oak leaf lettuce varieties. Sci. Hortic. 2017, 224, 265–271. [Google Scholar] [CrossRef]
- Avio, L.; Turrini, A.; Giovannetti, M.; Sbrana, C. Designing the Ideotype Mycorrhizal Symbiots for the Production of Healthy Food. Front. Plant. Sci. 2018, 9, 1089. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).