Biological Control of the Cucumber Downy Mildew Pathogen Pseudoperonospora cubensis
Abstract
1. Introduction
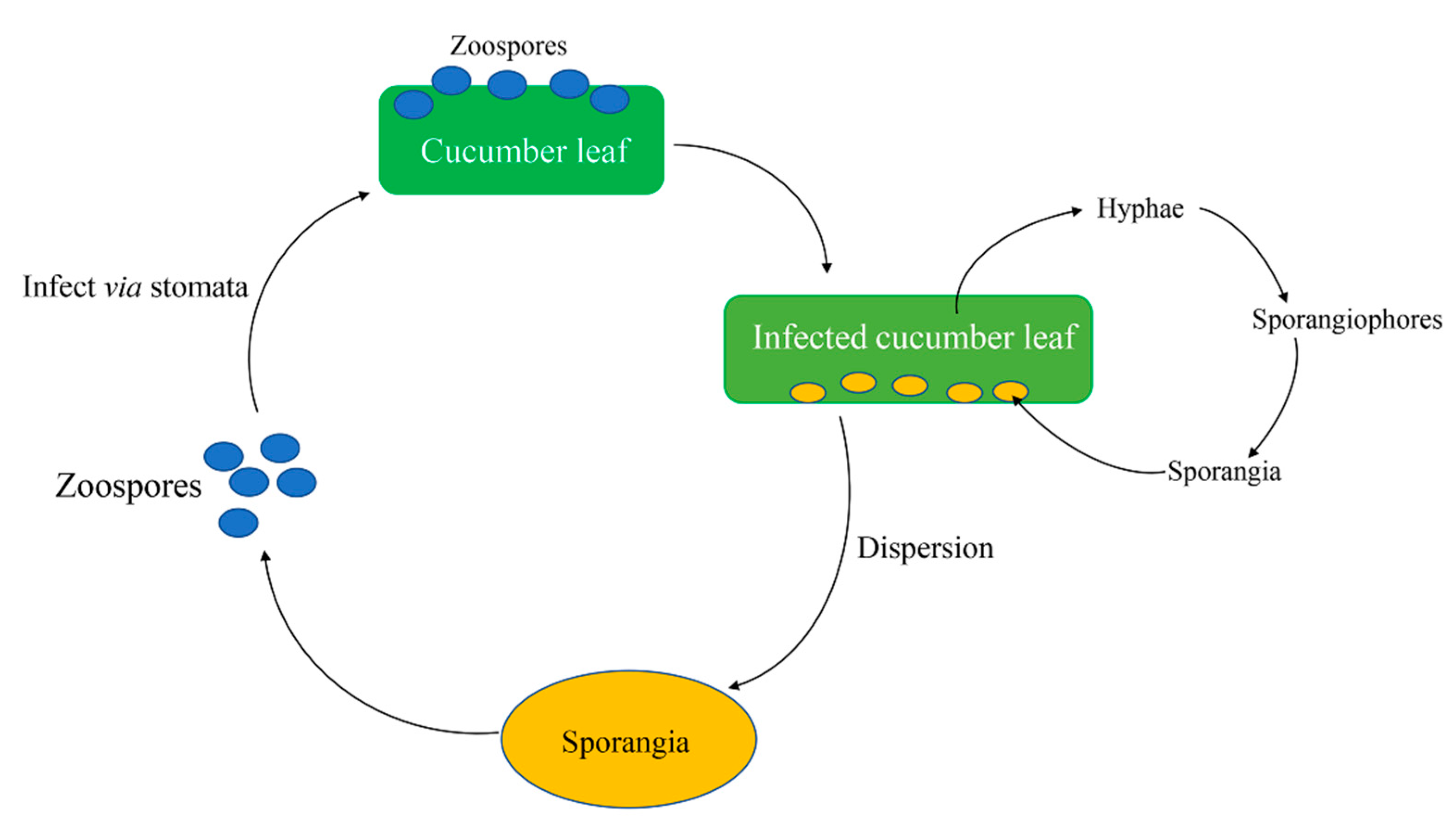
2. Pseudoperonospora cubensis
3. Strategy of Screening Biocontrol Agents
4. Biocontrol Agents
4.1. Biocontrol Fungi
4.2. Biocontrol Bacteria
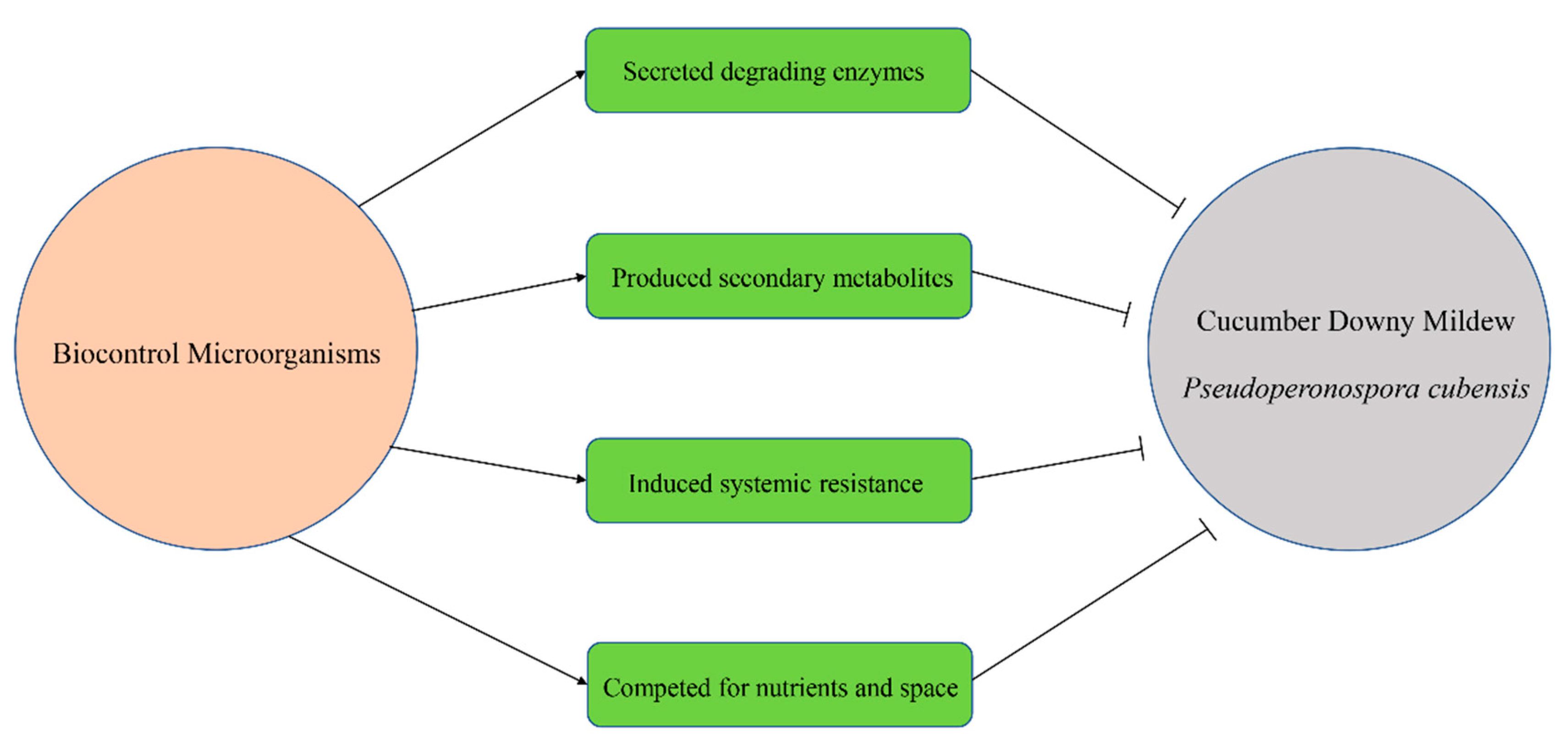
5. Biocontrol Mechanisms against CDM
6. Conclusions and Prospects
- (1)
- Study the molecular mechanism by which biocontrol agents prevent CDM. This could include investigating the transcriptome of biocontrol agents and analyzing the differentially expressed genes in biocontrol agents during the process of controlling CDM. The functions of differentially expressed genes in biocontrol agents preventing CDM can be studied through gene knockout, silencing, or overexpression.
- (2)
- Expand the variety and amount of biocontrol agents that effectively control CDM. Compared with controlling fungal plant diseases, the variety and amount of biocontrol agents that suppress CDM are small. The selection of a suitable screening method could be an effective way to expand the variety and amount of biocontrol agents. Another way is to investigate the control ability of CDM by known biocontrol agents with significant control ability against other fungal and oomycete plant diseases, e.g., other Bacillus strains, Clonostacys rosea, and Coniothyrium minitans [118,119,120].
- (3)
- Improve the control efficacy of existing biocontrol agents suppressing CDM. Several methods could be applied. One approach is to optimize the culture and inoculation conditions. In addition, genetic manipulations such as mutagenesis or genetic engineering could be used to improve the control ability of biocontrol agents suppressing CDM.
- (4)
- Study the synergistic effect of integrating multiple biocontrol agents or combining biocontrol agents and fungicides. We can expect to reach an ideal control efficacy for CDM by optimizing the proportion of each component.
