Abstract
Fruit ripening is a process that produces fruit with top sensory qualities that are ideal for consumption. For the plant, the final objective is seed dispersal. One of the fruit characteristics observed by consumers is texture, which is related to the ripening and softening of the fruit. Controlled and orchestrated events occur to regulate the expression of genes involved in disassembling and solubilizing the cell wall. Studies have shown that changes in pectins are closely related to the loss of firmness and fruit softening. For this reason, studying the mechanisms and enzymes that act on pectins could help to elucidate the molecular events that occur in the fruit. This paper provides a review of the enzyme rhamnogalacturonan endolyase (RGL; EC 4.2.2.23), which is responsible for cleavage of the pectin rhamnogalacturonan I (RGL-I) between rhamnose (Rha) and galacturonic acid (GalA) through the mechanism of β-elimination during fruit ripening. RGL promotes the loosening and weakening of the cell wall and exposes the backbone of the polysaccharide to the action of other enzymes. Investigations into RGL and its relationship with fruit ripening have reliably demonstrated that this enzyme has an important role in this process.
1. Introduction
Ripe fruit has organoleptic qualities, such as flavor, aroma, and texture, that are optimal for consumption [1]. Texture influences the molecular mechanisms that result in changes to phytohormone levels, protein synthesis and volatile compounds, chlorophyll degradation, the accumulation of fat-soluble pigments, solubilization, and disassembly of the polysaccharides of the cell wall and middle lamella [2]. During the fruit ripening process, changes associated with texture lead to reduced firmness, a short shelf life, and susceptibility to attack by pathogens and other organisms [3,4].
To understand this phenomenon from the primary cell wall degradation perspective, studies have been performed on enzymes that cleave links between polysaccharides such as cellulose, hemicellulose, and pectins. The cell wall composition varies between species and tissues, and conditions such as biotic and abiotic stress can affect their composition [5,6,7]. Among the pectins, rhamnogalacturonan I polysaccharide (RG-I) is an interesting polymer to study, because an increase in the activity of the enzymes α-arabinofuranosidases and β-galactosidases has been reported to alter RG-I levels at different stages of fruit ripening. These enzymes cleave ramifications of arabinans and galactans; as a consequence, during ripening, an increase in solubilization and depolymerization occurs in this type of pectin, as in the case of European and Chinese pear, banana, strawberry, and apple [8,9,10,11]. The rhamnogalacturonan endolyase enzyme (RGL), which is responsible for cleaving the backbone of RG-I, appears to be an active element in fruit ripening [12,13]. Therefore, RGL has been a target of RNAi experiments and heterologous expression to understand its relevance in plant development and fruit ripening [14,15].
This review offers a panoramic view of our current knowledge about α-L-rhamnopyranosyl-(1→4)-α-D-galactopyranosyluronate endolyase, also known as rhamnogalacturonan endolyase or RGL, which belongs to polysaccharide lyase group four. Here, we describe their role in fruit ripening and present new perspectives for investigation into these enzymes.
2. Rhamnogalacturonan-I in the Fruit Ripening
Pectins are abundant polysaccharides involved in the growth and development of cells; they are mainly present in the middle lamella, which are soft tissues in the joint between the primary and secondary cell walls [16]. Seventeen different polysaccharides and twenty types of links compose three types of pectins described in plants: homogalacturonan (HG), rhamnogalacturonan-I (RG-I), and rhamnogalacturonan-II (RG-II) (Figure 1) [17]. These polysaccharides are found in average proportions of 65%, 20–35%, and 10% [18]. Kaczmarska et al. [19] presented an elaborate review of RG-I focused on the structure and functionality of this polysaccharide in the plant cell wall.
