Volatile Oil Components of Laurel (Laurus nobilis L.) Leaves Obtained from Plants Cultivated under Salinity Stress Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Analysis of Volatile Compounds
2.2.1. Head Space-Solid Phase Microextraction (HS-SPME)
2.2.2. Gas Chromatography (GC×GC-FID) Analysis
3. Statistical Analysis
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Djilani, A.; Dicko, A. The therapeutic benefits of essential oil. Nutr. Well-Being Health 2012, 7, 155–179. [Google Scholar]
- Carvalho, I.I.; Estevinho, B.N.; Santos, L. Application of microencapsulated essential oils in cosmetic and personal healthcare products—A review. Int. J. Cosmet. Sci. 2016, 2, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Margaris, N.; Koedam, A.; Vokou, D. Aromatic Plants: Basic and Applied Aspects; Martinus Nijhoff Publishers: Leiden, The Netherlands, 1982. [Google Scholar]
- Tisserand, R.B. The Art of Aromatherapy; Healing Arts Press: Rochester, VT, USA, 1997. [Google Scholar]
- Baris, O.; Güllüce, M.; Sahin, F.; Ozer, H.; Kılıc, H.; Ozkan, H.; Sökmen, M.; Ozbek, T. Biological activities of the essential oil and methanol extract of Achillea biebersteini Afan Afan (Asteraceae). Turk. J. Biol. 2006, 30, 65–73. [Google Scholar]
- Wei, A.; Shibamoto, T. Antioxidant/Lipoxygenase Inhibitory activities and chemical compositions of selected essential oil. J. Agric. Food Chem. 2010, 58, 7218–7225. [Google Scholar] [CrossRef]
- Zouari, S.; Ayadi, I.; Fakhfakh, N.; Jdir, H.; Aloui, L.; Kossentini, M.; Rebai, A.; Zouari, N. Essential oil variation in wildpopulations of Artemisia saharae (Asteraceae) from Tunisia: Chemical composition, antibacterial and antioxidant properties. Bot. Stud. 2014, 55, 76. [Google Scholar] [CrossRef] [Green Version]
- Zouari, N. Essential oils: A less known side. Medic. Aromat. Plants 2013, 1, e145. [Google Scholar] [CrossRef] [Green Version]
- Esma’ilzadeh Behabadi, S.; Sharifi, M. Increase secondary metabolite production plants using biological alysytvrhay. J. Cells Tissues. 2013, 20, 119–128. [Google Scholar]
- Askary, M.; Talebi, S.M.; Amini, F.; Balout, A.D. Effect of NaCl and iron oxide nanoparticles on Mentha piperita essential oil composition. Environ. Exp. Biol. 2016, 14, 27–32. [Google Scholar] [CrossRef]
- Sarkik, A.; Stappen, I. Essential oils and their single compounds in cosmetics—A Critical Review. Cosmetics 2018, 5, 11. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Chang, K.S.; Su, M.S.; Huang, Y.S.; Jang, H.D. Effects of some chinese medicinal plant extracts on five different fungi. Food Control 2007, 18, 1547–1554. [Google Scholar] [CrossRef]
