Abstract
Red paddy soil is widely distributed in the south of China and has become an important production system for food and cash crops. However, the key factors limiting the quality of this soil type under the plastic shed cultivation system and the effective management strategies are still unclear. In the present study, the physicochemical and microbial properties of red paddy soil in a plastic shed (PS-Soil) and open-air (OA-Soil) cultivation systems were compared. Subsequently, reductive soil disinfestation (RSD) and organic fertilizer treatment (OF) were used to improve the soil properties in a representative PS-Soil. Results showed that the physicochemical and microbial properties in PS-Soil were significantly altered compared with those in the nearby OA-Soil, and those differences were primarily dominated by the cultivation system rather than the sampling site. Specifically, the electrical conductivity (EC) and available nutrients (NO3−-N, NH4+-N, available K, and available P) contents, as well as the abundances of fungi, potential fungal soil-borne pathogens (F. oxysporum and F. solani), and fungi/bacteria were significantly increased in PS-Soil. In addition, the OF treatment could not effectively improve the above-mentioned soil properties, which was mainly reflected by that soil EC and the abundances of potential fungal soil-borne pathogens were considerably increased in the OF-treated soil. In contrast, soil EC and NO3−-N content, the abundances of fungi, F. oxysporum, F. solani, and fungi/bacteria were remarkably decreased by 76%, 99%, 98%, 92%, 73%, and 85%, respectively. Moreover, soil pH, the abundance of bacteria, total microbial activity, metabolic activity, and carbon source utilization were significantly increased in the RSD-treated soil. Collectively, red paddy soil is significantly degraded under the plastic shed cultivation system, and RSD rather than OF can effectively improve the quality of this soil type.
1. Introduction
Paddy soil developed from the parent red soil material is one of the most important soil resources in China. It is widely distributed in the tropical and subtropical regions and has become an important production system for food and cash crops [1,2]. Therefore, the rational utilization of red paddy soil to maintain its quality is a major task in China.
Plastic shed cultivation, as a popular soil utilization strategy, can effectively avoid the loss of soil chemical elements and nutrients during rain leaching [3]. However, driven by economic benefits, plastic shed cultivation has gradually developed into an intensive cultivation system in the red paddy soil on a large scale, which is mainly characterized by continuous monoculture and over-fertilization, and thus often leads to soil degradation [4,5]. Many studies [4,5,6,7] have demonstrated that the severity of acidification, salinization, accumulation of plant soil-borne pathogens, such as Fusarium oxysporum, Fusarium solani, and Ralstonia solanacearum, and imbalance of microbial community are the common characteristics of the degraded soil under the plastic shed cultivation system, and ultimately lead to plant disease outbreaks and economic losses. For example, our previous study [7] has demonstrated that the abundance of soil-borne pathogens (Fusarium oxysporum) was significantly increased in the plastic shed cultivation system and caused severe Fusarium wilt disease in lisianthus.
Notably, the contributions of these degraded properties on plant health status may be different across diverse soil conditions. A previous study showed that the degradation of soil microbial properties, such as the accumulation of pathogens and imbalance of microbial community, rather than physicochemical properties, determined the plant health status [8]. However, other studies revealed that the degradation of soil physicochemical properties, such as acidification and salinization, can significantly affect the plant’s healthy growth [9,10]. Reflecting on these findings, it is necessary to investigate the main properties that are degraded in red paddy soil under the plastic shed cultivation system and then adopt reasonable management strategies to improve its quality.
Organic amendments, such as organic composts, wastes, and crop residues, have been documented as effective strategies to improve soil quality by increasing soil organic matter and improving soil structure [11,12,13,14]. However, their effects on the suppression of soil-borne pathogens remain inconsistent. For example, previous studies have reported that soil with organic amendments stimulated the proliferation of soil-borne pathogens and increased the incidence of plants disease [12,14]. In contrast, soil incorporated with the above-mentioned organic materials, irrigated to saturation, and covered with a plastic film can effectively suppress many soil-borne pathogens by creating a strongly reductive and anaerobic soil environment and producing diverse organic acids [15,16]. This management strategy is called reductive soil disinfestation (RSD), anaerobic soil disinfestation, or biological soil disinfestation in different countries [8,17,18]. Many studies further revealed that RSD can also significantly improve soil physicochemical properties and microbial communities, such as eliminating soil acidification and salinization and increasing soil organic carbon content and microbial diversities [5,8,19]. These results imply that RSD is a promising strategy in the improvement of soil quality.
Here, the physicochemical (soil pH, electrical conductivity, organic carbon and available nutrients contents) and microbial (the abundances of bacteria, fungi, and common soil-borne pathogens of wilt diseases, total microbial activity, and microbial metabolic activity) properties were used in this study as the indicators of soil quality. To reveal the main properties that degraded in red paddy soil under the plastic shed cultivation system (defined as PS-Soil), we collected 24 PS-Soils located in Yichun City, Jiangxi Province, China, and the nearby open-air cultivated soils (OA-Soil) were used as a control group. Subsequently, RSD and organic amendment (application of organic fertilizer) were employed as the management strategies for a representative PS-Soil, to clarify the improvement effects of RSD and organic amendment on the above properties that degradated in the PS-Soil.