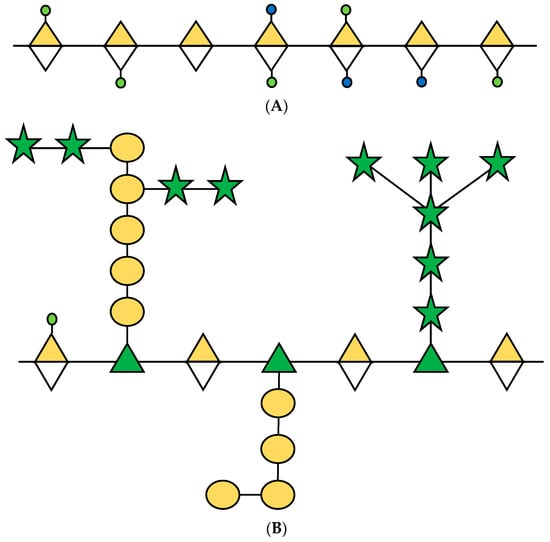
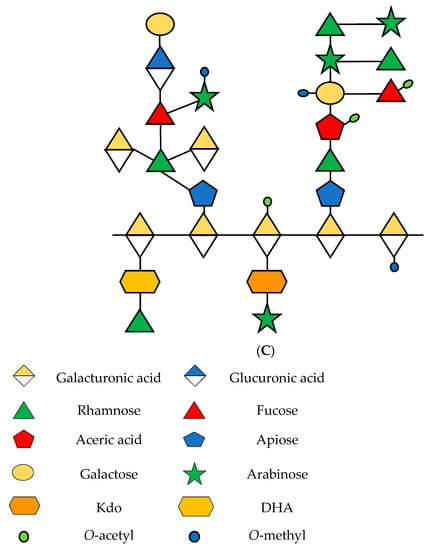
Figure 1.
Schematic representation of principal pectins in the primary cell wall of plants. (A) HG; (B) RG-I and (C) RG-II. Sugars are represented according to the Symbol Nomenclature for Glycans (SNFG) [20]. Based on [16,21,22].
The structure of RG-I is composed of alternating units of rhamnose (Rha) and galacturonic acid (GalA), linked to α-1,2 and α-1,4, respectively. In addition, Rha can be found in conjunction with a various proportions of galactose (Gal) and arabinose (Ara) [23].
The differences in fruit ripening and softening between different kinds of fruits depend on the cellular structure and composition, as well as enzymes present in the cell wall during this process [24].
Peña and Carpita [25] described RG-I debranching resulting in the loss of arabinans with high sugar units and correlated that loss with changes in the firmness and cell separation in the fruits of four cultivars of apple that had been stored for a long time. In olive fruit development, in the transition between the cherry and black stages during ripening, a release of the side chains of RG-I occurs [26]. In particular, the release of side chains of arabinans helps determine the ripening stage [27].
In Dacryodes edulis, a fruit from Africa, a 50% loss of the harvest has been described as a result of a rapid reduction in firmness. The analysis of the pectins that compose the fruit indicates that in the ripening stage, it is possible to find low molecular weight RG-I because of the modifications to this polysaccharide during the fruit ripening process [28].
3. Structural and Phylogenetic Classification of the Rhamnogalacturonan Endolyase Enzyme
The RGL enzyme is not only found in plants but has also been described in saprophytic fungi and bacteria of the genera Aspergillus, Bacillus, and Dickeya [29,30,31,32]. This species secretes enzymes into the extracellular environment that cleave the cell wall polysaccharides of plants for use as a carbon source [33]. Silva et al. [34] detailed a comprehensive review of endo- and exohydrolases that cleave RG-I. The authors built a phylogenetic tree in which RGL was classified into two groups: polysaccharide lyase 4 (PL4) and polysaccharide lyase 11 (PL11). This information is directly related to a report by Lombard et al. [35], which explains that enzymes in the same PL group have the same enzymatic activity but a different three-dimensional structure due to convergent evolution from different scaffolds. For this reason, the PL4 and PL11 enzyme groups cleave the same linkages, but their proteins differ structurally. In addition, PL4 is present in plants and fungi, and PL11 is present solely in bacteria. In particular, heterologous expression of two RGLs of the PL11 group from Bacteroides ovatus revealed a different preference for substrates: the Bo4416 clone prefers RG-I without substitutions in its side chains, while Bo3128 degrades polysaccharides with short substitutions in their side chains [36]. This result implies that different isoforms of the same enzyme have activity depending on the side chains of the substrate. This phenomenon has not been reported or studied in the RGLs of plants or fungi.
