A Fruit Colour Development Index (CDI) to Support Harvest Time Decisions in Peach and Nectarine Orchards
Abstract
1. Introduction
2. Materials and Methods
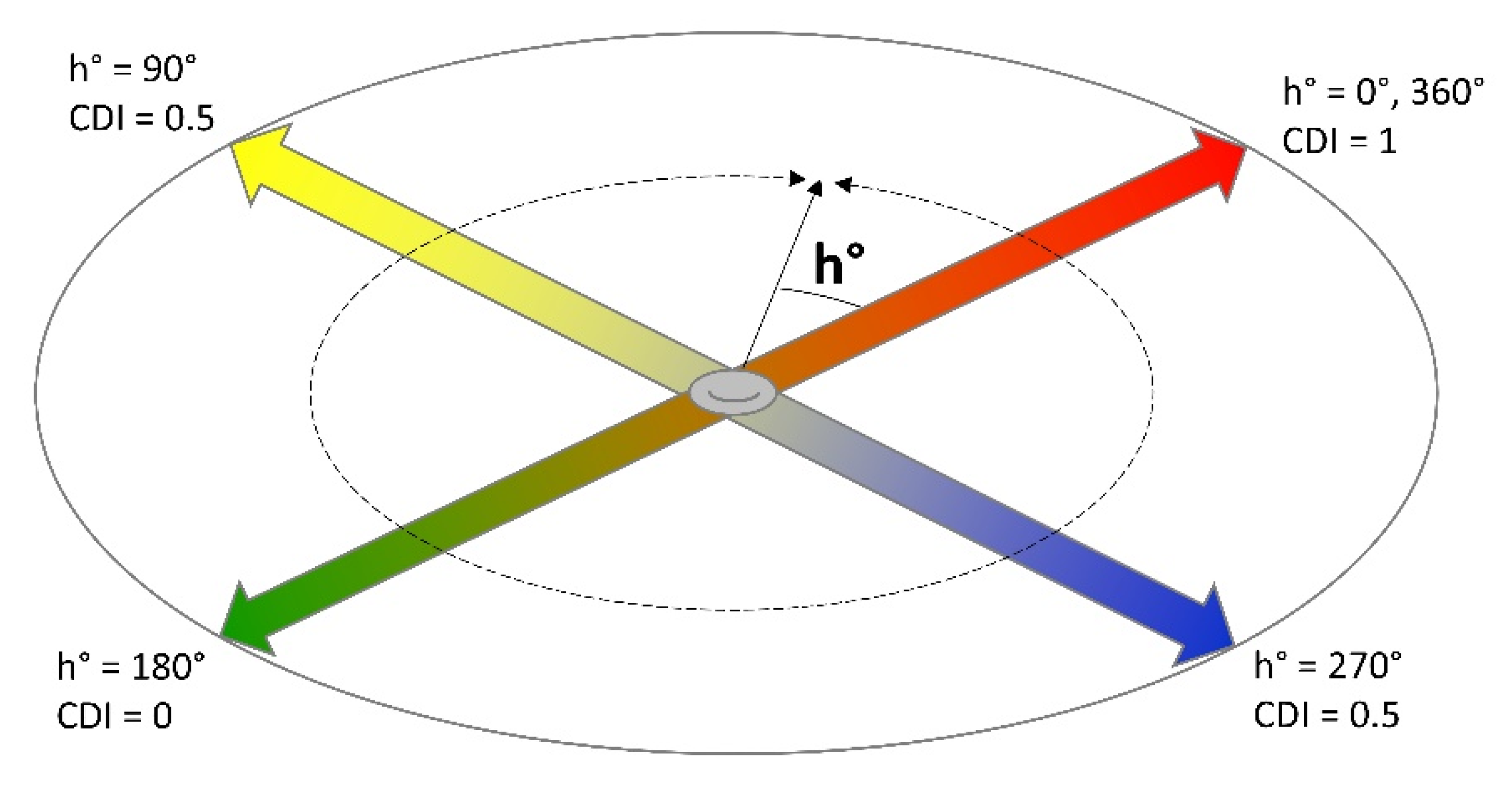
2.1. Colour Development Index (CDI)
2.2. Experimental Sites and Cultivars
2.3. Ground-Based Platform for Fruit Detection and Colour Recognition
2.4. Effects of Time of Scan, Cultivar and Canopy Side on Colour Measurements
2.5. Fruit Maturity and Harvest Time
2.6. CDI Relationship with Maturity and Time from Harvest
2.7. Geoprocessing, CDI Data Extraction and Spatial Mapping
2.8. Statistical Analysis
3. Results
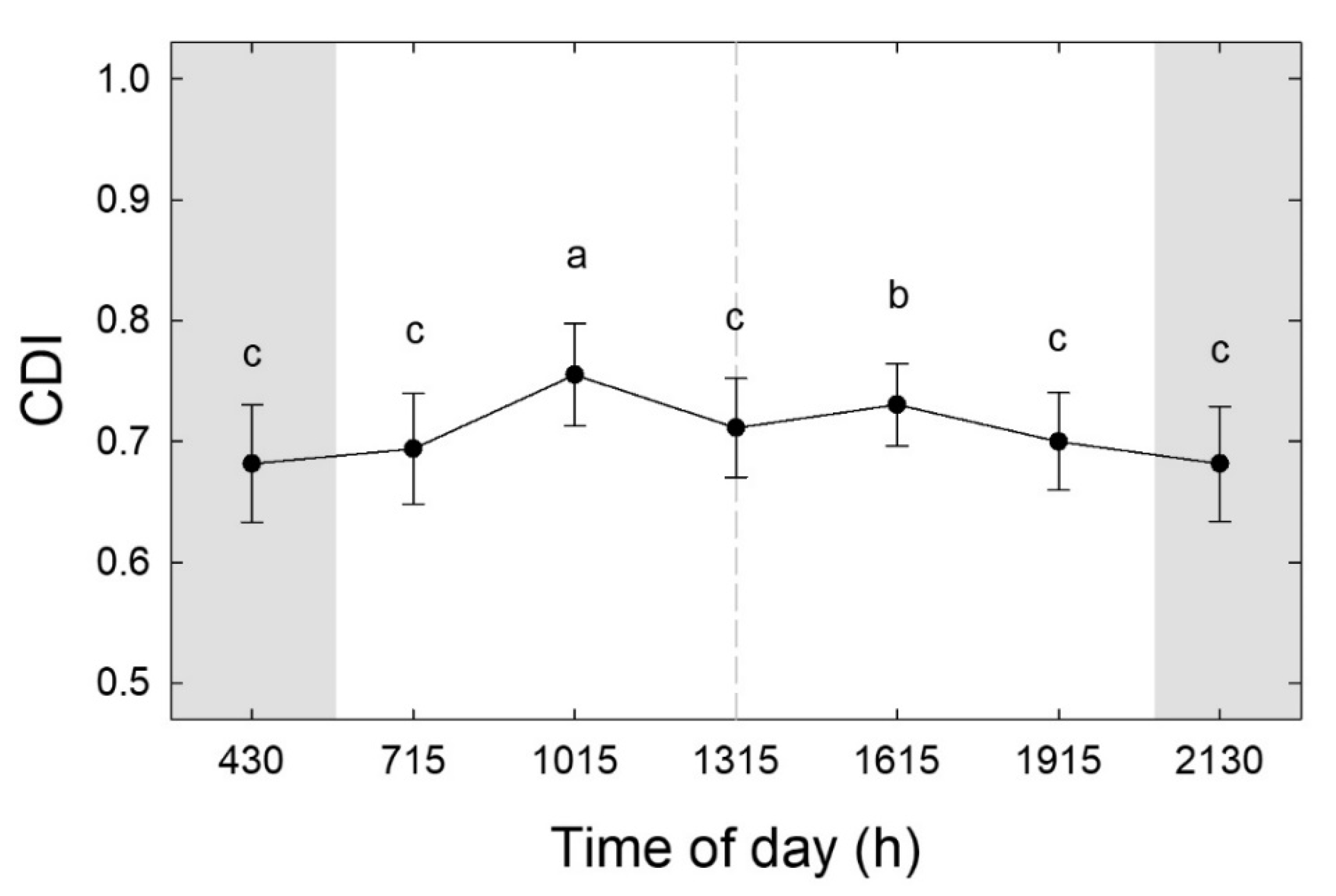
3.1. Effect of Time of Scan and Canopy Side on Colour Measurements
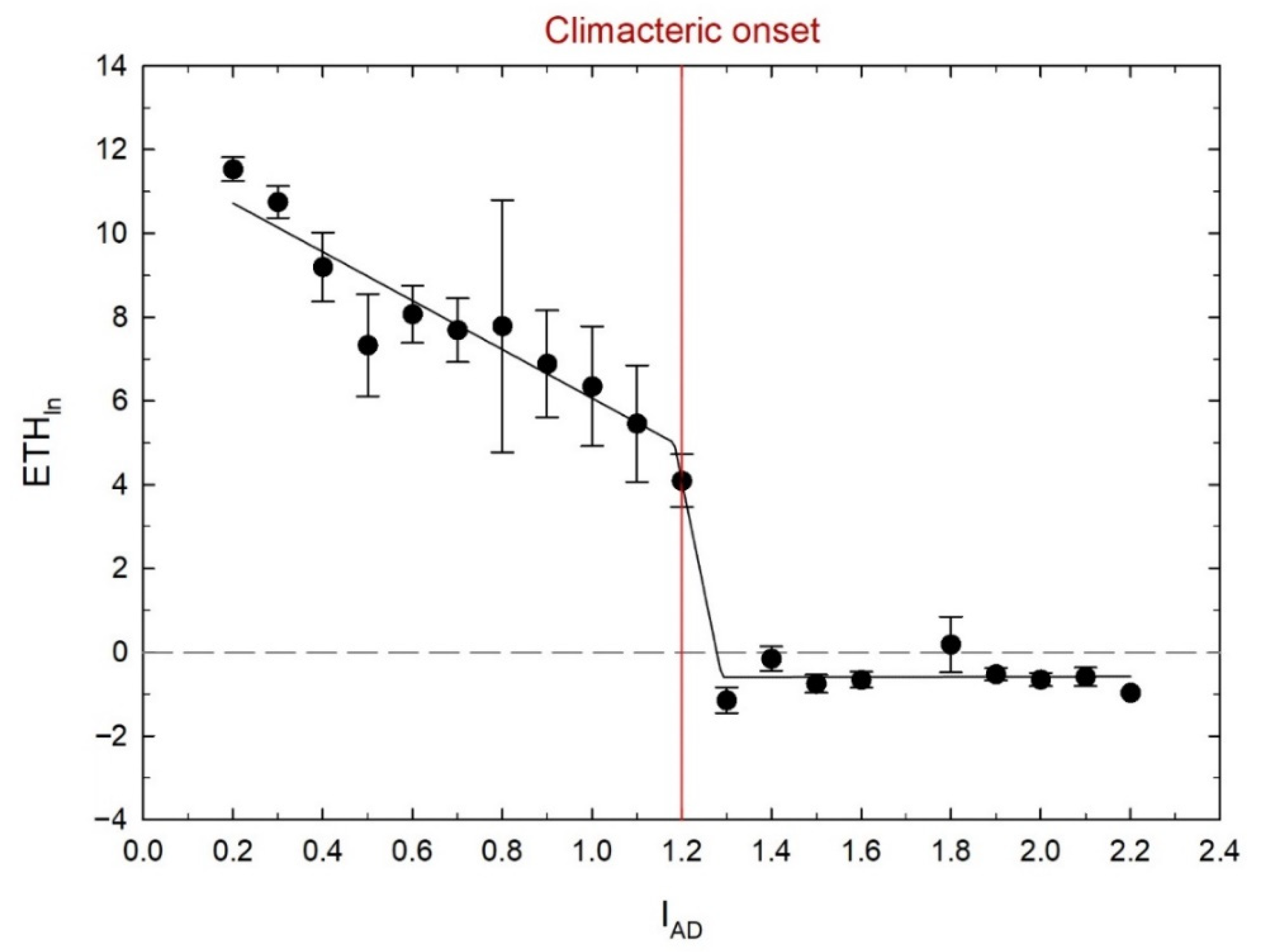
3.2. Relationship between IAD and Ethylene
3.3. CDI Relationship with IAD and Time from Harvest
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rose, D.C.; Chilvers, J. Agriculture 4.0: Broadening Responsible Innovation in an Era of Smart Farming. Front. Sustain. Food Syst. 2018, 2, 87. [Google Scholar] [CrossRef]