2. Materials and Methods
2.1. Sampling Site Description and Soil Collection
Three sampling sites, located at Nanmiao (114°42′ E, 28°09′ N), Xicun (114°21′ E, 28°13′ N), and Xinfang Towns (114°47′ E, 28°09′ N) in Yichun City, Jiangxi Province, China were selected to collect field soil samples (Figure S1). Each sampling site consisted of two cultivation system soils, PS-Soil and OA-Soil. The distance between each sampling site varied from 5 to 30 km. All of the sampling sites harbored the typical characteristics of red paddy soil and were classified as the Ferralic Cambisol based on the FAO classification system [20]. The soil in each cultivation system has a similar planting history. PS-Soil was primarily used for limited rotation of vegetables and melons. Chemical fertilizer combined with organic fertilizer was the most important fertilization pattern in the planting process. The soil-borne diseases in this soil type have exceeded 30% in recent years, and the yield of crops has also largely decreased. OA-Soil was used for the cultivation of grain crops, such as rice and rapeseed. Specifically, PS-Soils were converted from the open-air fields that are similar to the above-mentioned OA-Soil 4 years ago. Thus, the PS-Soils and OA-Soils used in this study were comparable.
The tillage layer soils (0–20 cm depth) were collected from eight greenhouses and three open-air cultivation fields nearby in each sampling site using sampling method according to Huang et al. [21], representing each sampling site contained 8 replicates of PS-Soil and 3 replicates of OA-Soil (regarded as the control group). These soils were then stored at 4 °C to measure physicochemical properties, total microbial activity, and microbial metabolic activity as well as −20 °C for DNA extraction and microbial quantification.
2.2. Field Experiment Design
After obtaining the main properties that degraded in PS-Soils, we selected a representative PS-Soil located in Nanmiao to conduct a management experiment. The initial properties of this soil were as follows: pH 4.11, electrical conductivity (EC) 406 µS cm−1, total organic carbon (TOC) 26 g kg−1, and available K and P 329 and 179 mg kg−1, respectively. Three treatments were performed in this soil: CK, soil without any treatment; OF, soil amended with 7.5 t ha−1 organic fertilizer (TOC 380 g kg−1, TN 8 g kg−1, and C/N 48) that comprised chicken manure and rice husks and fully mixed by a rotary cultivator; RSD [5,8], soil firstly incorporated with 15 t ha−1 grain chaff (size < 1 mm, TOC 348 g kg−1, TN 5 g kg−1, and C/N 70) and fully mixed by a rotary cultivator, and then irrigated to saturation and covered with a plastic film (transparent, thickness 0.08 mm) (Figure S2). These treatments were incubated in place for 18 days with an average temperature ranging from 28 °C to 40 °C. There were three replicates for each treatment, and each replicate covered an area of 60 m2 in a randomized complete block design. After treatment, the plastic film in the RSD treatment was removed, and the soil was drained. The soil samples (0–20 cm) were collected from each replicate and stored as described above. In addition to the above-mentioned soil parameters, microbial activity, and metabolic activity were further detected to clarify the effect of RSD treatment.
2.3. Physicochemical Properties Analysis
A total of 42 soil samples [(8 PS-Soils + 3 OA-Soils) × 3 sampling sites + 3 treatments × 3 replicates)] were used for physicochemical properties analysis. Soil pH and EC were detected in slurries (water: soil of 2.5:1 and 5:1) using the S220k and S230 electrode (Mettler, Greifense, Switzerland), respectively. Soil TOC was measured using wet digestion with H2SO4-K2Cr2O7 [22]. Soil NO3−-N and NH4+-N were extracted using the 2 mol L−1 KCl solution (solution: Soil of 5:1) and then detected using a continuous flow analyzer (Sann++; Skalar, Breda, The Netherlands). Available soil phosphorus (AP) and potassium (AK) were extracted using 0.5 mol L−1 NaHCO3 and 1 mol L−1 ammonium acetate, respectively [23,24]. Their concentrations were then determined by molybdenum-antimony resistance colorimetry and flame photometry, respectively.
2.4. DNA Extraction and Microbial Properties Measurement
Microbial NDA was extracted from 0.5 g soil of each replicate using the FastDNA® Spin Kit (MP Biomedicals, Santa Ana, CA, USA) according to the instruction and finally dissolved in 100 µL of elution buffer. The quality of DNA was determined using a DS-11 spectrophotometer (Denovix, Wilmington, DE, USA). The abundances of soil bacteria, fungi, and potential soil-borne pathogens (Fusarium oxysporum, Fusarium solani, and Ralstonia solanacearum) were quantified using the QuanStudio 3 Real-Time PCR system (Applied Biosystems, Waltham, MA, USA). The amplification mixtures in each gene were prepared using 10 µL of SYBR Green premix Taq (2×), 6 µL of sterile distilled water, 2 µL of soil DNA, and 1 µL of forward and reverse primers. The amplification primers (Eub338/Eub518 for bacterial 16S rDNA, Flic-F/Flic-R for R. solanacearum, ITS1f/ITS2R for fungal ITS, ITS1f/AFP346 for F. solani, ITS1f/AFP308 for F. oxysporum) and protocols are listed in Table S1. The standard curves and amplification specificity values were generated as previously described [7,8].