Garron et al. [37] described the structure of the PL4 group of enzymes as having three β-sandwich domains and that of the PL11 group as having eight bladed β-propellers. Furthermore, both groups depend on Ca2+ ions for enzymatic activity, where the β-elimination catalytic mechanism requires a Ca2+ ion coordinated between sites −1 and +1 [38]. Nevertheless, other reports indicate that Ca2+ is a hexacoordinated ion that interacts with five protein ligands and one water molecule and plays a structural role in promoting catalysis [39]. The enzymatic activity of RGL in plants is suppressed in the absence of Ca2+ ions. A 2 mM concentration of CaCl2 recovered the activity to 100% [15].
In the Protein Data Bank (https://www.rcsb.org/, accessed on 25 January 2022), four crystallographic structures of RGL from the PL4 group exist in fungi and bacteria. All reported structures share Ca2+ ions as single molecules coordinated by protein-ligand residues [31,39] (Table 1). Although there are no crystallographic structures of RGL in plants, sequence alignment between RGL from Fragaria chiloensis and Aspergillus aculeatus shows a high percentage of identity in the carbohydrate binding module (CBM) [15].

Table 1.
Crystallographic structures of RGL belonging to the PL4 group, deposited in the Protein Data Bank.
The PL4 group of RGL enzymes (EC 4.2.2.23) cleave the backbone of RG-I internally between L-α-rhamnopyranosyl-(1→4)-α-D-galactopyranosyluronic acid bonds. The result of this cleavage is the formation of L-rhamnopyranose at the reduced end and 4-deoxy-4,5-unsaturated D-galactopyranosyluronic acid at the nonreduced end [40]. Three functional domains have been reported in plant RGLs, similar to those in A. aculeatus: domain I or the catalytic domain; domain II or the fibronectin type III-like domain (FnIII); and domain III or the carbohydrate binding module (CBM) (Figure 2) [15,35].
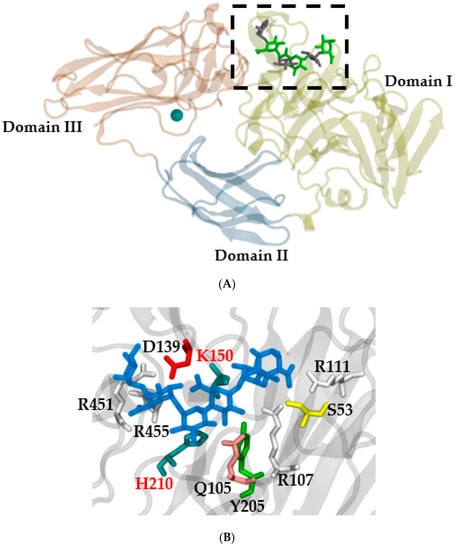
Figure 2.
(A) Three-dimensional protein structure of RGL from A. aculeatus (PDB ID 1NKG) with a crystalized ligand from PDB ID 3NJV with the K150A mutation. Every domain is tagged, and the calcium ion is represented in VDW (green). The active site is shown in the dotted box. (B) Active site zoom (dotted box Figure 1A); the ligand is in blue, and the different amino acids are presented in different colors. H210 and K150 are key amino acids for enzyme activity [31]. The representation was created with Visual Molecular Dynamics [42].
To determine the phylogenetic classification of RGLs, Mokshina et al. [41] built a phylogenetic tree using 98 plant-derived sequences containing the three functional domains. Six classification groups were generated. Similar to dicotyledonous plants, monocotyledonous plants are classified into two clades tagged as Groups I and II. Vascular plants and primitive angiosperms are classified into two separate clades. In F. chiloensis, similar results have been obtained, where dicot plants formed two clades, while monocots formed a different group [15]. Unfortunately, the authors of both reports did not establish which sequences or regions were key to the differences in clade classification. However, monocots could be classified into another group, due to their low level of pectins; for that reason, there could be a smaller number of isoforms.