- (5)
- Develop biocontrol agent products. Although many potential biocontrol agents were screened, commercially available products are very few. Therefore, developing biocontrol agent products is crucial for the wide application of biocontrol agents to control CDM.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lebeda, A.; Urban, J. Temporal changes in pathogenicity and fungicide resistance in Pseudoperonospora cubensis populations. Acta Hortic. 2007, 731, 327–336. [Google Scholar] [CrossRef]
- Miao, J.Q.; Dong, X.; Chi, Y.D.; Lin, D.; Chen, F.R.; Du, Y.X.; Liu, P.F.; Liu, X.L. Pseudoperonospora cubensis in China: Its sensitivity to and control by oxathiapiprolin. Pestic. Biochem. Physiol. 2018, 147, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.L.; Lian, S.; Feng, S.; Dong, X.; Wang, C.; Li, B.; Liang, W. Effects of temperature and moisture on sporulation and infection by Pseudoperonospora cubensis. Plant Dis. 2017, 101, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Colucci, S.J.; Webner, T.C.; Holmes, G.J. The downy mildew epidemic of 2004 and 2005 in the eastern United States. In Proceedings of the Cucurbitaceae 2006, Asheville, NC, USA, 17–21 September 2006; pp. 403–411. [Google Scholar]
- Palti, J.; Cohen, Y. Downy mildew of cucurbits Pseudoperonospora.cubensis. The fungus and its hosts, distribution, epidemiology and control. Phytoparasitica 1980, 8, 109–147. [Google Scholar] [CrossRef]
- Thomas, C.E. Downy mildew. In Compendium of Cucurbit Diseases; Zitter, T.A., Ed.; Cornell University Press: Ithaca, NY, USA, 1996; pp. 25–27. [Google Scholar]
- Wang, H.C.; Zhou, M.G.; Wang, J.X.; Chen, C.J.; Li, H.X.; Sun, H.Y. Biological mode of action of dimethomorph on Pseudoperonospora cubensis and its systemic activity in cucumber. Agric. Sci. China 2009, 8, 172–181. [Google Scholar] [CrossRef]
- Yang, X.T.; Li, M.; Zhao, C.J.; Zhang, Z.; Hou, Y.L. Early warning model for cucumber downy mildew in unheated greenhouses. N. Z. J. Agric. Res. 2007, 50, 1261–1268. [Google Scholar] [CrossRef]
- Ahamad, S.; Narain, U.; Prajapati, R.K.; Lal, C. Management of downy mildew of cucumber. Ann. Plant Prot. Sci. 2000, 8, 254–255. [Google Scholar]
- Burkhardt, A.; Day, B. A genomics perspective on cucurbit-oomycete interactions. Plant Biotechnol. 2013, 30, 265–271. [Google Scholar] [CrossRef]
- Holmes, G.J.; Main, C.E.; Keever, Z.T. Cucurbit downy mildew: A unique pathosystem for disease forecasting. In Advances in Downy Mildew Research; Spencer-Phillips, P.T.N., Jeger, M., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; Volume 2, pp. 69–80. [Google Scholar]
- Lebeda, A.; Schwinn, F.J. The downy mildews–an overview of recent.research progress. J. Plant Dis. Prot. 1994, 101, 225–254. [Google Scholar]
- Shetty, N.V.; Wehner, T.C.; Thomas, C.E.; Doruchowski, R.W.; Shetty, K.V. Evidence for downy mildew races in cucumber tested in Asia, Europe, and North America. Sci. Hortic. 2002, 94, 231–239. [Google Scholar] [CrossRef]
- Lebeda, A.; Pavelková, J.; Urban, J.; Sedláková, B. Distribution, host.range and disease severity of Pseudoperonospora cubensis on cucurbits in the Czech Republic. J. Phytopathol. 2011, 159, 589–596. [Google Scholar] [CrossRef]
- Oerke, E.C.; Steiner, U.; Dehne, H.W.; Lindenthal, M. Thermal imaging of.cucumber leaves affected by downy mildew and environmental conditions. J. Exp. Bot. 2006, 57, 2121–2132. [Google Scholar] [CrossRef]
- Savory, E.A.; Granke, L.L.; Quesada-Ocampo, L.M.; Varbanova, M.; Hausbeck, M.K.; Day, B. The cucurbit downy mildew pathogen Pseudoperonospora cubensis. Mol. Plant Pathol. 2011, 12, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.C.; Wang, H.B.; Feng, J.J.; Xiang, H.F.; Wu, M.D.; Wang, Z.M.; Wei, D.Y.; Zhang, H.C.; Tang, Q.L. Progress in cucumber downy mildew and mechanisms of host resistance. Chin. J. Biotechnol. 2022. [Google Scholar] [CrossRef]
- Zhao, X.J.; Lin, Z.M.; Zhao, Z.J. Studies on diversity of symptoms of cucumber downy mildew. J. Shanxi Agric. Sci. 2000, 28, 72–74. [Google Scholar]
- Cohen, Y.; Van Den Langenberg, K.M.; Wehner, T.C.; Ojiambo, P.S.; Hausbeck, M.; Quesada-Ocampo, L.M.; Lebeda, A.; Sierotzki, H.; Gisi, U. Resurgence of Pseudoperonospora cubensis: The causalagent of cucurbit downy mildew. Phytopathology 2015, 105, 998–1012. [Google Scholar] [CrossRef]