- Verástegui, A.; Verde, J.; Garcia, S.; Heredia, N.; Oranday, A.; Rivas, C. Species of agave with antimicrobial activity against selected pathogenic bacteria and fungi. World J. Microbiol. Biotechnol. 2008, 24, 1249–1252. [Google Scholar] [CrossRef]
- Santas, J.; Almajano, M.P.; Carbo, R. Antimicrobial and antioxidant activity of crude onion (L.) Allium cepa extracts. Inter. J. Food Sci. Technol. 2010, 45, 403–409. [Google Scholar] [CrossRef]
- Mir, S.A.; Qureshi, A.H. Antifungal activity of oil against plant pathogenic fungi isolated Zingiber officinale from solanaceous vegetable fruits. Asian J. Pharm. Pharmacol. 2017, 3, 121–124. [Google Scholar]
- Yu, J.; Whitelaw, C.A.; Nierman, W.C.; Bhatnagar, D.; Cleveland, T.E. expressed sequence tags Aspergillus flavus for identification of genes with putative roles in aflatoxin contamination of crops. FEMS Microbiol. Lett. 2004, 237, 333340. [Google Scholar] [CrossRef] [Green Version]
- Ignjatov, M.; Milošević, D.; Nikolić, Z.; Tamindžić, G.; Gvozdanović-Varga, J.; Ivanović, Z.; Popović, T. First report of Fusarium sp. FIESC 3 on onion seed in Serbia. Plant Disease 2015, 99, 1277–1278. [Google Scholar] [CrossRef]
- Pavela, R. History, presence and perspective of using plant extracts as commercial botanical insecticides and farm products for protection against insects—A Review. Plant Protect. Sci. 2016, 4, 229–241. [Google Scholar]
- Morita, T.; Jinno, K.; Kawagishi, H.; Arimoto, Y.; Suganuma, H.; Inakuma, T.; Sugiyama, K. Hepatoprotective effect of myristicin from nutmeg (Myristica fragrans) on lipopolysaccharide/d-galactosamine-induced liver injury. J. Agric. Food Chem. 2003, 51, 1560–1565. [Google Scholar] [CrossRef]
- Milner, J.A. A historical perspective on garlic and cancer. Nutr. J. 2001, 131, 1027S–1031S. [Google Scholar] [CrossRef] [Green Version]
- Ali, B.H.; Blunden, G. Pharmacological and toxicological properties of Nigella sativa. Phytother. Res. 2003, 17, 299–305. [Google Scholar] [CrossRef]
- Barla, A.; Topcu, G.; Oksuz, S.; Tumen, G.; Kingston, D. Identification of cytotoxic sesquiterpenes from Laurus nobilis L. Food Chem. 2007, 104, 1478–1484. [Google Scholar] [CrossRef]
- Gayda, A. Etude des Principales Huiles Essentielles Utilisées en Rhumatologie. Ph.D. Thesis, Université Toulouse III Paul Sabatier, Toulouse, France, 2013. [Google Scholar]
- Duke, J.A. The Green Pharmacy: New Discoveries in Herbal Remedies for Common Diseases and Conditions from the Worlds for Most Authority on Healing Herbs; Rodal Press: New York, NY, USA, 1997. [Google Scholar]
- Knobloch, K.; Pauli, A.; Iberl, N.; Weigand, N.; Weis, H.M. Antibacterial and antifungal properties of essential oil components. J. Ess. Oil Res. 1989, 1, 119–128. [Google Scholar] [CrossRef]
- Ozcan, M.; Erkmen, O. Antimicrobial activity of the essential oils of Turkish plant spices. Eur. Food Res. Technol. 2001, 212, 658–660. [Google Scholar]