Total microbial activity of the soil (3 treatments × 3 replicates) was determined using the fluorescein diacetate hydrolysis method [25]. Microbial metabolic activity (CK and RSD × 3 replicates) was determined by measuring the carbon source utilization pattern using Biolog EcoPlates™ (Biolog, Inc., Hayward, CA, USA) [26]. Briefly, 10 g of fresh soil sample in each treatment was shaken in 90 mL of sterile saline for 30 min at 200 rpm and then diluted to 10−3. Subsequently, 125 μL aliquots of 10−3 dilution were inoculated into each well of the ECO MicroPlate and incubated in the dark at 25 °C for 6 days. The optical density (OD) at 590 nm was measured at 0, 24, 48, 72, 96, 120, and 144 h by using an automated microplate reader (BioTek Instruments Inc., Winooski, VT, USA). The average well color development (AWCD) of all carbon sources was calculated as described by the previous study [27], and then clustered as carboxylic acids, carbohydrates, phenolic acids, amino acids, polymers, and amines. This carbon source utilization pattern was used to indicate soil microbial metabolic activity.
2.5. Data Analysis
To compare the differences in physciochemical and microbial properties (Tables S2 and S3) between PS-Soil and OA-Soil, nonmetric multidimensional scaling (NMDS) analysis based on Bray–Curtis distance matrices was performed using the R “phyloseq” package [28]. Relative contributions of sampling site and cultivation system on the differences in physicochemical and microbial properties were calculated using the variance partitioning analysis (VPA) and permutational multivariate analysis of variance (PERMANOVA) with R “vegan” package [29]. Relationships between physicochemical and microbial properties were analyzed using the redundancy analysis and Pearson correlation of heatmap analysis through Wekemo Bioincloud (https://www.bioincloud.tech, accessed date: 24 February 2022). The statistical differences in physicochemical properties and microbial abundance (log10-transformed) between PS-Soil and OA-Soil, as well as metabolic activity and microbial activity among different treatments, were determined using the two independent-samples t-test and Duncan’s test, respectively.
3. Results
3.1. Differences in Physicochemical and Microbial Properties between PS-Soil and OA-Soil and Their Contributors
The physicochemical and microbial properties of PS-Soil and OA-Soil collected from each sampling site were listed in Tables S2 and S3, which showed that these properties of the soils between different cultivation systems were largely different. Except for pH, TOC, the abundance of R. solanacearum, R. solanacearum/bacteria, and F. solani/fungi, the rest of physicochemical and microbial properties exhibited significant (p < 0.05) differences between all of the PS-Soils and OA-Soils (p < 0.05) (Table 1). Compared with that in OA-Soil, the abundance of bacteria was significantly (p < 0.05) reduced by 53.34% in the PS-Soil (Table 1). However, soil EC and contents of NH4+-N, NO3−-N, AK, AP, and the abundances of fungi, F. oxysporum and F. solani, as well as the fungi/bacteria and F. oxysporum/fungi were significantly (p < 0.05) increased in the PS-Soil (Table 1). Moreover, the potential fungal pathogens F. oxysporum and F. solani were significantly (p < 0.05) increased by 89.23% and 97.87% in the PS-Soil, respectively.

Table 1.
The compositions of physicochemical and microbial properties between PS-Soil and OA-Soil.
NMDS results also showed that the compositions of physicochemical and microbial properties between PS-Soil and OA-Soil were significantly (p < 0.05) different (Figure 1a,b). The differences in physicochemical and microbial properties between PS-Soil and OA-Soil were remarkably (p < 0.05) affected by sampling site and cultivation system, while the relative contributions of cultivation system on the differences in soil physicochemical and microbial properties were higher than that of the sampling site (Figure 1c,d).
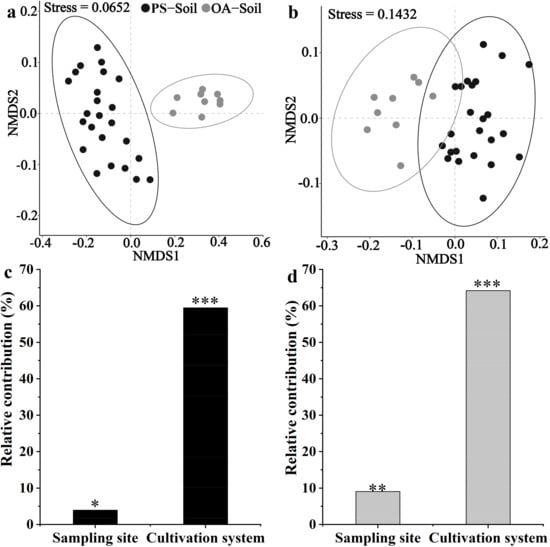
Figure 1.
The dissimilarities in physicochemical (a) and microbial (b) properties between plastic shed and open-air cultivation soils and their contributors (c,d). Nonmetric Multidimensional Scaling (NMDS) analyses were conducted using the Bray-Curtis matrices of the physicochemical and microbial properties among different soils. The ellipses indicate confidence intervals of 0.95. The relative contributions of the sampling site and cultivation system on the dissimilarities in physicochemical (c) and microbial (d) properties were analyzed by variance partitioning analyses (VPA). * p < 0.05, ** p < 0.01, and *** p < 0.001, which were calculated with 999 permutations using PERMANOVA. PS-Soil and OA-Soil represent the plastic shed and open-air cultivation soils, respectively.
3.2. Relationships between Physicochemical and Microbial Properties in the PS-Soil and OA-Soil
RDA result indicated that the microbial properties in the PS-Soil were significantly and negatively (p < 0.05) correlated with the pH and moisture content, whereas there was a significant (p < 0.05) and positive correlation with the soil EC and contents of NH4+-N, NO3−-N, AK, and AP (Figure 2a). Furthermore, the abundances of soil bacteria and R. solanacearum were significantly (p < 0.05) and positively correlated with the soil pH and moisture content, whereas the abundances of fungi, F. oxysporum, and F. solani, and fungi/bacteria, F. solani/fungi were considerably (p < 0.05) and positively correlated with soil EC and contents of NO3−-N, AK, and AP (Figure 2b).