- Granke, L.L.; Morrice, J.J.; Hausbeck, M.K. Relationships between airborne Pseudoperonospora cubensis sporangia, environmental conditions, and cucumber downy mildew severity. Plant Dis. 2014, 98, 674–681. [Google Scholar] [CrossRef]
- Holmes, G.J.; Ojiambo, P.S.; Hausbeck, M.K.; Quesada-Ocampo, L.; Keinath, A.P. Resurgence of cucurbit downy mildew in the United States: A watershed event for research and extension. Plant Dis. 2015, 99, 428–441. [Google Scholar] [CrossRef]
- Lebeda, A.; Urban, J. Distribution, harmfulness and pathogenic variability of cucurbit downy mildew in the Czech Republic. Acta Fytotech. Zootech. 2004, 7, 170–173. [Google Scholar]
- Rondomański, W.; Żurek, B.; Sekuł, S. Downy mildew of cucumber a new problem on cucumber in Poland Roczn. Nauk. Roln seria ET 1987, 17, 200–209. [Google Scholar]
- Guan, A.; Wang, M.; Yang, J.; Wang, L.; Xie, Y.; Lan, J.; Liu, C. Discovery of a new fungicide candidate through lead optimization of pyrimidinamine derivatives and its activity against cucumber downy mildew. J. Agric. Food Chem. 2017, 65, 10829–10835. [Google Scholar] [CrossRef] [PubMed]
- Ishii, H.; Fraaije, B.A.; Sugiyama, T.; Noguchi, K.; Nishimura, K.; Takeda, T.; Amano, T.; Hollomon, D.W. Occurrence and molecular characterization of strobilurin resistance in cucumber powdery mildew and downy mildew. Phytopathology 2001, 91, 1166–1171. [Google Scholar] [CrossRef] [PubMed]
- Keinath, A.P. Utility of a cucumber plant bioassay to assess fungicide efficacy against Pseudoperonospora cubensis. Plant Dis. 2016, 100, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Mitani, S.; Kamachi, K.; Sugimoto, K.; Araki, S.; Yamaguchi, T. Control of cucumber downy mildew by cyazofamid. J. Pestic. Sci. 2003, 28, 64–68. [Google Scholar] [CrossRef][Green Version]
- Salas, S.E.; Shepherd, C.P.; Ngugi, H.K.; Genet, J.L. Disease control attributes of oxathiapiprolin fungicides for management of cucurbit downy mildew. Plant Dis. 2019, 103, 2812–2820. [Google Scholar] [CrossRef]
- Chen, Z.J.; Zhang, S.L.; Zhang, F.; Liang, Y.L.; Xu, F.L. Present situateion of chemical pesticides controlling cucumber pests in sunlight greenhouse and its controlling countermeasures. Chin. J. Eco-Agric. 2006, 14, 141–143. [Google Scholar]
- Zhang, F.; Qin, Z.; Zhou, X.; Xin, M.; Li, S.; Luan, J. Expression and functional analysis of the propamocarb-related gene CsMAPEG in cucumber. BMC Plant Biol. 2019, 19, 371. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, W.; Zhang, Y.L.; Ma, L.G.; Qi, J.S.; Li, C.S. Control effect of five kinds of preparations and combinations of cucumber downy mildew and its evaluation. Agrochemicals 2015, 54, 456–457. [Google Scholar]
- Blum, M.; Waldner, M.; Olaya, G.; Cohen, Y.; Gisi, U.; Sierotzki, H. Resistance mechanism to carboxylic acid amide fungicides in the cucurbit downy mildew pathogen Pseudoperonospora cubensis. Pest Manag. Sci. 2011, 67, 1211–1214. [Google Scholar] [CrossRef]
- Okada, K.; Furukawa, M. Occurrence and countermeasure of fungicide-resistant pathogens in vegetable field of Osaka prefecture. J. Pestic. Sci. 2008, 33, 326–329. [Google Scholar] [CrossRef][Green Version]
- Reuveni, M.; Eyal, M.; Cohen, Y. Development of resistance to metalaxyl in Pseudoperonospora cubensis. Plant Dis. 1980, 64, 1108–1109. [Google Scholar] [CrossRef]
- Urban, J.; Lebeda, A. Fungicide resistance in cucurbit downy mildew-methodological, biological and population aspects. Ann. Appl. Biol. 2006, 149, 63–75. [Google Scholar] [CrossRef]
- Zhu, S.S.; Liu, X.L.; Wang, Y.; Wu, X.H.; Liu, P.F.; Li, J.Q.; Yuan, S.K.; Si, N.G. Resistance of Pseudoperonospora cubensis to flumorph on cucumber in plastic houses. Plant Pathol. 2007, 56, 967–975. [Google Scholar] [CrossRef]
- Call, A.D.; Criswell, A.D.; Weimer, T.C.; Klosinska, U.; Kozik, E.U. Screening cucumber for resistance to downy mildew caused by Pseudoperonospora cubensis (Berk. and Curt.) Rostov. Crop Sci. 2012, 52, 577–592. [Google Scholar] [CrossRef]
- Holdsworth, W.L.; Summers, C.F.; Glos, M.; Smart, C.D.; Mazourek, M. Development of downy mildew-resistant cucumbersfor late-season production in the northeastern United States. HortScience 2014, 49, 10–17. [Google Scholar] [CrossRef]