4. Rhamnogalacturonan Endolyase in Fruit Ripening
As mentioned earlier, changes in fruit texture are directly associated with the molecular event that alters the cell wall composition, either by depolymerization of glycans and polysaccharides, pectin solubilization, or the loss of neutral sugars during pectin ramification [43] (Table 2). Wang et al. [44] summarized genes that code for enzymes that participate in the fruit ripening process as those with activity on fruit pectins, where the publication by Molina-Hidalgo et al. [14] focused solely on an RGL of F. x ananassa. At the molecular level, the gene that encodes FaRGlyase1 is located in a locus related to fruit hardness and firmness. The transcript levels of this gene increase as the fruit ripens, whereas silencing of this gene by agroinfiltration of RNAi into fruits indicates that no phenotypic differences are observed; however, at the histological level observed by pectin staining, the cell wall remains more intact with RGL inhibition. Similar experimental results were reported by Méndez-Yáñez et al. (2020) [15], who observed an increase in the transcript levels of the gene that codes for RGL during fruit ripening. Using the heterologous expression of RGL from F. chiloensis in Pichia pastoris yeast, the biochemical parameters and enzymatic characterization of the enzyme were obtained. An assay in seeds of A. thaliana dyed with ruthenium red demonstrated that the heterologously expressed enzyme was active and could cleave seed mucilage, which is rich in non-ramified pectins [45]. Promoter analysis of Fragaria vesca accounts for the increased regulation of response elements for phytohormones, such as abscisic acid (ABA), methyl jasmonate, gibberellins, salicylic acid, and auxin, and stress response, such as drought, defense, and low temperature [46].
RGL enzymes have also been studied in the fruit ripening process of tomato (Table 2). Unlike strawberry, tomato is a climacteric fruit, and for this reason, the crosstalk between phytohormones and transcription factors associated with the ripening process differs considerably [47]. Promoter analysis of the gene that encodes RGL in S. lycopersicum and A. thaliana demonstrated a response to ABA, dehydration, and light [48]. In another study, the expression of the RGL promoter Solyc11g011300 was constitutively induced in fruits via the CaMV35S sequence and tagged with GUS as a reporter gene; the results showed that gene expression was first observed in the red ripe stage [49]. On the other hand, constitutive expression of three RGL isoenzymes during the development and ripening of S. lycopersicum fruit resulted in firmer tomatoes with a longer shelf life due to a cosuppression mechanism [12]. One of the genes evaluated displays a strong correlation with the increase in ethylene production and therefore could be important for the loss of firmness. Regarding the expression of the other genes, the authors suggest that they participate in the modification of RG-I at the initial stages of the ripening [13].

Table 2.
Summary of results obtained in research on RGL, until the year 2022.
Table 2.
Summary of results obtained in research on RGL, until the year 2022.
| Specie | Results | Reference |
|---|---|---|
| F. x ananassa | Changes in the spatio-temporal expression of FaRGlyase1 across the fruit ripening. Transient silencing of FaRGlyase1 induces a high conservation of cell wall. FaRGlyase1 expression could be induced by abscisic acid. FaRGlyase1 gene is located in a QTL linked to firmness. | [14] |
| F. chiloensis | Structural characterization and biochemical parameters of RGL enzyme. Transcripts levels increase during fruit ripening. Phylogenetic tree with same results according to Mokshina et al. (2018) [37]. Calcium dependence on enzymatic activity. | [15] |
| F. chiloensis | Elements of response to phytohormones: ABA, methyl jasmonate, gibberellins, salicylic acid, and auxin; response elements to biotic and abiotic stress such as defense, low temperatures, and drought. | [46] |
| S. lycopersicum L. | GUS under the control of an RGL promoter induces expression in the red ripe stage of fruit ripening. Increased expression levels of GUS 40 days after anthesis. | [49] |
| S. lycopersicum L. | Constitutive expression of RGL promoter to produce tomatoes with greater firmness and a longer shelf life. Overexpression under CaMV35S of Solyc11g011300 promoter reduces viability and pollen germination and decreases the number of seeds and fruits. Delay in fruit ripening of one week. | [12] |
| S. lycopersicum L. and A. thaliana | RGL genes respond to abscisic acid, dehydration, and light. | [48] |
| S. lycopersicum L. | Solyc11g011300 gene increases activity in fruit ripening, correlated with ethylene production increasing. Solyc04g076630 and Solyc04g076660 could participate in re-engineering on RG-I pectin at the beginning of fruit ripening. | [13] |
5. New Perspectives and Challenges
A large battery of enzymes participate in the fruit ripening process, acting in a coordinated manner to disassemble the cell wall. Environmental and other signaling conditions, phytohormones, and transcription factors, among others, are responsible for regulating the protein level and activity of these enzymes. The matrix formed by cellulose, hemicellulose, and pectin constitutes one of the most important components of the plant cell wall and is a key target for enzymes and proteins during the developmental processes of fruit ripening and softening. In this matrix, RG-I accounts for almost 30% of the pectic fraction and is of paramount importance for understanding the structural dynamics during different physiological processes. The challenge for researchers is to identify the key enzymes that take part in these different processes with special attention given to fruit ripening and conditions needed for adequate enzyme performance. The integrative understanding of the participation of each of these members, specifically RGLs, in the changes in fruit texture could help to create strategies that delay their emergence or inhibit their activity, thus prolonging the shelf life of the fruit. Possible strategies will be generated depending on the role each enzyme plays in modulating the functional properties of the cell wall at a specific developmental stage.