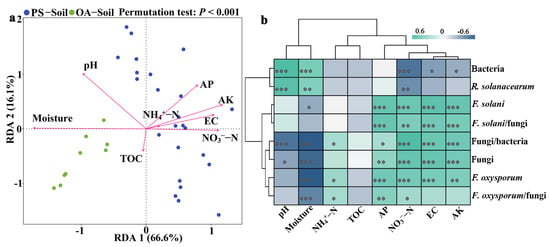
Figure 2.
RDA (redundancy analysis) ordination plot (a) and correlation heatmap (b) showing the relationships between physicochemical and microbial properties in different soils. * p < 0.05, ** p < 0.01, *** p < 0.001, and a blank represents a nonsignificant (p > 0.05) correlation. The key from green (positive correlation) to blue (negative correlation) indicates the changes of Pearson correlation coefficients (r). EC: electrical conductivity; TOC: total organic carbon; AK: available potassium; AP: available phosphorus. PS-Soil and OA-Soil represent the plastic shed and open-air cultivation soils, respectively.
3.3. Effects of Different Treatments on Soil Physicochemical Properties
Compared with that in the CK soil, soil pH was significantly increased (p < 0.05) by 0.54 and 1.12 after OF and RSD treatment, respectively (Table 2). Soil EC and content of NO3−-N were significantly (p < 0.05) decreased by 76.04% and 99.47% after RSD treatment in comparison to CK soil, respectively, while which showed an increasing trend after OF treatment (Table 2).

Table 2.
Soil physicochemical properties among different treatments.
3.4. Effects of Different Treatments on Soil Microbial Abundances
After treatment, the abundances of bacteria in OF-treated (3.23 × 1010 copies g−1 soil) and RSD-treated (4.42 × 1010 copies g−1 soil) soils were significantly (p < 0.05) increased by 0.29 and 0.77 times compared with that in the CK (2.49 × 1010 copies g−1 soil) soil, respectively (Table 3). Compared with CK, the abundance of fungi (1.17 × 109 copies g−1 soil) and the ratio of fungi/bacteria in OF-treated soil were increased (p < 0.05) by 6.79 and 4.97 times, respectively, whereas the abundance of fungi (4.01 × 107 copies g−1 soil) and the ratio of fungi/bacteria in RSD-treated soil were decreased by 73.47% and 84.80%, respectively (p < 0.05) (Table 3).

Table 3.
Soil microbial abundances among different treatments.
The abundances of F. oxysporum (1.81 × 107 copies g−1 soil) and F. solani (1.31 × 107 copies g−1 soil) in OF-treated soil were significantly (p < 0.05) increased by 2.53 and 3.03 times, respectively, compared with those in the CK (5.13 × 106 and 3.25 × 106 copies g−1 soil) soil, whereas they considerably (p < 0.05) decreased by 98.35% and 91.95%, respectively, in the RSD-treated soil (2.19 × 104 and 6.71 × 104 copies g−1 soil) (Table 3). Furthermore, the ratios of F. oxysporum/fungi and F. solani/fungi were remarkably (p < 0.05) decreased in the RSD-treated soil in comparison to CK, while the non-significant differences of these parameters were observed between CK and OF-treated soil (Table 3).
3.5. Effects of Different Treatments on Soil Microbial Activity and Metabolic Activity
Compared with that in CK soil, the total microbial activity was significantly (p < 0.05) increased in RSD-treated soil, whereas the OF treatment did not affect microbial activity (Figure 3a). Moreover, RSD also significantly (p < 0.05) increased the microbial metabolic activity and changed the carbon source utilization pattern compared to that of CK soil (Figure 3b,c). The AWCD values of the carbon sources, such as carbohydrates, carboxylic acids, amino acids, phenolic acids, amines, and polymers, were considerably (p < 0.05) increased in the RSD-treated soil (Figure 3c).
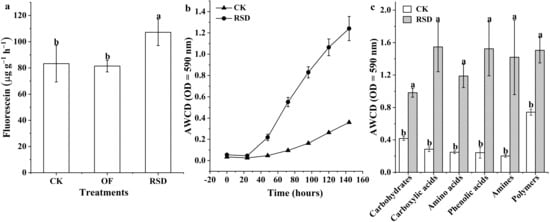
Figure 3.
Changes in the microbial activity (a), average well color development (AWCD) values (b), and categorized carbon utilization pattern at the end of incubation time (c). Error bars indicate the standard deviations. Different letters represent significant differences at p < 0.05 according to the Duncan test and independent-samples t-test. OD: optical density. CK: soil without any treatment; OF: soil incorporated with organic fertilizer; RSD: soil incorporated with grain chaff, irrigated to saturation, and covered with plastic film.