- Derwich, E.; Benziane, Z.; Boukir, A. Chemical composition and antibacterial activity of leaves essential oil of Laurus nobilis from Morocco. AJBAS 2009, 3, 3818–3824. [Google Scholar]
- Chmit, M.; Kanaan, H.; Habib, J.; Abbass, M.; Mcheik, A.; Chokr, A. Antibacterial and antibiofilm activities of polysaccharides, essential oil, and fatty oil extracted from Laurus nobilis growing in Lebanon. Asian Pac J Trop Med. 2014, 7, S546–S552. [Google Scholar] [CrossRef] [Green Version]
- Sangun, M.K.; Aydin, E.; Timur, M.; Karadeniz, H.; Caliskan, M.; Ozkan, A. Comparison of chemical composition of the essential oil of Laurus nobilis L. leaves and fruits from different regions of Hatay, Turkey. J. Environ. Boil. 2007, 28, 731–733. [Google Scholar]
- Sarmoum, R.; Haid, S.; Biche, M.; Djazouli, Z.; Zebib, B.; Merah, O. Effect of salinity and water stress on the essential oil components of rosemary (Rosmarinus officinalis L.). Agronomy 2019, 9, 214. [Google Scholar] [CrossRef] [Green Version]
- Ulas, A.; Aydin, A.; Ulas, F.; Yetisir, H.; Miano, T.F. Cucurbita rootstocks improve salt tolerance of melon scions by inducing physiological, biochemical and nutritional responses. Horticulturae 2020, 6, 66. [Google Scholar] [CrossRef]
- Heuer, B.; Yaniv, Z.; Ravina, I. Effect of late salinization of chia (Salvia hispanica), stock (Matthiola tricuspidata) and evening primrose (Oenothera biennis) on their oil content and quality. Ind. Crops Prod. 2002, 15, 163–167. [Google Scholar] [CrossRef]
- Hendawy, S.F.; Khalid, K.A. Response of sage (Salvia officinalis L.) plants to zinc application under different salinity levels. J. Appl. Sci. Res. 2005, 1, 147–155. [Google Scholar]
- Akula, R.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef]
- Nagazi, K.; Masmoudi, M.M.; Ben Mechlia, N. Effects of deficit drip-irrigation scheduling regimes with saline water on pepper yield, water productivity and soil salinity under arid conditions of Tunisia. JAEID 2012, 106, 85–103. [Google Scholar] [CrossRef]
- Hachicha, M. Les sols salés et leur mise en valeur en Tunisie. Sci. Et Changements Planétaires/Sécheresse 2007, 18, 45–50. [Google Scholar]
- Acosta, J.A.; Faz, A.; Jansen, B.; Kalbitz, K.; Martinez-Martinez, S. Assessment of salinity status in intensively cultivated soils under semiarid climate, Murcia, SE Spain. J. Arid Environ. 2011, 75, 1056–1066. [Google Scholar] [CrossRef]
- Louati, D.; Majdoub, R.; Rigane, H.; Abida, H. Effects of irrigating with saline water on soil salinization (Eastern Tunisia). Arab. J. Sci. Eng. 2018, 43, 3793–3805. [Google Scholar] [CrossRef]
- Maatallah, S.; Nasri, N.; Hajlaoui, H.; Albouchi, A.; Elaissi, A. Evaluation changing of essential oil of laurel (Laurus nobilis L.) under water deficit stress conditions. Ind. Crops Prod. 2016, 99, 170–178. [Google Scholar] [CrossRef]