Author Contributions
L.M.-Q., P.R. and A.M.-Y., writing—original draft preparation; L.M.-Q., P.R. and A.M.-Y., writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
The Agencia Nacional de Investigación y Desarrollo (ANID, Chile) [grants REDES #190093 to L.M.-Q.; FONDECYT #1220782 to L.M.-Q.; FONDECYT PostDoctoral #3220284 to A.M.-Y.; REDES #190078 to P.R.; FONDECYT #1211057 to P.R.] supported the work. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare the absence of any commercial or financial relationships as a potential conflict of interest.
References
- Kader, A.A. Flavor quality of fruits and vegetables. J. Sci. Food Agric. 2008, 88, 1863–1868. [Google Scholar] [CrossRef]
- Giovannoni, J.J. Genetic regulation of fruit development and ripening. Plant Cell 2004, 16, S170–S180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, P.; Powell, A.L.T.; Orlando, R.; Bergmann, C.; Gutiérrez-Sánchez, G. A proteomic analysis of ripening tomato fruit infected by Botrytis cinerea. J. Proteome Res. 2012, 11, 2178–2192. [Google Scholar] [CrossRef] [Green Version]
- Alkan, N.; Fortes, A.M. Insights into molecular and metabolic events associated with fruit response to post-harvest fungal pathogens. Front. Plant Sci. 2015, 6, 889. [Google Scholar] [CrossRef] [Green Version]
- Baldwin, L.; Domon, J.; Klimek, J.F.; Fournet, F.; Sellier, H.; Gillet, F.; Pelloux, J.; Lejeune-Hénaut, I.; Carpita, N.C.; Rayon, C. Structural alteration of cell wall pectins accompanies pea development in response to cold. Phytochemistry 2014, 104, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Amos, R.A.; Mohnen, D. Critical review of plant cell wall matrix polysaccharide glycosyltransferase activities verified by heterologous protein expression. Front. Plant Sci. 2019, 10, 915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bacete, L.; Hamann, T. The role of mechanoperception in plant cell wall integrity maintenance. Plants 2020, 9, 574. [Google Scholar] [CrossRef]
- Mwaniki, M.W.; Mathooko, F.M.; Hiwasa, K.; Tateishi, A.; Yokotani, N.; Ushijima, K.; Nakano, R.; Inaba, A. β-galactosidase and α-L-arabinofuranosidase activities and gene expression in European and Chinese pear fruit during ripening. J. Jpn. Soc. Hortic. Sci. 2007, 76, 85–90. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, J.P.; Su, J.; Li, X.P.; Chen, W.X. Changes in alpha-L-arabinofuranosidase activity in peel and pulp of banana (Musa sp.) fruits during ripening and softening. J. Plant Physiol. Mol. Biol. 2007, 33, 131–136. [Google Scholar]
- Rosli, H.G.; Civello, P.M.; Martínez, G.A. Alpha-L-arabinofuranosidase from strawberry fruit: Cloning of three cDNAs, characterization of their expression and analysis of enzymatic activity in cultivars with contrasting firmness. Plant Physiol. Biochem. 2009, 47, 272–281. [Google Scholar] [CrossRef]