4. Discussion
The primary soil properties restricting its quality are often inconsistent under different conditions [30,31]. In the present study, we found that the physicochemical and microbial properties between PS-Soil and OA-Soil were significantly different, while the differences were mainly dominated by the cultivation system rather than the sampling site. This result was primarily due to the dissimilarities in micro-environments, such as temperature, crop type, and cultivation intensity, as well as water and fertilizer regime, between these two cultivation systems [32,33,34]. Specifically, many studies have revealed that soil pH and EC are the crucial physicochemical properties affecting soil quality and plant health status [8,9,30]. For example, the soil disease resistance could be significantly inhibited at pH below 5.0, and meanwhile, the crops were susceptible to the toxic effects of aluminum [35]. Furthermore, soil with high EC would increase its osmotic potential and thereby hinder crop uptake of water needed for healthy growth [36]. In this study, we found that the plastic shed cultivation system significantly increased soil EC but had an insignificant effect on soil pH. The increase in soil EC might be primarily caused by overfertilization and lack of rain leaching, which lead to the accumulation of NO3− and SO42− [37]. In addition, previous studies have reported that the red paddy soils distributed in Jian City, Jiangxi Province, and Hangzhou City, Zhejiang Province, China also had a low pH value (5.03 and 5.97, respectively) [38,39], which is similar to our study. However, the soil organic carbon contents (5.50 and 2.64 g kg−1, respectively) in these studies were obviously lower than our study, which might be related to the differences in artificial management between these areas.
In addition, microbial properties, such as microbial abundance, diversity, and community composition, are the key indicators for evaluating soil quality and are often used to predict plant health status [7,21]. Previous studies have indicated that healthy soils were often dominated by a beneficial bacterial microbiome, such as plant growth-promoting rhizobacteria [40,41], whereas the opposite result was found in the long-term continuous cropping soils that mainly controlled by a harmful fungal microbiome, such as the potential fungal soil-borne pathogens [21,42]. Although the microbial communities were not investigated in this study, we found that the ratio of fungi to bacteria in PS-Soil was significantly increased compared to that in OA-Soil. This result is consistent with the above-mentioned studies and indirectly indicated that the microbial communities could be considerably changed in the PS-Soil. In particular, the potential plant soil-borne pathogens in PS-Soil were dominated by fungal pathogens, such as F. oxysporum and F. solani, whereas the abundance of the bacterial pathogen (R. solanacearum) between PS-Soil and OA-Soil had no significant differences. This result further confirmed our speculation and this difference may be related to the root exudates that are released by melons or some vegetables rather than solanaceae crops. As we know, the melons, such as watermelon and cucumber, are the main host crops of F. oxysporum and F. solani, while solanaceae crops, such as tomato and eggplant, are the host crops of R. solanacearum. Consistently, the PS-Soils used in this study were primarily characterized by limited rotations of melons and vegetables. Collectively, these results indicated that the quality of red paddy soil has been significantly degraded under the plastic shed cultivation system, especially soil salinization (EC and NO3−-N), dominant microbiome alteration, and fungal soil-borne pathogens accumulation were the key factors that degraded in the PS-Soil.
Improving soil quality and maintaining soil health by reliable management strategies is essential for the sustainable development of agriculture. In this study, we found that soil amended with organic fertilizer significantly increased the soil EC, abundances of fungi, F. oxysporum and F. solani, and fungi/bacteria, indicating that the soil quality could be reduced by organic fertilizer treatment. This result was unexpected because the application of organic fertilizer is a common soil management strategy by local farmers. Moreover, although a few similar results with us have been reported in recent years [12,14], the overall evaluation of organic fertilizers are positive [11,13,43]. The results of this study may be related to the following: (1) this organic fertilizer may carry more salt and a larger number of potential soil-borne pathogens or (2) the nutrients, such as carbon sources, of this organic fertilizer were easily utilized by these indigenous soil-borne pathogens. As a result, the salinization and proliferation of soil-borne pathogens could be accelerated in the soil after applying such organic fertilizers. These results further indicate that the application of suitable organic amendments, such as bio-organic fertilizers, is important for improving soil quality.
In contrast, soil salinization, acidification, and the accumulation of soil-borne pathogens, were significantly improved in the RSD-treated soil. This result indicated that RSD is an effective strategy for the improvement of soil quality and is also consistent with many previous studies [8,16,17,18,19]. Specifically, the elimination of soil salinization and acidification by RSD was primarily due to the formation of a reductive and anaerobic environment, which can effectively convert the soil nitrate and sulfate into gases of N2O, N2, and H2S and consume a large amount of H+ [37,44]. The anaerobic environment and organic acids (acetic, propionic, butyric, and isovaleric acids) produced during the RSD process can significantly kill the soil-borne pathogens [18,19]. Meanwhile, we found that the total microbial activity was significantly increased after RSD treatment, while the ratio of fungi to bacteria showed an opposite trend. This result showed that the soil microbial community was changed significantly and may be dominated by a bacterial microbiome. Moreover, previous studies have demonstrated that the dominant microbes during the RSD treatments included many carbon sources decomposers, such as Clostridium, Cytophaga, Ruminococcus, Chaetomium, and Caulobacteracere [45,46,47]. It is consistent with our study that the utilization abilities of carbohydrates, carboxylic acids, amino acids, phenolic acids, amines, and polymers by soil microbes were significantly increased in the RSD treatment. Therefore, RSD rather than organic fertilizer can significantly and effectively increase the quality of red paddy soil.