- Ben Ayed, A.; Zanin, G.; Aissa, E.; Haouala, F. Effect of NaCl on growth and mineral nutrition of laurel (Laurus nobilis L.). IJAAST 2018, 9, 20–37. [Google Scholar]
- Tabatabaie, S.J.; Nazari, J. Influence of nutrient concentrations and NaCl salinity on the growth, photosynthesis, and essential oil content of peppermint and lemon verbena. Turk. J. Agri. For. 2007, 31, 245–253. [Google Scholar]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Haider, F.; Kumar, N.; Banerjee, S.; Naqvi, A.A.; Bagchi, G.D. Effect of altitude on the essential oil constituents of Artemisia roxburghiana Besser var. purpurascens (Jacq.) Hook. J. Essent. Oil Res. 2011, 21, 303–304. [Google Scholar] [CrossRef]
- Zgheib, R.; Chaillou, S.; Ouaini, N.; Kassouf, A.; Rutledge, D.; Azzi, D.; El Beyrouthy, M. Chemometric tools to highlight the variability of the chemical composition and yield of Lebanese Origanum syriacum L. essential oil. Chem. Biodivers. 2016, 13, 1326–1347. [Google Scholar] [CrossRef]
- El-Alam, I.; Zgheib, R.; Iriti, M.; El Beyrouthy, M.; Hattouny, P.; Verdin, A.; Fontaine, J.; Chahine, R.; Lounès-Hadj Sahraoui, A.; Makhlouf, H. Origanum syriacum Essential oil chemical polymorphism according to soil type. Foods 2019, 8, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nurzyńska-Wierdak, R. Does mineral fertilization modify essential oil content and chemical composition in medicinal plants? Acta Sci. Pol. Hort. Cult. 2013, 12, 3–16. [Google Scholar]
- Omer, E.A. Response of wild Egyptian oregano to nitrogen fertilization in a sandy soil. J. Plant Nutr. 2000, 22, 103–114. [Google Scholar] [CrossRef]
- Schaller, R.G.; Schnitzler, W.H. Nitrogen nutrition and flavour compounds of carrots (Daucus carota) cultivated in Mitscher-lich pots. J. Sci. Food Agric. 2000, 80, 49–56. [Google Scholar]
- Moghtader, M.; Mansouri, I.; Farahmand, A.; Mansouri, S.H. Evaluation of antibacterial potential of rosemary extracts for therapeutic agents. In Book of the 3rd Congress of Medicinal Plants; Shahed University: Tehran, Iran, 2007; pp. 535–536. [Google Scholar]
- Heidari, F.; Zehtab Salmasi, S.; Javanshir, A.; Aliari, H.; Dadpoor, M.R. The effects of application microelements and plant density on yield and essential oil of peppermint (Mentha piperita L.). Iran. J. Medic. Aromat. Plants 2008, 24, 1–9. [Google Scholar]
- Razmjoo, K.; Heydarizadeh, P.; Sabzalian, M.R. Effect of salinity and drought stresses on growth parameters and essential oil Content of Matricaria chamomila. Int. J. Agric. Biol. 2008, 10, 1560–8530. [Google Scholar]