- Horejsi, T.; Staub, J.E.; Thomas, C. Linkage of random amplified polymorphic DNA markers to downy mildew resistance in cucumber (Cucumis.sativus L.). Euphytica 2000, 115, 105–113. [Google Scholar] [CrossRef]
- Innark, P.; Ratanachan, T.; Khanobdee, C.; Samipak, S.; Jantasuriyarat, C. Downy mildew resistant/susceptible cucumber germplasm (Cucumis sativus L.) genetic diversity assessment using ISSR markers. Crop Prot. 2014, 60, 56–61. [Google Scholar] [CrossRef]
- Olczak-Woltman, H.; Marcinkowska, J.; Niemirowicz-Szczytt, K. The genetic basis of resistance to downy mildew in Cucumis spp.—latest developments and prospects. J. Appl. Genet. 2011, 52, 249–255. [Google Scholar] [CrossRef]
- Cohen, Y. The combined effects of temperature, leaf wetness, and inoculum concentration on infection of cucumbers with Pseudoperonospora cubensis. Can. J. Bot. 1977, 55, 1478–1487. [Google Scholar] [CrossRef]
- Göker, M.; Voglmayr, H.; Riethmüller, A.; Oberwinkler, F. How do obligate parasites evolve? A multi-gene phylogenetic analysis of downy mildews. Fungal Genet. Biol. 2007, 44, 105–122. [Google Scholar] [CrossRef]
- Lange, L.; Eden, U.; Olson, L.W. Zoosporogenesis in Pseudoperonospora cubensis, the causal agent of cucurbit downy mildew. Nord. J. Bot. 1989, 8, 497–504. [Google Scholar] [CrossRef]
- Ojiambo, P.S.; Holmes, G.J. Spatiotemporal spread of cucurbit downy mildew in the eastern United States. Phytopathology 2011, 101, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Runge, F.; Thines, M. Host matrix has major impact on the morphology of Pseudoperonospora cubensis. Eur. J. Plant Pathol. 2011, 129, 147–156. [Google Scholar] [CrossRef]
- Salcedo, A.F.; Purayannur, S.; Standish, J.R.; Miles, T.; Thiessen, L.; Quesada-Ocampo, L.M. Fantastic downy mildew pathogens and how to find them: Advances in detection and diagnostics. Plants 2021, 10, 435. [Google Scholar] [CrossRef]
- Bedlan, G. Erstmaliger nachweis von oosporen von Pseudoperonospora cubensis (Berk. et Curt.) Rost. an Gewächshausgurken in Österreich. Pflanzenschutzberichte 1989, 50, 119–120. [Google Scholar]
- Cohen, Y.; Meron, I.; Mor, N.; Zuriel, S. A new pathotype of Pseudoperonospora cubensis causing downy mildew in cucurbits in Israel. Phytoparasitica 2003, 31, 458–466. [Google Scholar] [CrossRef]
- Cohen, Y.; Rubin, A.E.; Galperin, M. Host preference of mating type in Pseudoperonospora cubensis, the downy mildew causal agent of cucurbits. Plant Dis. 2013, 97, 292. [Google Scholar] [CrossRef]
- Runge, F.; Thines, M. Reevaluation of host specificity of the closely related species Pseudoperonospora humuli and P. cubensis. Plant Dis. 2012, 96, 55–61. [Google Scholar] [CrossRef]
- Runge, F.; Thines, M. A potential perennial host for Pseudoperonospora cubensis in temperate regions. Eur. J. Plant Pathol. 2009, 123, 483–486. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.; Zhang, Y.J.; Zhou, M.G. Occurrence and molecular characterization of azoxystrobin resistance in cucumber downy mildew in Shandong province in China. Phytoparasitica 2008, 36, 136–143. [Google Scholar] [CrossRef]
- Forsberg, A.S. Downy mildew-Pseudoperonospora cubensis in Swedish cucumber fields. Växtskyddsnotiser 1986, 50, 17–19. [Google Scholar]
- Neufeld, K.N.; Isard, S.A.; Ojiambo, P.S. Relationship between disease severity and escape of P.cubensis sporangia from a cucumber canopy during downy mildew epidemics. Plant Pathol. 2013, 62, 1366–1377. [Google Scholar] [CrossRef]
- Niu, D.; Fu, J.; Wang, L.J. Latest research development of cucumber downy mildew. J. Northeast F. Univ. 2008, 9, 94–98. [Google Scholar]
- Spring, O.; Gomez-Zeledon, J.; Hadziabdic, D.; Trigiano, R.N.; Thines, M.; Lebeda, A. Biological characteristics and assessment of virulence diversity in pathosystems of economically important biotrophic oomycetes. Crit. Rev. Plant. Sci. 2018, 37, 439–495. [Google Scholar] [CrossRef]
- Kitner, M.; Lebeda, A.; Sharma, R.; Runge, F.; Dvorak, P.; Tahir, A.; Choi, Y.J.; Sedlakova, B.; Thines, M. Coincidence of virulence shifts and population genetic changes of Pseudoperonospora cubensis in the Czech Republic. Plant Pathol. 2015, 64, 1467–1470. [Google Scholar] [CrossRef]