5. Conclusions
In conclusion, our study revealed that the quality of red paddy soil decreased significantly in the plastic shed cultivation system, reflected by severe soil salinization and fungal pathogens accumulation. Organic fertilizer could not effectively remediate soil degradation. In contrast, RSD is an effective tool to eliminate soil salinization and fungal pathogens, increase soil microbial activity and metabolic activity, and improve the quality of red paddy soil under the plastic shed cultivation system.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae8040279/s1, Table S1: Primers list, Table S2: Physicochemical properties of each PS-Soil and OA-Soil, Table S3: Microbial properties of each PS-Soil and OA-Soil. References [48,49,50,51,52,53,54] are cited in the supplementary materials. Figure S1: Map of soil sampling sites. PS-Soil and OA-Soil represent the plastic shed and open air cultivation soils, respectively. Figure S2: The operation processes of RSD treatment. (a), soil incorporated with organic material; (b), the fully mixed of soil and organic material using a rotary cultivator; (c), soil irrigated to saturation; and (d), soil covered with a plastic film. This illustration was taken from our previous RSD experiment rather than from this study, but it was not published anywhere.
Author Contributions
Conceptualization, L.L. and S.L.; methodology, B.D.; software, B.D. and J.K.; validation, K.W., T.L. and Z.B.; formal analysis, J.K. and Z.B.; investigation, S.L.; resources, L.L.; data curation, S.L.; writing—original draft preparation, L.L.; writing—review and editing, Q.S.; visualization, L.L.; supervision, Q.S.; project administration, L.L. and Q.S.; funding acquisition, L.L. and Q.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Key-Area Research and Development Program of Guangdong Province (2020B0202010006), the National Natural Science Foundation of China (32160748, 32160716), the China Postdoctoral Science Foundation (2021M691625), and the Key Research and Development Project (Agriculture) of Yichun City, Jiangxi Province (20211YFN4240).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gao, S.J.; Zhang, R.G.; Cao, W.D.; Fan, Y.Y.; Gao, J.S.; Huang, J.; Bai, J.S.; Zeng, N.H.; Chang, D.N.; Shimizu, K.Y.; et al. Long-term rice-rice-green manure rotation changing the microbial communities in typical red paddy soil in south china. J. Integr. Agric. 2015, 14, 2512–2520. [Google Scholar]
- Huang, B.; Li, Z.W.; Li, D.Q.; Yuan, Z.J.; Nie, X.D.; Huang, J.Q.; Zhou, Y.Y. Effect of moisture condition on the immobilization of cd in red paddy soil using passivators. Environ. Technol. 2018, 40, 2705–2714. [Google Scholar] [PubMed]
- Chen, X.M.; Zhang, Q.; Zeng, S.M.; Chen, Y.; Guo, Y.Y.; Huang, X.Z. Rain-shelter cultivation affects fruit quality of pear, and the chemical properties and microbial diversity of rhizosphere soil. Can. J. Plant Sci. 2020, 100, 683–691. [Google Scholar]
- Runia, W.T.; Molendijk, L.P.G. Physical methods for soil disinfestation in intensive agriculture: Old methods and new approaches. Acta Hortic. 2010, 883, 249–258. [Google Scholar]
- Meng, T.Z.; Ren, G.D.; Wang, G.F.; Ma, Y. Impacts on soil microbial characteristics and their restorability with different soil disinfestation approaches in intensively cropped greenhouse soils. Appl. Microbiol. Biotechnol. 2019, 103, 6369–6383. [Google Scholar]
- Zhao, J.; Li, Y.L.; Wang, B.Y.; Huang, X.Q.; Yang, L.; Lan, T.; Zhang, J.B.; Cai, Z.C. Comparative soil microbial communities and activities in adjacent sanqi ginseng monoculture and maize-sanqi ginseng systems. Appl. Soil Ecol. 2017, 120, 89–96. [Google Scholar]
- Liu, L.L.; Yan, Y.Y.; Ding, H.X.; Zhao, J.; Cai, Z.C.; Dai, C.C.; Huang, X.Q. The fungal community outperforms the bacterial community in predicting plant health status. Appl. Microbiol. Biotechnol. 2021, 105, 6499–6513. [Google Scholar]
- Liu, L.L.; Huang, X.Q.; Zhao, J.; Zhang, J.B.; Cai, Z.C. Characterizing the key agents in a disease-suppressed soil managed by reductive soil disinfestation. Appl. Environ. Microb. 2019, 85, e02992-18. [Google Scholar]
- Li, S.L.; Liu, Y.Q.; Wang, J.; Yang, L.; Zhang, S.T.; Xu, C.; Ding, W. Soil Acidification Aggravates the Occurrence of Bacterial Wilt in South China. Front. Microbiol. 2017, 8, 703. [Google Scholar]
- Xun, W.B.; Huang, T.; Zhao, J.; Ran, W.; Wang, B.R.; Shen, Q.R.; Zhang, R.F. Environmental conditions rather than microbial inoculum composition determine the bacterial composition, microbial biomass and enzymatic activity of reconstructed soil microbial communities. Soil Biol. Biochem. 2015, 90, 10–18. [Google Scholar]
- Coventry, E.; Noble, R.; Mead, A.; Whipps, J.M. Suppression of allium white rot (Sclerotium cepivorum) in different soils using vegetable wastes. Eur. J. Plant Pathol. 2005, 111, 101–112. [Google Scholar]