- Ozturk, A.; Unlukara, A.; Ipek, A.; Gurbuz, B. Effects of salt and water deficit on plant growth and essential oil content of lemon Balm (Melissa Officinalis L.). Pak. J. Bot. 2004, 36, 787–792. [Google Scholar]
- Khalid, A.K.; Teixeira da Silva, J.A. Yield, essential oil and pigment content of Calendula officinalis L. flower heads cultivated under salt stress conditions. Sci. Hortic. 2010, 126, 297–305. [Google Scholar] [CrossRef]
- Roodbari, N.; Roodbari, S.; Ganjali, A.; Ansarifar, M. The effect of salinity stress on growth parameters and essential oil percentage of peppermint (Mentha piperita L.). Int. J. Basic Appl. Sci. 2013, 2, 294–299. [Google Scholar]
- Tounekti, T.; Vadel, A.M.; Bedoui, A.; Khemira, H. NaCl stress affects growth and essential oil composition in rosemary (Rosmarinus officinalis L.). J. Hortic. Sci. Biotechnol. 2008, 83, 267–273. [Google Scholar] [CrossRef]
- Alaei, S.; Khosh-Khui, M.; Kobraee, S.; Zaji, B. Effect of different salinity levels on essential oil content and composition of Dracocephalum moldavica. Agricult. Commun. 2014, 2, 42–46. [Google Scholar]
- Del Rosario Cappellari, R.; Chiappero, J.; Belén Palermo, T.; Giordano, W.; Banchio, E. Volatile organic compounds from rhizobacteria increase the biosynthesis of secondary metabolites and improve the antioxidant status in Mentha piperita L. Grown under Salt Stress. Agronomy 2020, 10, 1094. [Google Scholar] [CrossRef]
- Ben Taarit, M.; Msaada, K.; Hosni, K.; Hammami, M.; Kchouk, M.E.; Marzouk, B. Plant growth, essential oil yield and composition of sage (Salvia officinalis L.) fruits cultivated under salt stress conditions. Ind. Crop Prod. 2009, 30, 333–337. [Google Scholar] [CrossRef]
- Seufert, G.; Kotzias, D.; Spartà, C.; Versino, B. Volatile organics in mediterranean shrubs and their potential role in a changing environment. In Global Change and Mediterranean-Type Ecosystems; Springer: New York, NY, USA, 1995; pp. 343–370. [Google Scholar]
- Pichersky, E.; Sharkey, T.D.; Gershenzon, J. Plant volatiles a lack of function or a lack of knowledge? Trends Plant Sci. 2006, 11, 421. [Google Scholar] [CrossRef]
- Sokame, B.M.; Ntiri, E.S.; Ahuya, P.; Torto, B.; LeRu, B.P.; Kilalo, D.C.; Juma, G.; Calatayud, P.-A. Caterpillar-induced plant volatiles attract conspecific and heterospecific adults for oviposition within a community of lepidopteran stemborers on maize plant. Chemoecology 2019, 29, 89–101. [Google Scholar] [CrossRef]
- Romagni, J.G.; Allen, S.N.; Dayan, F.E. Allelopathic effects of volatile cineoles on two weedy plant species. J. Chem. Ecol. 2000, 26, 303–313. [Google Scholar] [CrossRef]
- Abdelmajeed, N.A.; Danial, E.N.; Ayad, H.S. The effect of environmental stress on qualitative and quantitative essential oil of aromatic and medicinal plants. Arch. Des. Sci. 2013, 66, 100–120. [Google Scholar]