- Yang, H.; Liu, J.; Dang, M.; Zhang, B.; Li, H.; Meng, R.; Qu, D.; Yang, Y.; Zhao, Z. Analysis of β-galactosidase during fruit development and ripening in two different texture types of apple cultivars. Front. Plant Sci. 2018, 9, 539. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Jiménez, V.; Berumen-Varela, G.; Burgara-Estrella, A.; Orozco-Avitia, J.; Ojeda-Contreras, A.; Trillo-Hernández, E.; Rivera-Domínguez, M.; Troncoso-Rojas, R.; Báez-Sañudo, R.; Datsenka, T.; et al. Functional analysis of tomato rhamnogalacturonan lyase gene Solyc11g011300 during fruit ripening development and ripening. J. Plant Physiol. 2018, 231, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Trillo-Hernández, E.A.; Orozco-Avitia, J.A.; Ojeda-Contreras, A.J.; Berumen-Varela, G.; Ochoa-Jiménez, V.A.; Troncoso-Rojas, R.; Rivera-Domínguez, M.; Baez-Flores, M.E.; Hernández-Oñate, M.A.; Tiznado-Hernández, M.E. Análisis de expresión de genes codificantes de isoenzimas de ramnogalacturonano liasa durante el desarrollo y la madurez del fruto de tomate. Rev. Fitotec. Mex. 2021, 44, 529–536. [Google Scholar] [CrossRef]
- Molina-Hidalgo, F.J.; Franco, A.R.; Villatoro, C.; Medina-Puche, L.; Mercado, J.A.; Hidalgo, M.A.; Monfort, A.; Caballero, J.L.; Muñoz-Blanco, J.; Blanco-Portales, R. The strawberry (Fragaria x ananassa) fruit-specific rhamnogalacturonate lyase 1 (FaRGLyase1) gene encodes an enzyme involved in the degradation of cell wall middle lamellae. J. Exp. Bot. 2013, 64, 1471–1483. [Google Scholar] [CrossRef] [Green Version]
- Méndez-Yáñez, A.; González, M.; Carrasco-Orellana, C.; Herrera, R.; Moya-León, M.A. Isolation of a rhamnogalacturonan lyase from the Chilean strawberry fruit and its biochemical characterization. Plant Physiol. Biochem. 2020, 146, 411–419. [Google Scholar] [CrossRef]
- Mohnen, D. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef]
- Voragen, A.G.J.; Coenen, G.; Verhoef, R.P.; Schols, H.A. Pectin, a versatile polysaccharide present in plant cell walls. Struct. Chem. 2009, 20, 263–275. [Google Scholar] [CrossRef] [Green Version]
- Atmodjo, M.A.; Hao, Z.; Mohnen, D. Evolving views of pectin biosynthesis. Annu. Rev. Plant Biol. 2013, 64, 747–779. [Google Scholar] [CrossRef] [Green Version]
- Kaczmarska, A.; Pieczywek, P.M.; Cybulska, J.; Zdunek, A. Structure and functionality of Rhamnogalacturonan I in the cell wall and in solution: A review. Carbohydr. Polym. 2021, 278, 118909. [Google Scholar] [CrossRef]
- Varki, A.; Cummings, R.D.; Aebi, M.; Packer, N.H.; Seeberger, P.H.; Esko, J.D.; Stanley, P.; Hart, G.; Darvill, A.; Kinoshita, T.; et al. Symbol Nomenclature for Graphical Representations of Glycans. Glycobiology 2015, 25, 1323–1324. [Google Scholar] [CrossRef] [Green Version]
- Nepogodiev, S.; Fais, M.; Hughes, D.; Field, R.A. Synthesis of apiose-containing oligosaccharide fragments of the plant cell wall: Fragments of rhamnogalacturonan-II side chains A and B, and apiogalacturonan. Org. Biomol. Chem. 2011, 9, 6670. [Google Scholar] [CrossRef] [PubMed]
- Yapo, B. Rhamnogalacturonan-I: A structurally puzzling and functionally versatile polysaccharide from plant cell walls and mucilages. Polym. Rev. 2011, 52, 391–413. [Google Scholar] [CrossRef]
- Van Audenhove, J.; Bernaerts, T.; De Smet, V.; Delbaere, S.; Van Loey, A.M.; Hendrickx, M.E. The structure and composition of extracted pectin and residual cell wall material from processing tomato: The role of a stepwise approach versus high-pressure homogenization-facilitated acid extraction. Foods 2021, 10, 1064. [Google Scholar] [CrossRef] [PubMed]