- Tilston, E.L.; Pitt, D.; Groenhof, A.C. Composted recycled organic matter suppresses soil-borne diseases of field crops. New Phytol. 2002, 154, 731–740. [Google Scholar] [PubMed]
- Bonanomi, G.; Antignani, V.; Capodilupo, M.; Scala, F. Identifying the characteristics of organic soil amendments that suppress soilborne plant diseases. Soil Biol. Biochem. 2010, 42, 136–144. [Google Scholar]
- Mazzola, M.; Granatstein, D.M.; Elfving, D.C.; Mullinix, K. Suppression of specific apple root pathogens by brassica napus seed meal amendment regardless of glucosinolate content. Phytopathology 2007, 91, 673–679. [Google Scholar]
- Blok, W.J.; Lamers, J.G.; Termorshuizen, A.J.; Bollen, G.J. Control of soilborne plant pathogens by incorporating fresh organic amendments followed by tarping. Phytopathology 2000, 90, 253–259. [Google Scholar]
- Momma, N.; Momma, M.; Kobara, Y. Biological soil disinfestation using ethanol: Effect on Fusarium oxysporum f. sp. lycopersici and soil microorganisms. J. Gen. Plant Pathol. 2010, 76, 336–344. [Google Scholar]
- Butler, D.M.; Kokalis-Burelle, N.; Muramoto, J.; Shennan, C.; McCollum, T.G.; Rosskopf, E.N. Impact of anaerobic soil disinfestation combined with soil solarization on plant–parasitic nematodes and introduced inoculum of soilborne plant pathogens in raised-bed vegetable production. Crop Prot. 2012, 39, 33–40. [Google Scholar]
- Momma, N. Biological soil disinfestation (BSD) of soilborne pathogens and its possible mechanisms. Jpn. Agr. Res. Q. 2008, 42, 7–12. [Google Scholar]
- Zhu, R.; Huang, X.Q.; Zhang, J.B.; Cai, Z.C.; Li, X.; Wen, T. Efficiency of Reductive Soil Disinfestation Affected by Soil Water Content and Organic Amendment Rate. Horticulturae 2021, 7, 559. [Google Scholar]
- IUSS Working Group. World Reference Base for Soil Resources 2006; World Soil Resources Reports; FAO: Rome, Italy, 2007; First Update 2007; NO. 103. [Google Scholar]
- Huang, X.Q.; Zhou, X.; Zhang, J.B.; Cai, Z.C. Highly connected taxa located in the microbial network are prevalent in the rhizosphere soil of healthy plant. Biol. Fert. Soils 2019, 55, 299–312. [Google Scholar]
- Bremner, J.M.; Jenkinson, D.S. Determination of organic carbon in soil. I. Oxidation by dichromate of organic matter in soil and plant materials. Eur. J. Soil Sci. 1960, 11, 394–402. [Google Scholar]
- Robertson, J.A. Comparison of an acid and an alkaline extracting solution for measuring available phosphorus in alberta soils. Can. J. Soil Sci. 1962, 42, 115–121. [Google Scholar]
- Peck, N.H.; Macdonald, G.E. Table beet responses to residual and band-applied phosphorus and potassium. Agron. J. 1981, 73, 1037–1041. [Google Scholar]
- Adam, G.; Duncan, H. Development of a sensitive and rapid method for the measurement of total microbial activity using fluorescein diacetate (FDA) in a range of soils. Soil Biol. Biochem. 2001, 33, 943–951. [Google Scholar]
- Garland, J.L.; Mills, A.L. Classification and characterization of heterotrophic microbial communities on the basis of patterns of community-level sole-carbon-source utilization. Appl. Environ. Microbiol. 1991, 57, 2351–2359. [Google Scholar]
- Garland, J.L. Analytical approaches to the characterization of samples of microbial communities using patterns of potential C source utilization. Soil Biol. Biochem. 1996, 28, 213–221. [Google Scholar]
- McMurdie, P.J.; Holmes, S.; Michael, W. Phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friednly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.5–7. The R Journal. Published 28 November 2020. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 28 February 2022).
- Janvier, C.; Villeneuve, F.; Alabouvette, C.; Edel-Hermann, V.; Mateille, T.; Steinberg, C. Soil health through soil disease suppression: Which strategy from descriptors to indicators? Soil Biol. Biochem. 2007, 39, 1–23. [Google Scholar]
- Raaijmakers, J.M.; Paulitz, T.C.; Steinberg, C.; Alabouvette, C.; Moenneloccoz, Y. The rhizosphere: A playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil 2009, 321, 341–361. [Google Scholar]
- Liu, L.L.; Huang, X.Q.; Zhang, J.B.; Cai, Z.C.; Jiang, K.; Chang, Y.Y. Deciphering the relative importance of soil and plant traits on the development of rhizosphere microbial communities. Soil Biol. Biochem. 2020, 148, 107909. [Google Scholar]
- Preem, J.K.; Truu, J.; Truu, M.; Mander, Ü.; Oopkaup, K.; Lõhmus, K.; Helmisaari, H.S.; Uri, V.; Zobel, M. Bacterial community structure and its relationship to soil physico-chemical characteristics in alder stands with different management histories. Ecol. Eng. 2012, 49, 10–17. [Google Scholar]