- Ramezani, S.; Abbasi, A.; Sobhanverdi, S.; Shojaeiyan, A.; Ahmadi, N. The effects of water deficit on the expression of monoterpene synthases and essential oils composition in Salvia ecotypes. Physiol. Mol. Biol. Plants 2020, 26, 2199–2207. [Google Scholar] [CrossRef]
- Najar, B.; Pistelli, L.; Marchioni, I.; Pistelli, L.; Muscatello, B.; De Leo, M.; Scartazza, A. Salinity-induced changes of photosynthetic performance, lawsone, VOCs, and antioxidant metabolism in Lawsonia inermis L. Plants 2020, 9, 1797. [Google Scholar] [CrossRef]
- Zhou, Y.; Tang, N.; Huang, L.; Zhao, Y.; Tang, X.; Wang, K. Effects of salt stress on plant growth, antioxidant capacity, glandular trichome density, and volatile exudates of Schizonepeta tenuifolia Briq. Int. J. Mol. Sci. 2018, 19, 252. [Google Scholar] [CrossRef] [Green Version]
- Valifard, M.; Mohsenzadeh, S.; Kholdebarin, B.; Rowshan, V.; Niazi, A.; Moghadam, A. Effect of salt stress on terpenoid biosynthesis in Salvia mirzayanii: From gene to metabolite. J. Hortic. Sci. Biotechnol. 2019, 94, 389–399. [Google Scholar] [CrossRef]
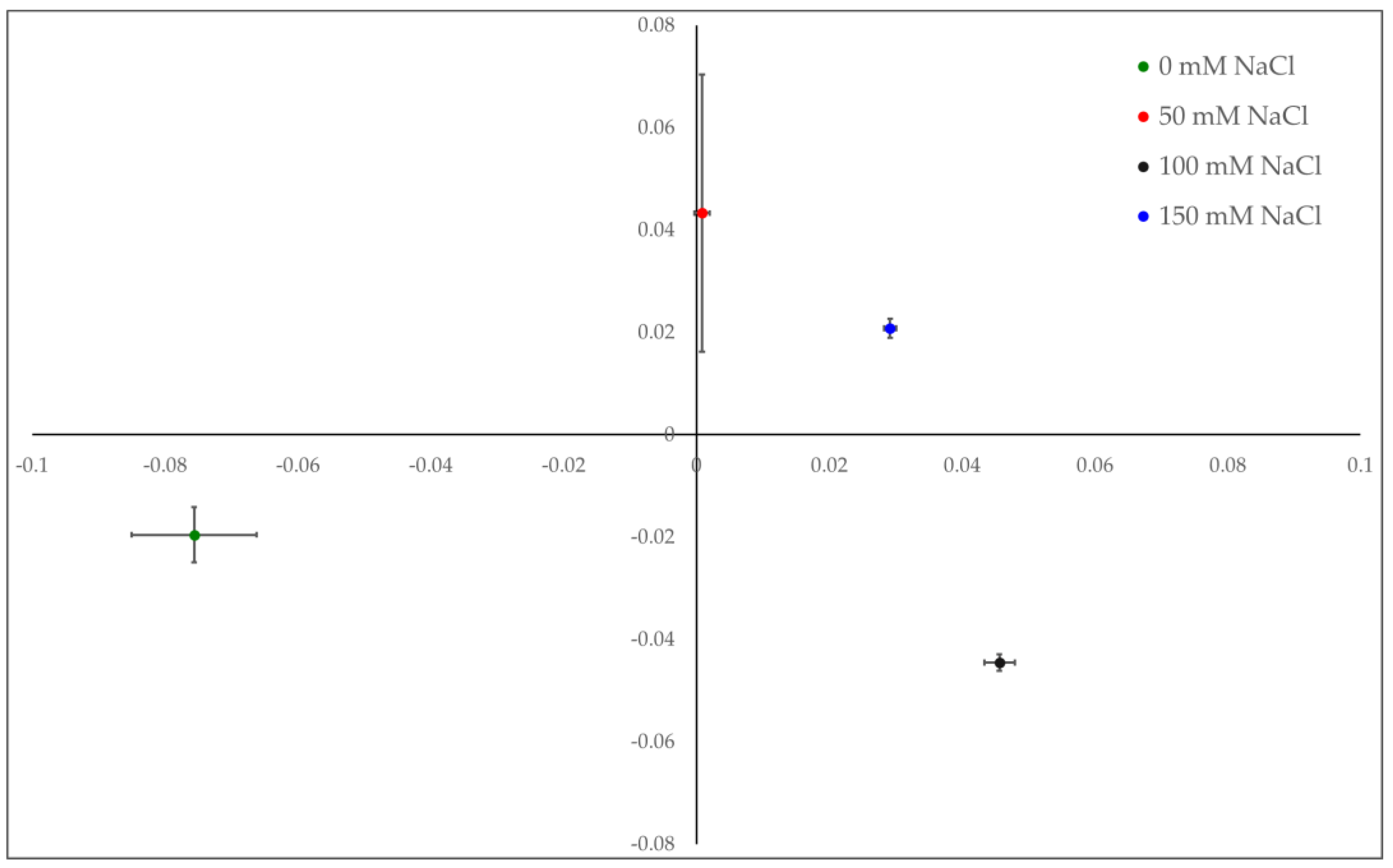
| NaCl (mM) | ||||||
|---|---|---|---|---|---|---|
| No | Compounds | Kavats Index | 0 | 50 | 100 | 150 |
| 1 | α-Thujene | 930 | 0.24 ± 0.01 b | 0.36 ± 0.08 a | 0.38 ± 0.1 a | 0.26 ± 0.03 b |
| 2 | α-Pinene | 941 | 2.22 ± 0.25 a | 1.26 ± 0.21 b | 1.18 ± 0.11 b | 1.20 ± 0.17 b |
| 3 | β-Pinene | 986 | 2.10 ± 0.31 a | 0.91 ± 0.36 b | 0.7 ± 0.18 c | 0.62 ± 0.11 c |
| 4 | Sabinene | 979 | 0.85 ± 0.3 c | 0.90 ± 0.07 b | 0.93 ± 0.36 b | 1.20 ± 0.5 a |
| 5 | α-Terpenyl acetate | 1357 | 5.70 ± 0.13 b | 6.74 ± 0.63 ab | 7.63 ± 0.7 a | 7.98 ± 0.55 a |
| 6 | 1,8-Cineole | 1045 | 18.43 ± 0.18 a | 16.83 ± 0.22 b | 12.67 ± 0.03 c | 15.42 ± 0.09 b |
| 7 | Camphene | 964 | 0.13 ± 0.07 a | 0.11 ± 0.02 ab | 0.10 ± 0.05 ab | 0.07 ± 0.01 b |
| 8 | Linalool | 1201 | 4.74 ± 0.12 a | 4.60 ± 0.23 a | 4.55 ± 0.09 a | 3.08 ± 0.01 b |
| 9 | α-Terpineol | 1208 | 2.00 ± 1.01 a | 1.24 ± 0.89 b | 1.25 ± 0.4 b | 1.20 ± 0.19 b |
| 10 | Bornyl acetate | 1294 | 0.63 ± 0.01 ab | 0.65 ± 0.02 ab | 0.78 ± 0.06 a | 0.57 ± 0.02 b |
| 11 | γ-Terpinene | 1064 | 0.22 ± 0.8 a | 0.22 ± 0.06 a | 0.21 ± 0.01 a | 0.24 ± 0.1 a |
| 12 | p-Cymene | 1035 | 2.61 ± 0.25 a | 2.06 ± 0.11 b | 1.96 ± 0.63 b | 1.94 ± 0.14 b |
| 13 | Borneol | 1189 | 0.31 ± 0.14 a | 0.22 ± 0.06 ab | 0.12 ± 0.01 b | 0.10 ± 0.21 b |
| 14 | Methyl eugenol | 1412 | 5.13 ± 0.13 b | 5.14 ± 0.11 b | 6.55 ± 0.26 a | 5.90 ± 0.19 ab |
| 15 | α-Terpinolene | 1093 | 0.45 ± 0.01 a | 0.44 ± 0.03 a | 0.41 ± 0.09 a | 0.45 ± 0.02 a |
| 16 | Limonene | 1039 | 1.99 ± 0.07 a | 2.00 ± 0.18 a | 2.00 ± 0.26 a | 2.10 ± 0.32 a |
| 17 | α-Terpinene | 1025 | 0.81 ± 0.11 a | 0.74 ± 0.08 a | 0.70 ± 0.04 a | 0.70 ± 0.02 a |