- Tucker, G.; Yin, X.; Zhang, A.; Wang, M.; Zhu, Q.; Liu, X.; Xie, X.; Chen, K.; Grierson, D. Ethylene and fruit softening. Food Qual. Saf. 2017, 1, 253–267. [Google Scholar] [CrossRef]
- Peña, M.J.; Carpita, N.C. Loss of highly branched arabinans and debranching of rhamnogalacturonan I accompany loss of firm texture and cell separation during prolonged storage of apple. Plant Physiol. 2004, 135, 1305–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiménez, A.; Rodríguez, R.; Fernández-Caro, I.; Guillén, R.; Fernández-Bolaños, J.; Heredia, A. Olive fruit cell wall: Degradation of pectic polysaccharides during ripening. J. Agric. Food Chem. 2001, 49, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Coimbra, M.A.; Cardoso, S.M.; Lopes-da-Silva, J.A. Olive pomace, a source for valuable arabinan-rich pectic polysaccharides. Top. Curr. Chem. 2010, 294, 129–141. [Google Scholar]
- Missang, C.E.; Renard, C.; Baron, A.; Drilleau, J. Changes in the pectic fraction of bush butter (Dacryodes edulis (G Don) HJ Lam) fruit pulp during ripening. J. Sci. Food Agric. 2001, 81, 781–789. [Google Scholar] [CrossRef]
- Laatu, M.; Condemine, G. Rhamnogalacturonate lyase RhiE is secreted by the out system in Erwinia chrysanthemi. J. Bacteriol. 2003, 185, 1642–1649. [Google Scholar] [CrossRef] [Green Version]
- Ochiai, A.; Itoh, T.; Kawamata, A.; Hashimoto, W.; Murata, K. Plant cell wall degradation by saprophytic Bacillus subtilis strains: Gene clusters responsible for rhamnogalacturonan depolymerization. J. Appl. Environ. Microbiol. 2007, 73, 3803–3813. [Google Scholar] [CrossRef] [Green Version]
- Jensen, M.H.; Otten, H.; Christensen, U.; Borchert, T.V.; Christensen, L.L.H.; Larsen, S.; Lo Leggio, L. Structural and biochemical studies elucidate the mechanism of rhamnogalacturonan lyase from Aspergillus aculeatus. J. Mol. Biol. Mol. 2010, 404, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.R.; Larsen, D.M.; Meyer, A.S.; Mikkelsen, J.D. Identification, expression and characterization of a novel bacterial RGI Lyase enzyme for the production of biofunctional fibers. Enzym. Microb. Technol. 2011, 49, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Gardner, J.G. Polysaccharide degradation systems of the saprophytic bacterium Cellvibrio japonicus. World J. Microbiol. Biotechnol. 2016, 32, 121. [Google Scholar] [CrossRef]
- Silva, I.R.; Jers, C.; Meyer, A.S.; Mikkelsen, J.D. Rhamnogalacturonan I modifying enzymes: An update. New Biotechnol. 2016, 33, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Lombard, V.; Bernard, T.; Rancurel, C.; Brumer, H.; Coutinho, P.; Henrissat, B. A hierarchical classification of polysaccharide lyases for glycogenomics. Biochem. J. 2010, 432, 437–444. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Wang, Y.; Yi, H.; Zhang, G.; Zhang, L.; Mayo, K.H.; Yuan, Y.; Zhou, Y. Biochemical characterization of two rhamnogalacturonan lyases from Bacteroides ovatus ATCC 8483 with preference for RG-I substrates. Front. Microbiol. 2022, 12, 799875. [Google Scholar] [CrossRef] [PubMed]
- Garron, M.; Cygler, M. Structural and mechanistic classification of uronic acid-containing polysaccharide lyases. Glycobiology 2010, 20, 1547–1573. [Google Scholar] [CrossRef] [Green Version]