- Cubero, S.; Aleixos, N.; Moltó, E.; Gómez-Sanchis, J.; Blasco, J. Advances in Machine Vision Applications for Automatic Inspection and Quality Evaluation of Fruits and Vegetables. Food Bioprocess Technol. 2011, 4, 487–504. [Google Scholar] [CrossRef]
- Robertson, J.A.; Meredith, F.I.; Horvat, R.J.; Senter, S.D. Effect of Cold Storage and Maturity on the Physical and Chemical Characteristics and Volatile Constituents of Peaches (Cv. Cresthaven). J. Agric. Food Chem. 1990, 38, 620–624. [Google Scholar] [CrossRef]
- Byrne, D.H.; Nikolic, A.N.; Burns, E.E. Variability in Sugars, Acids, Firmness, and Color Characteristics of 12 Peach Genotypes. J. Am. Soc. Hortic. Sci. 1991, 116, 1004–1006. [Google Scholar] [CrossRef]
- Luchsinger, L.E.; Walsh, C.S. Development of an objective and non-destructive harvest maturity index for peaches and nectarines. Acta Hortic. 1998, 465, 679–687. [Google Scholar] [CrossRef]
- Ferrer, A.; Remón, S.; Negueruela, A.I.; Oria, R. Changes during the ripening of the very late season Spanish peach cultivar Calanda: Feasibility of using CIELAB coordinates as maturity indices. Sci. Hortic. 2005, 105, 435–446. [Google Scholar] [CrossRef]
- Do Nascimento Nunes, M.C. Color Atlas of Postharvest Quality of Fruits and Vegetables; Wiley-Blackwell: Ames, IA, USA, 2008. [Google Scholar]
- Scalisi, A.; O’Connell, M.G.; Pelliccia, D.; Plozza, T.; Frisina, C.; Chandra, S.; Goodwin, I. Reliability of a Handheld Bluetooth Colourimeter and Its Application to Measuring the Effects of Time from Harvest, Row Orientation and Training System on Nectarine Skin Colour. Horticulturae 2021, 7, 255. [Google Scholar] [CrossRef]
- Crisosto, C.H. Stone fruit maturity indices: A descriptive review. Postharvest News Inf. 1994, 5, 65N–68N. [Google Scholar]
- Mirbod, O.; Choi, D.; Thomas, R.; He, L. Overcurrent-driven LEDs for consistent image colour and brightness in agricultural machine vision applications. Comput. Electron. Agric. 2021, 187, 106266. [Google Scholar] [CrossRef]
- Silwal, A.; Parhar, T.; Yandun, F.; Kantor, G. A Robust Illumination-Invariant Camera System for Agricultural Applications. In Proceedings of the 2021 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), Prague, Czech Republic, 27 September–1 October 2021; pp. 3292–3298. [Google Scholar] [CrossRef]
- Scalisi, A.; McClymont, L.; Underwood, J.; Morton, P.; Scheding, S.; Goodwin, I. Reliability of a commercial platform for estimating flower cluster and fruit number, yield, tree geometry and light interception in apple trees under different rootstocks and row orientations. Comput. Electron. Agric. 2021, 191, 106519. [Google Scholar] [CrossRef]
- Islam, M.S.; Scalisi, A.; O’Connell, M.G.; Morton, P.; Scheding, S.; Underwood, J.; Goodwin, I. A ground-based platform for reliable estimates of fruit number, size and colour in stone fruit orchards. Comput. Electron. Agric. 2022. [Google Scholar]
- Islam, M.S. RGB-to-CIELab Conversion. Available online: https://github.com/sirajulislam/RGB-to-CIELab/blob/main/rgb2_labrgblch.py/ (accessed on 20 December 2021).