| 18 | Terpinen-4-ol | 1196 | 1.50 ± 0.07 a | 0.73 ± 0.21 b | 0.69 ± 0.05 b | 0.86 ± 0.1 b |
| 19 | neo-Isopulegol | 1306 | 2.67 ± 0.03 a | 2.35 ± 0.12 b | 2.72 ± 0.09 a | 2.62 ± 0.1 a |
| 20 | α-Phellandrene | 1013 | 0.21 ± 0.15 a | 0.11 ± 0.03 b | 0.18 ± 0.06 a | 0.10 ± 0.09 b |
| 21 | trans-Sabinene hydrate | 1111 | 0.18 ± 0.23 a | 0.09 ± 0.08 b | 0.15 ± 0.06 a | 0.13 ± 0.05 ab |
| 22 | trans-Pinocarveol | 1139 | 3.35 ± 0.22 a | 3.43 ± 0.09 a | 3.86 ± 0.13 a | 3.05 ± 0.41 b |
| 24 | γ-Cadinene | 1491 | 0.24 ± 0.11 b | 0.43 ± 0.10 a | 0.32 ± 0.09 ab | 0.48 ± 0.31 a |
| 23 | Valencene | 1490 | 0.10 ± 0.02 a | 0.13 ± 0.06 a | 0.09 ± 0.02 a | 0.11 ± 0.03 a |
| 24 | trans-Piperitol | 1225 | 0.20 ± 0.01 ab | 0.22 ± 0.09 ab | 0.27 ± 0.18 a | 0.15 ± 0.06 b |
| 25 | Zingiberene | 1497 | 0.21 ± 0.11 b | 0.25 ± 0.02 b | 0.29 ± 0.01 b | 0.36 ± 0.16 a |
| 26 | Tricyclene | 934 | 0.93 ± 0.07 b | 1.06 ± 0.28 a | 0.92 ± 0.13 b | 0.93 ± 0.03 b |
| 27 | E-Linalool oxide | 1079 | 0.24 ± 0.12 b | 0.24 ± 0.03 b | 0.31 ± 0.01 a | 0.26 ± 0.10 b |
| 28 | (Z)-β-Ocimene | 1034 | 0.33 ± 0.16 a | 0.31 ± 0.09 a | 0.3 ± 0.22 a | 0.31 ± 0.03 a |
| 29 | o-Cymene | 1024 | 0.09 ± 0.07 a | 0.09 ± 0.01 a | 0.11 ± 0.25 a | 0.14 ± 0.03 a |
| 30 | Myrcene | 989 | 0.16 ± 0.1 b | 0.33 ± 0.20 a | 0.33 ± 0.12 a | 0.26 ± 0.07 ab |
| 31 | cis-p-Mentha-1,3,8-triene | 1121 | 0.27 ± 0.11 b | 0.25 ± 0.22 b | 0.31 ± 0.41 a | 0.25 ± 0.17 b |
| 32 | Bicyclogermacrene | 1516 | 0.44 ± 0.14 b | 0.76 ± 0.28 a | 0.64 ± 0.09 ab | 0.74 ± 0.19 a |
| 33 | β-Elemene | 1401 | 0.54 ± 0.21 b | 0.63 ± 0.54 a | 0.49 ± 0.18 b | 0.61 ± 0.24 a |
| 34 | trans-Caryophyllene | 1445 | 0.74 ± 0.13 a | 0.60 ± 0.03 a | 0.75 ± 0.06 a | 0.66 ± 0.17 a |
| 35 | δ-Terpinyl acetate | 1322 | 1.39 ± 0.12 a | 1.40 ± 0.09 a | 1.48 ± 0.03 a | 1.44 ± 0.02 a |
| 36 | m-Cymene | 1031 | 0.10 ± 0.13 b | 0.06 ± 0.22 b | 0.12 ± 0.07 a | 0.06 ± 0.02 b |
| 37 | 3-Ethyl-2,5-dimethylhexane | 981 | 1.25 ± 0.02 a | 1.09 ± 0.04 b | 1.00 ± 0.11 b | 1.05 ± 0.01 b |
| 38 | β-Bisabolene | 1443 | 0.19 ± 0.14 b | 0.28 ± 0.06 a | 0.16 ± 0.11 b | 0.19 ± 0.07 b |
| 39 | (E)-α-Bergamotene | 1447 | 0.15 ± 0.13 a | 0.14 ± 0.09 a | 0.17 ± 0.06 a | 0.14 ± 0.18 a |
| 40 | Aromadendrene | 1456 | 0.19 ± 0.12 a | 0.11 ± 0.02 b | 0.13 ± 0.07 b | 0.21 ± 0.05 a |
| 41 | 2,5-Dimethoxy-p-cymene | 1424 | 0.87 ± 0.11 c | 1.30 ± 0.33 b | 4.02 ± 0.23 a | 1.05 ± 0.18 c |
| 42 | Valencene | 1490 | 0.10 ± 0.06 a | 0.13 ± 0.18 a | 0.09 ± 0.01 a | 0.11 ± 0.03 a |
| 43 | α-Copaene | 1391 | 0.36 ± 0.66 b | 0.57 ± 0.17 a | 0.53 ± 1.01 a | 0.62 ± 0.09 a |
| 44 | α-Humulene | 1476 | 0.12 ± 0.03 a | 0.14 ± 0.01 a | 0.14 ± 0.08 a | 0.16 ± 0.25 a |
| 45 | γ-Curcumene | 1487 | 0.08 ± 0.11 a | 0.10 ± 0.04 a | 0.08 ± 0.1 a | 0.04 ± 0.18 a |