- Lebeda, A.; Widrlechner, M. A set of Cucurbitaceae taxa for differentiation of Pseudoperonospora cubensis pathotypes. J. Plant Dis. Prot. 2003, 110, 337–349. [Google Scholar]
- Lebeda, A.; Cohen, Y. Cucurbit downy mildew (Pseudoperonospora cubensis)-biology, ecology, epidemiology, host-pathogen interaction and control. Eur. J. Plant Pathol. 2011, 129, 157–192. [Google Scholar] [CrossRef]
- Bains, S.S.; Jhooty, J.S. Over wintering of Pseudoperonospora cubensis causing downy mildew of muskmelon. Indian Phytopathol. 1976, 29, 213–214. [Google Scholar]
- Hall, G.S. Modern approaches to species concepts in downy mildews. Plant Pathol. 1996, 45, 1009–1026. [Google Scholar] [CrossRef]
- Thines, M. Phylogeny and evolution of plant pathogenic oomycetes: A global overview. Eur. J. Plant Pathol. 2014, 138, 431–447. [Google Scholar] [CrossRef]
- Cohen, Y. Downy mildew of cucurbits. In The Downy Mildews; Spencer, D.M., Ed.; Academic Press: London, UK, 1981; pp. 341–354. [Google Scholar]
- Sun, Y.J. Occurrence regularity, environmental influencing factors and control techniques of cucumber downy mildew. J. Seed Ind. Guide 2021, 6, 35–38. [Google Scholar]
- Zhu, J.X.; Wang, Y.P.; Guo, P.Y.; Gao, F.J. Progress of study on downy mildew in cucumber. Nor. Horticul. 2008, 4, 74–78. [Google Scholar]
- Bouwmeester, K.; van Poppel, P.M.J.A.; Govers, F. Genome biology cracks enigmas of oomycete plant pathogens. Annu. Plant Rev. 2009, 34, 102–133. [Google Scholar]
- Choi, Y.J.; Hong, S.B.; Shin, H.D. A re-consideration of Pseudoperonospora cubensis and P. humuli based on molecular and morphological data. Mycol. Res. 2005, 109, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Granke, L.L.; Hausbeck, M.K. Dynamics of Pseudoperonospora cubensis sporangia in commercial cucurbit fields in Michigan. Plant Dis. 2011, 95, 1392–1400. [Google Scholar] [CrossRef] [PubMed]
- Arauz, L.F.; Neufeld, K.N.; Lloyd, A.L.; Ojiambo, P.S. Quantitative models for germination and infection of Pseudoperonospora cubensis in response to temperature and duration of leaf wetness. Phytopathology 2010, 100, 959–967. [Google Scholar] [CrossRef][Green Version]
- Cohen, Y.; Rubin, A.E.; Galperin, M. Formation and infectivity of oospores of Pseudoperonospora cubensis, the causal agent of downy mildew in cucurbits. Plant Dis. 2011, 95, 874. [Google Scholar] [CrossRef]
- Kanetis, L.; Holmes, G.J.; Ojiambo, P.S. Survival of Pseudoperonospora cubensis sporangia exposed to solar radiation. Plant Pathol. 2009, 59, 313–323. [Google Scholar] [CrossRef]
- Neufeld, K.N.; Ojiambo, P.S. Interactive effects of temperature and leaf wetness duration on sporangia germination and infection of cucurbit hosts by Pseudoperonospora cubensis. Sci. Hortic. 2011, 96, 345–353. [Google Scholar] [CrossRef]
- Shi, Y.X.; Li, B.J.; Liu, X.M. The study of cucumber downy mildew. J. Northeast Agric. Univ. 2002, 33, 391–395. [Google Scholar]
- Adhikari, B.N.; Savory, E.A.; Vaillancourt, B.; Childs, K.L.; Hamilton, J.P.; Day, B.; Buell, C.R. Expression profiling of Cucumis sativus in response to infection by Pseudoperonospora cubensis. PLoS ONE 2012, 7, e34954. [Google Scholar] [CrossRef] [PubMed]
- Savory, E.A.; Adhikari, B.N.; Hamilton, J.P.; Vaillancourt, B.; Buell, C.R.; Day, B. mRNA-Seq analysis of the Pseudoperonospora cubensis transcriptome during cucumber (Cucumis sativus L.) infection. PLoS ONE 2012, 7, e35796. [Google Scholar] [CrossRef] [PubMed]
- Savory, E.A.; Zou, C.; Adhikari, B.N.; Hamilton, J.P.; Buell, C.R.; Shiu, S.H.; Day, B. Alternative splicing of a multi-drug transporter from Pseudoperonospora cubensis generates an RXLR effector protein that elicits a rapid cell death. PLoS ONE 2012, 7, e34701. [Google Scholar] [CrossRef]
- Zhang, P.; Zhu, Y.; Luo, X.; Zhou, S. Comparative proteomic analysis provides insights into the complex responses to Pseudoperonospora cubensis infection of cucumber (Cucumis sativus L.). Sci. Rep. 2019, 9, 9433. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Gu, C.; Cao, J.; Li, S.M.; Wang, G.; Luo, Y.M.; Guo, J.H. Selecting bacterial antagonists for cucurbit downy mildew and developing an effective application method. Plant Dis. 2018, 102, 628–639. [Google Scholar] [CrossRef]