- Stres, B.; Danevcic, T.; Pal, L.; Fuka, M.M.; Resman, L.; Leskovec, S.; Hacin, J.; Stopar, D.; Mahne, I.; Mandic-Mulec, I. Influence of temperature and soil water content on bacterial, archaeal and denitrifying microbial communities in drained fen grassland soil microcosms. FEMS Microbiol. Ecol. 2010, 66, 110–122. [Google Scholar]
- Watanabe, K.; Matsui, M.; Honjo, H.; Becker, J.O.; Fukui, R. Effects of soil pH on rhizoctonia damping-off of sugar beet and disease suppression induced by soil amendment with crop residues. Plant Soil 2011, 347, 255–268. [Google Scholar]
- Nachmias, A.; Kaufman, Z.; Livescu, L.; Tsror, L.; Meiri, A.; Caligari, P.D.S. Effects of salinity and its interactions with disease incidence on potatoes grown in hot climates. Phytoparasitica 1993, 21, 245–255. [Google Scholar]
- Meng, T.Z.; Zhu, T.B.; Zhang, J.B.; Cai, Z.C. Effect of liming on sulfate transformation and sulfur gas emissions in degraded vegetable soil treated by reductive soil disinfestation. J. Environ. Sci. 2015, 36, 112–120. [Google Scholar]
- Zhong, W.H.; Cai, Z.C.; Zhang, H. Effects of Long-Term Application of Inorganic Fertilizers on Biochemical Properties of a Rice-Planting Red Soil. Pedosphere 2007, 17, 419–428. [Google Scholar]
- Dong, W.; Zhang, X.; Wang, H.; Dai, X.; Sun, X.; Qiu, W.; Yang, F. Effect of Different Fertilizer Application on the Soil Fertility of Paddy Soils in Red Soil Region of Suothern China. PLoS ONE 2012, 7, e44504. [Google Scholar]
- Wang, T.T.; Hao, Y.W.; Zhu, M.Z.; Yu, S.T.; Ran, W.; Xue, C.; Ling, N.; Shen, Q.R. Characterizing differences in microbial community composition and function between fusarium wilt diseased and healthy soils under watermelon cultivation. Plant Soil 2019, 438, 421–433. [Google Scholar]
- Wei, Z.; Gu, Y.A.; Friman, V.; Kowalchuk, G.; Xu, Y.C.; Shen, Q.R.; Jousset, A. Initial soil microbiome composition and functioning predetermine future plant health. Sci. Adv. 2019, 5, eaaw0759. [Google Scholar]
- Huang, X.Q.; Liu, S.Z.; Liu, X.; Zhang, S.R.; Li, L.; Zhao, H.T.; Zhao, J.; Zhang, J.B.; Cai, Z.C. Plant pathological condition is associated with fungal community succession triggered by root exudates in the plant-soil system. Soil Biol. Biochem. 2020, 151, 108046. [Google Scholar]
- Li, B.Y.; Zhou, D.M.; Cang, L.; Zhang, H.L.; Fan, X.H.; Qin, S.W. Soil micronutrient availability to crops as affected by long-term inorganic and organic fertilizer applications. Soil Tillage Res. 2007, 96, 166–173. [Google Scholar]
- Zhu, T.B.; Dang, Q.; Zhang, J.B.; Müller, C.; Cai, Z.C. Reductive soil disinfestation (RSD) alters gross N transformation rates and reduces NO and N2O emissions in degraded vegetable soils. Plant Soil 2014, 382, 269–280. [Google Scholar]
- Huang, X.Q.; Liu, L.L.; Zhao, J.; Zhang, J.B.; Cai, Z.C. The families Ruminococcaceae, Lachnospiraceae, and Clostridiaceae are the dominant bacterial groups during reductive soil disinfestation with incorporated plant residues. Appl. Soil Ecol. 2019, 135, 65–72. [Google Scholar]
- Huang, X.Q.; Zhao, J.; Zhou, X.; Zhang, J.B.; Cai, Z.C. Differential responses of soil bacterial community and functional diversity to reductive soil disinfestation and chemical soil disinfestation. Geoderma 2019, 348, 124–134. [Google Scholar]
- Liu, L.L.; Kong, J.J.; Cui, H.L.; Zhang, J.B.; Wang, F.H.; Cai, Z.C.; Huang, X.Q. Relationships of decomposability and C/N ratio in different types of organic matter with suppression of Fusarium oxysporum and microbial communities during reductive soil disinfestation. Biol. Control 2016, 101, 103–113. [Google Scholar]
- Lane, D.J. 16S/23S rRNA sequencing. In Nucleic Acid Techniques in Bacterial Systematics, 2nd ed.; Stackenbrandt, E., Goodfellow, M., Eds.; John Wiley and Sons, Inc.: Chichester, UK, 1991; pp. 115–175. [Google Scholar]
- Muyzer, G.; de Waal, E.C.; Uitterlinden, A.G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microb. 1993, 59, 695–700. [Google Scholar]
- Schonfeld, J.; Heuer, H.; van Elsas, J.D.; Smalla, K. Specific and Sensitive Detection of Ralstonia solanacearum in Soil on the Basis of PCR Amplification of fliC Fragments. Appl. Environ. Microb. 2003, 69, 7248–7256. [Google Scholar]
- Gardes, M.; Bruns, T.D. TS primers with enhanced specificity for basidiomycetes–application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar]
- Lievens, B.; Brouwer, M.; Vanachter, A.C.R.C.; Cammue, B.P.A.; Thomma, B.P.H.J. Real-time PCR for detection and quantification of fungal and oomycete tomato pathogens in plant and soil samples. Plant Sci. 2006, 171, 155–165. [Google Scholar]
- Lievens, B.; Brouwer, M.; Vanachter, A.C.R.C.; Lévesque, C.A.; Cammue, B.P.A.; Thomma, B.P.H.J. Quantitative assessment of phytopathogenic fungi in various substrates using a DNA macroarray. Environ. Microbiol. 2005, 7, 1698–1710. [Google Scholar] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).