| 46 | δ-Cadinene | 1513 | 0.24 ± 0.13 b | 0.43 ± 0.03 a | 0.32 ± 0.09 b | 0.47 ± 0.09 a |
| 47 | Safrole | 1358 | 0.30 ± 0.10 b | 0.35 ± 0.18 b | 0.41 ± 0.09 a | 0.46 ± 0.15 a |
| 48 | (E)-γ-bisabolene | 1443 | 0.26 ± 0.11 a | 0.27 ± 0.22 a | 0.28 ± 0.09 a | 0.27 ± 0.14 a |
| 49 | Sabina ketone | 1177 | 0.42 ± 0.13 a | 0.27 ± 0.07 b | 0.25 ± 0.10 b | 0.27 ± 0.11 b |
| 50 | cis-p-Mentha-1,5-dien-8-ol | 1171 | 0.18 ± 0.22 a | 0.08 ± 0.13 b | 0.08 ± 0.25 b | 0.09 ± 0.11 b |
| 51 | Neryl propionate | 1424 | 0.23 ± 0.64 a | 0.25 ± 0.10 a | 0.20 ± 0.06 a | 0.23 ± 0.64 a |
| 52 | 6-Methyl-hept-5-en-2-one | 992 | 0.49 ± 0.08 a | 0.50 ± 0.03 a | 0.54 ± 0.01 a | 0.55 ± 0.10 a |
| 53 | E-Cinnamaldehyde | 1268 | 0.10 ± 0.08 b | 0.11 ± 0.15 b | 0.16 ± 0.32 a | 0.08 ± 0.08 b |
| Total | 67.98 | 66.29 | 65.01 | 62.67 | ||
| Other identified compounds | 7.97 | 4.57 | 7.87 | 8.14 | ||
| Unidentified compounds | 24.05 | 29.14 | 27.12 | 29.19 | ||
| NaCl (mM) | ||||
|---|---|---|---|---|
| Grouped Compounds | 0 | 50 | 100 | 150 |
| Monoterpene hydrocarbons | 13.71 ± 0.61 a | 12.21 ± 0.55 ab | 11.84 ± 0.41 b | 10.83 ± 0.36 b |
| Oxygenated monoterpenes | 41.52 ± 0.32 a | 40.82 ± 0.25 ab | 39.56 ± 0.21 b | 38.95 ± 0.1 b |
| Sesquiterpene hydrocarbons | 3.92 ± 1.02 c | 5.92 ± 1.51 b | 7.45 ± 1.78 a | 7.16 ± 1.65 a |
| Oxygenated sesquiterpenes | 0.13 ± 0.04 a | 0.15 ± 0.02 a | 0.12 ± 0.23 a | 0.13 ± 0.2 a |
| Aromatic compound | 5.23 ± 0.24 a | 5.25 ± 0.06 a | 6.68 ± 0.14 a | 6.05 ± 0.16 a |
| Alcohol | 0.23 ± 0.02 a | 0.25 ± 0.07 a | 0.21± 0.15 a | 0.26 ± 0.20 a |
| Aliphatic aldehydes | 1.35 ± 0.01 a | 1.20 ± 0.04 b | 1.16 ± 0.00 b | 1.13 ± 0.02 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben Ayed, A.; Zanin, G.; Aissa, E.; Haouala, F. Volatile Oil Components of Laurel (Laurus nobilis L.) Leaves Obtained from Plants Cultivated under Salinity Stress Conditions. Horticulturae 2022, 8, 442. https://doi.org/10.3390/horticulturae8050442
Ben Ayed A, Zanin G, Aissa E, Haouala F. Volatile Oil Components of Laurel (Laurus nobilis L.) Leaves Obtained from Plants Cultivated under Salinity Stress Conditions. Horticulturae. 2022; 8(5):442. https://doi.org/10.3390/horticulturae8050442
Chicago/Turabian StyleBen Ayed, Amina, Giampaolo Zanin, Echrak Aissa, and Faouzi Haouala. 2022. "Volatile Oil Components of Laurel (Laurus nobilis L.) Leaves Obtained from Plants Cultivated under Salinity Stress Conditions" Horticulturae 8, no. 5: 442. https://doi.org/10.3390/horticulturae8050442
APA StyleBen Ayed, A., Zanin, G., Aissa, E., & Haouala, F. (2022). Volatile Oil Components of Laurel (Laurus nobilis L.) Leaves Obtained from Plants Cultivated under Salinity Stress Conditions. Horticulturae, 8(5), 442. https://doi.org/10.3390/horticulturae8050442