- Charnock, S.J.; Brown, I.E.; Turkenburg, J.P.; Black, G.W.; Davies, G.J. Convergent evolution sheds light on the anti- β-elimination mechanism common to family 1 and 10 polysaccharide lyases. Proc. Natl. Acad. Sci. USA 2002, 99, 12067–12072. [Google Scholar] [CrossRef] [Green Version]
- McDonough, M.A.; Kadirvelraj, R.; Harris, P.; Poulsen, J.; Larsen, S. Rhamnogalacturonan lyase reveals a unique three-domain modular structure for polysaccharide lyase family 4. FEBS Lett. 2004, 565, 188–194. [Google Scholar] [CrossRef]
- Azadi, P.; O’Neill, M.; Bergmann, C.; Darvill, A.G.; Albersheim, P. The backbone of the pectic polysaccharide rhamnogalacturonan I is cleaved by an endohydrolase and an endolyase. Glycobiology 1995, 5, 783–789. [Google Scholar] [CrossRef]
- Mokshina, N.; Makshanova, O.; Nazipova, A.; Gorshkov, O.; Gorshkova, T. Flax rhamnogalacturonan lyases: Phylogeny, differential expression and modeling of protein structure. Physiol. Plant. 2018, 167, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Humprey, W.; Dalke, A.; Schulten, K. VMD—Visual Molecular Dynamics. J. Mol. Graph. Model. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Posé, S.; Paniagua, C.; Matas, A.J.; Gunning, P.; Morris, V.J.; Quesada, M.A.; Mercado, J.A. A nanostructural view of the cell wall disassembly process during fruit ripening and postharvest process during fruit ripening and postharvest storage by atomic force microscopy. Trends Food Sci. Technol. 2019, 87, 47–58. [Google Scholar] [CrossRef]
- Wang, D.; Yeasts, T.H.; Uluisik, S.; Rose, J.K.C.; Seymour, G.B. Fruit softening: Revisiting the role of pectin. Trends Plant Sci. 2018, 23, 302–310. [Google Scholar] [CrossRef]
- Ajayi, O.O.; Held, M.A.; Showalter, A.M. Two β-glucuronosyltransferases involved in the biosynthesis of type II arabinogalactans function in mucilage polysaccharide matrix organization in Arabidopsis thaliana. BMC Plant Biol. 2021, 21, 245. [Google Scholar] [CrossRef]
- Méndez-Yañez, A. Aislamiento y caracterización de la enzima ramnogalacturonano endoliasa inducida durante la maduración de frutos de Fragaria chiloensis (L.) Mill. In Tesis Doctorado en Ciencias, Mención Ingeniería Genética Vegetal; Universidad de Talca: Región del Maule, Chile, 2019. [Google Scholar]
- Symons, G.M.; Chua, Y.J.; Ross, J.J.; Quittenden, L.J.; Davies, N.W.; Reid, J.B. Hormonal changes during non-climateric ripening in strawberry. J. Exp. Bot. 2012, 63, 4741–4750. [Google Scholar] [CrossRef] [Green Version]
- Berumen-Varela, G.; Rivera-Domínguez, M.; Troncoso-Rojas, R.; Báez-Sañudo, R.; Tiznado-Hernández, M. Physiological function of rhamnogalacturonan lyase genes based in the analysis of cis-acting elements located in the promoter region. Res. J. Biotechnol. 2017, 12, 77–108. [Google Scholar]
- Berumen-Varela, G.; Ochoa-Jiménez, V.; Burgara-Estrella, A.; Trillo-Hernández, E.; Ojeda-Contreras, A.; Orozco-Avitia, A.; Rivera-Domínguez, M.; Troncoso-Rojas, R.; Báez-Sañudo, R.; Datsenka, T.; et al. Functional analysis of a tomato (Solanum lycopersicum L.) rhamnogalacturonan lyase promoter. J. Plant Physiol. 2018, 229, 175–184. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).