- Costa, G.; Noferini, M.; Fiori, G.; Torrigiani, P. Use of Vis/NIR Spectroscopy to Assess Fruit Ripening Stage and Improve Management in Post-Harvest Chain Invited Mini-Review Fresh Produce Use of Vis/NIR Spectroscopy to Assess Fruit Ripening Stage and Improve Management in Post-Harvest Chain. Fresh Prod. 2009, 1, 35–41. [Google Scholar]
- Soto, A.; Ruiz, K.B.; Ziosi, V.; Costa, G.; Torrigiani, P. Ethylene and auxin biosynthesis and signaling are impaired by methyl jasmonate leading to a transient slowing down of ripening in peach fruit. J. Plant Physiol. 2012, 169, 1858–1865. [Google Scholar] [CrossRef] [PubMed]
- Bonora, E.; Stefanelli, D.; Costa, G. Nectarine fruit ripening and quality assessed using the index of absorbance difference (IAD). Int. J. Agron. 2013, 2013, 242461. [Google Scholar] [CrossRef]
- Bonora, E.; Noferini, M.; Vidoni, S.; Costa, G. Modeling fruit ripening for improving peach homogeneity in planta. Sci. Hortic. 2013, 159, 166–171. [Google Scholar] [CrossRef]
- Spadoni, A.; Cameldi, I.; Noferini, M.; Bonora, E.; Costa, G.; Mari, M. An innovative use of DA-meter for peach fruit postharvest management. Sci. Hortic. 2016, 201, 140–144. [Google Scholar] [CrossRef]
- Stefanelli, D.; Lopresti, J.; Hale, G.; Jaeger, J.; Frisina, C.; Jones, R.; Tomkins, B. Modelling peach and nectarine ripening during storage using the IAD maturity index. Acta Hortic. 2017, 1154, 17–23. [Google Scholar] [CrossRef]
- Zhang, B.; Peng, B.; Zhang, C.; Song, Z.; Ma, R. Determination of fruit maturity and its prediction model based on the pericarp index of absorbance difference (IAD) for peaches. PLoS ONE 2017, 12, e0177511. [Google Scholar] [CrossRef]
- Victorian Horticulture Industry Network DA Meter IAD Maturity Classes: Database—HIN. Available online: http://www.hin.com.au/networks/profitable-stonefruit-research/stonefruit-maturity-and-fruit-quality/da-meter-iad-maturity-classes-database (accessed on 10 February 2022).
- Zhang, P.; Wei, Y.; Xu, F.; Wang, H.; Chen, M.; Shao, X. Changes in the chlorophyll absorbance index (IAD) are related to peach fruit maturity. N. Z. J. Crop Hortic. Sci. 2020, 48, 34–46. [Google Scholar] [CrossRef]
- Scalisi, A.; O’Connell, M.G. Application of visible/NIR spectroscopy for the estimation of soluble solids, dry matter and flesh firmness in stone fruits. J. Sci. Food Agric. 2021, 101, 2100–2107. [Google Scholar] [CrossRef]
- Frisina, C.; Stefanelli, D.; Giri, K.; Tomkins, B. A revised method for the field collection and storage of fruit ethylene samples using evacuated vials. Sci. Hortic. 2018, 236, 123–126. [Google Scholar] [CrossRef]
- Scalisi, A.; Pelliccia, D.; O’Connell, M.G. Maturity Prediction in Yellow Peach (Prunus persica L.) Cultivars Using a Fluorescence Spectrometer. Sensors 2020, 20, 6555. [Google Scholar] [CrossRef] [PubMed]
- Gallucci, M. General Analyses for Linear Models. Available online: https://gamlj.github.io/ (accessed on 15 March 2022).