- Sun, Z.B.; Yuan, X.F.; Zhang, H.; Wu, L.F.; Liang, C.; Feng, Y.J. Isolation, screening and identification of antagonistic downy mildew endophytic bacteria from cucumber. Eur. J. Plant Pathol. 2013, 137, 847–857. [Google Scholar] [CrossRef]
- Alfiky, A.; Weisskopf, L. Deciphering Trichoderma-plant-pathogen interactions for better development of biocontrol applications. J. Fungi 2021, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, M.; Atanasova, L.; Jensen, D.F.; Zeilinger, S. Necrotrophic mycoparasites and their genomes. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef]
- Mukherjee, M.; Mukherjee, P.K.; Horwitz, B.A.; Zachow, C.; Berg, G.; Zeilinger, S. Trichoderma-plant-pathogen interactions: Advances in genetics of biological control. Indian J. Microbiol. 2012, 52, 522–529. [Google Scholar] [CrossRef]
- Sood, M.; Kapoor, D.; Kumar, V.; Sheteiwy, M.S.; Ramakrishnan, M.; Landi, M.; Araniti, F.; Sharma, A. Trichoderma: The “secrets” of a multitalented biocontrol agent. Plants 2020, 9, 762. [Google Scholar] [CrossRef]
- Tyśkiewicz, R.; Nowak, A.; Ozimek, E.; Jaroszuk-Ściseł, J. Trichoderma: The current status of its application in agriculture for the biocontrol of fungal phytopathogens and stimulation of plant growth. Int. J. Mol. Sci. 2022, 23, 2329. [Google Scholar] [CrossRef] [PubMed]
- Al-Aswad, R.M.A.; Al-Azzawi, Q.K.Z. Control of downy mildew disease on cucumber caused by the fungus Psuedoperonospora cubensis by using environmentally friendly materials. Euphrates J. Agric. Sci. 2021, 13, 98–110. [Google Scholar]
- Elsharkawy, M.M.; Kamel, S.M.; El-Khateeb, N.M.M. Biocontrol control of powdery and downy mildews of cucumber under greenhouse conditions. Egypt. J. Biol. Pest Co. 2014, 24, 301–308. [Google Scholar]
- El-khalily, M.R.; Gazar, T.A.; EL-Sheshtawi, M. Ability of some antagonistic fungi for controlling cucumber downy mildew disease caused by Pseudoperonospora cubensis. J. Plant Protect. Pathol. 2021, 12, 67–69. [Google Scholar]
- Alvarado-Aguayo, A.; Pilaloa-David, W.; Torres-Sánchez, S.; Torres-Sánchez, K. Efecto de Trichoderma harzianum en el control de mildiu (Pseudoperonospora cubensis) en pepino. Agron. Costarric. 2019, 43, 101–111. [Google Scholar] [CrossRef]
- Martínez, B.; González, E.; Infante, D. Nuevas evidencias de la accion Antagonista de Trichoderma asperellum Samuels. Rev. Protección Veg. 2011, 26, 131–132. [Google Scholar]
- Szczech, M.; Nawrocka, J.; Felczynski, K.; Malolepsza, U.; Sobolewski, J.; Kowalska, B.; Maciorowski, R.; Jas, K.; Kancelista, A. Trichodermaatroviride TRS25 isolate reduces downy mildew and induces systemic defence responses in cucumber in field conditions. Sci. Hortic. 2017, 224, 17–26. [Google Scholar] [CrossRef]
- Abd-El-Moity, T.H.; Tia, M.M.M.; Aly, A.Z.; Tohamy, M.R.A. Biological control of some cucumber diseases under organic agriculture. In Proceedings of the International Symposium on the Horizons of Using Organic Matter and Substrates in Horticulture, Cairo, Egypt, 6–9 April 2002; Volume 608, pp. 227–236. [Google Scholar]
- Yi, X.H.; Feng, J.T.; Fang, X.L.; Li, Y.P.; Zhang, X. Biological characteristics and biological activity of Pestalotiopsis sp. obtained from leaf of Pyrethrum cinerariafolium Trey. J. Zhejiang Univ. 2008, 34, 516–524. [Google Scholar]
- Yi, X.H.; Feng, J.T.; Wang, Y.H.; Guo, X.W.; Zhang, X. The preliminary study on screening and identification of endophytic fungi Y2 from Pyrethrum cinerariifolium and antifungal activity of Y2 fermentation products. Chin. J. Pestic. Sci. 2007, 9, 193–196. [Google Scholar]
- Yang, M.; Zong, Z.F.; Guo, X.F.; Yao, J.; Claude, A. Effect of biocontrol agents FO47 and FO47B10 on watermelon and cucumber. J. Northwest Sci. Tech. Univ. Agric. For. 2005, 33, 57–60. [Google Scholar]
- Andrić, S.; Meyer, T.; Ongena, M. Bacillus responses to plant-associated fungal and bacterial communities. Front. Microbiol. 2020, 11, 1350. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Wang, C.; Song, X.; Ding, X.; Wu, L.; Wu, H.; Gao, X.; Borriss, R. Bacillus velezensis FZB42 in 2018: The gram-positive model strain for plant growth promotion and biocontrol. Front. Microbiol. 2018, 9, 2491. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.R.; Patel, D.D.; Thakor, P.; Patel, B.; Thakkar, V.R. Alleviation of salt stress in germination of Vigna radiate L. by two halotolerant Bacillus sp. isolated from saline habitats of Gujarat. Plant Growth Regul. 2014, 76, 51–60. [Google Scholar] [CrossRef]