- Mendoza, F.; Dejmek, P.; Aguilera, J.M. Calibrated color measurements of agricultural foods using image analysis. Postharvest Biol. Technol. 2006, 41, 285–295. [Google Scholar] [CrossRef]
- Kang, S.P.; East, A.R.; Trujillo, F.J. Colour vision system evaluation of bicolour fruit: A case study with ‘B74′ mango. Postharvest Biol. Technol. 2008, 49, 77–85. [Google Scholar] [CrossRef]
- Gardner, J.L. Comparison of calibration methods for tristimulus colorimeters. J. Res. Natl. Inst. Stand. Technol. 2007, 112, 129–138. [Google Scholar] [CrossRef]
- Bible, B.B.; Singha, S. Canopy Position Influences CIELAB Coordinates of Peach Color. HortScience 1993, 28, 992–993. [Google Scholar] [CrossRef]
- Herrero-Langreo, A.; Fernández-Ahumada, E.; Roger, J.M.; Palagós, B.; Lleó, L. Combination of optical and non-destructive mechanical techniques for the measurement of maturity in peach. J. Food Eng. 2012, 108, 150–157. [Google Scholar] [CrossRef][Green Version]
- Kader, A.A. Fruit maturity, ripening, and quality relationships. Acta Hortic. 1999, 485, 203–208. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Costa, G. Preharvest factors affecting peach quality. In The Peach: Botany, Production and Uses; CABI: Wallingford, UK, 2008; pp. 536–549. [Google Scholar] [CrossRef]
- Anthony, B.M.; Minas, I.S. Redefining the impact of preharvest factors on peach fruit quality development and metabolism: A review. Sci. Hortic. 2022, 297, 110919. [Google Scholar] [CrossRef]

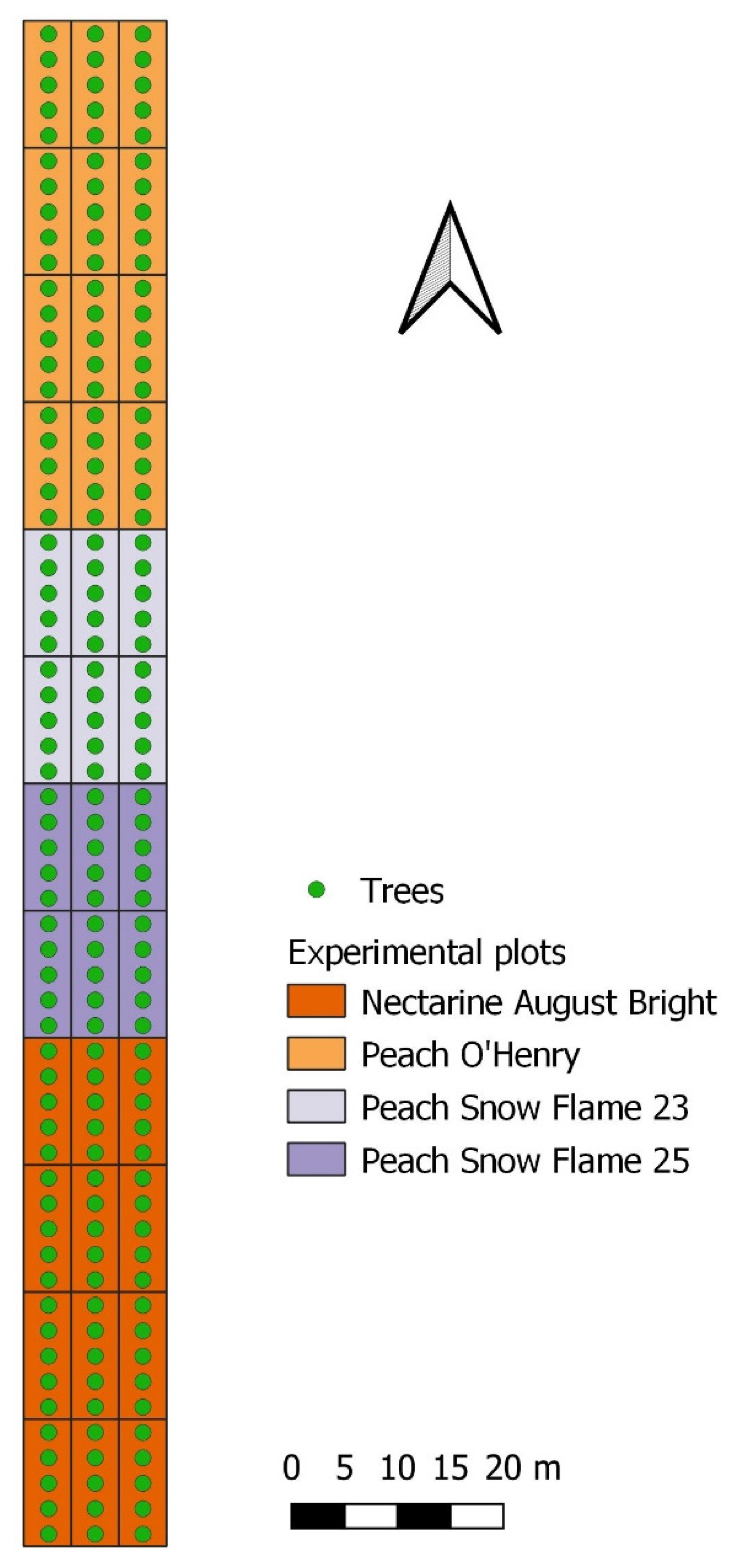





| Cultivar | Images/Cultivar | Images/Experimental Plot | Fruit Detections/Image |
|---|---|---|---|
| Peach ‘O’Henry’ | 395 | 33 (3) | 175 (53) |
| Peach ‘Snow Flame 23’ | 194 | 32 (2) | 282 (83) |
| Peach ‘Snow Flame 25’ | 187 | 31 (2) | 152 (38) |
| Nectarine ‘August Bright’ | 412 | 34 (3) | 75 (20) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scalisi, A.; O’Connell, M.G.; Islam, M.S.; Goodwin, I. A Fruit Colour Development Index (CDI) to Support Harvest Time Decisions in Peach and Nectarine Orchards. Horticulturae 2022, 8, 459. https://doi.org/10.3390/horticulturae8050459
Scalisi A, O’Connell MG, Islam MS, Goodwin I. A Fruit Colour Development Index (CDI) to Support Harvest Time Decisions in Peach and Nectarine Orchards. Horticulturae. 2022; 8(5):459. https://doi.org/10.3390/horticulturae8050459
Chicago/Turabian StyleScalisi, Alessio, Mark G. O’Connell, Muhammad S. Islam, and Ian Goodwin. 2022. "A Fruit Colour Development Index (CDI) to Support Harvest Time Decisions in Peach and Nectarine Orchards" Horticulturae 8, no. 5: 459. https://doi.org/10.3390/horticulturae8050459
APA StyleScalisi, A., O’Connell, M. G., Islam, M. S., & Goodwin, I. (2022). A Fruit Colour Development Index (CDI) to Support Harvest Time Decisions in Peach and Nectarine Orchards. Horticulturae, 8(5), 459. https://doi.org/10.3390/horticulturae8050459