- Fira, D.; Dimkić, I.; Berić, T.; Lozo, J.; Stanković, S. Biological control of plant pathogens by Bacillus species. J. Biotechnol. 2018, 285, 44–55. [Google Scholar] [CrossRef]
- Miljaković, D.; Marinković, J.; Balešević-Tubić, S. The significance of Bacillus spp. in disease suppression and growth promotion of field and vegetable crops. Microorganisms 2020, 8, 1037. [Google Scholar] [CrossRef]
- Mohamed, A.; Hamza, A.; Derbalah, A. Recent approaches for controlling downy mildew of cucumber under greenhouse conditions. Plant Protect. Sci. 2016, 52, 1–9. [Google Scholar] [CrossRef]
- Zheng, L.; Xu, L.; Luo, Y.M.; Liu, H.X.; Guo, J.H. Preliminary study on the mechanism of a srain Bacillus licheniformis HS10 against cucumber downy mildew. Guangdong Agric. Sci. 2020, 47, 81–87. [Google Scholar]
- Wang, Z.X.; Wang, Y.P.; Zheng, L.; Yang, X.N.; Liu, H.X.; Guo, J.H. Isolation and characterization of an antifungal protein from Bacillus licheniformis HS10. Biochem. Biophys. Res. Commun. 2014, 454, 48–52. [Google Scholar] [CrossRef]
- Xiao, Q.; Li, S.W.; Liang, C.; Li, W.Y.; Mao, Y.W. Action mode and duration of Bacillus velezensis against cucumber downy mildew. Agrochemicals 2021, 60, 829–831. [Google Scholar]
- Ketta, H.A.; Kamel, S.M.; Ismail, A.M.; Ibrahem, E.S. Control of downy mildew disease of cucumber using Bacillus chitinosporus. Egypt. J. Biol. Pest Control 2016, 26, 839–845. [Google Scholar]
- Li, X.; Zhang, D.; Yang, W.X.; Dong, L.; Liu, D.Q. Studies on the effect of Bacillus on downy mildew of cucumber. Plant Protect. 2003, 29, 25–27. [Google Scholar]
- Anand, T.; Chandrasekaran, A.; Kuttalam, S.; Raguchander, T.; Samiyappan, R. Management of cucumber (Cucumis sativus L.) mildews through azoxystrobin-tolerant Pseudomonas fluorescens. J. Agric. Sci. Technol. 2009, 11, 211–226. [Google Scholar]
- Anand, T.; Raguchander, T.; Karthikeyan, G.; Prakasam, V.; Samiyappan, R. Chemically and biologically mediated systemic resistance in cucumber (Cucumis sativus L.) against Pseudoperonospora cubensis and Erysiphe cichoracearum. Phytopathol. Mediterr. 2007, 46, 259–271. [Google Scholar]
- Tian, X.W.; Long, J.Y.; Bai, H.J.; Wu, W.J. Studies on the fungicidal activity of secondary metabolic products of actinomycetes. Plant Protect. 2004, 30, 51–54. [Google Scholar]
- Patel, R.R.; Thakkar, V.R.; Subramanian, B.R. A Pseudomonas guariconensis strain capable of promoting growth and controlling collar rot disease in Arachis hypogaea L. Plant Soil 2015, 390, 369–381. [Google Scholar] [CrossRef]
- Ye, N.W.; Wang, C.F.; Gan, H.L.; Wu, Z.Y.; Mi, F.; Mao, W.L. Control effect of Paenibacilus polymyxa strain P1 against cucumber downy mildew. Plant Protect. 2021, 47, 271–275. [Google Scholar]
- Fan, Y.T.; Chung, K.R.; Huang, J.W. Fungichromin production by Streptomyces padanus PMS-702 for controlling cucumber downy mildew. Plant Pathol. J. 2019, 35, 341–350. [Google Scholar] [CrossRef]
- Schuster, C.; Schmitt, A. Efficacy of a bacterial preparation of Aneurinibacillus migulanus against downy mildew of cucumber (Pseudoperonospora cubensis). Eur. J. Plant Pathol. 2018, 151, 439–450. [Google Scholar] [CrossRef]
- Scherf, A.; Schuster, C.; Marx, P.; Gärber, U.; Konstantinidou-Doltsinis, S.; Schmitt, A. Control of downy mildew (Pseudoperonospora cubensis) of greenhouse grown cucumbers with alternative biological agents. Commun. Agric. Appl. Biol. Sci. 2010, 75, 541–554. [Google Scholar]
- Georgieva, O.A.; Georgiev, G.A. Biological control of diseases on main vegetables-researches and practice in maritsa vegetable crops institute. In Proceedings of the IV Balkan Symposium on Vegetables and Potatoes, Plovdiv, Bulgaria, 17 June 2009; Volume 830, pp. 511–517. [Google Scholar]
- Wolk, M.; Sorkar, S. Antagonism in vivo of Bacillus spp. against Rhizoctoniasolani and Pythium spp. Anz. Fur Schadl. Skundey Pflanzenschutz Umweltschutz 1993, 66, 121–125. [Google Scholar]
- Loper, J.E. Role of fluorescent siderophore production in biological control of Pythium ultimum by a Pseudomonas fluorescens strain. Phytopathology 1988, 78, 166–172. [Google Scholar] [CrossRef]
- Abbas, A.; Khan, S.U.; Khan, W.U.; Saleh, T.A.; Khan, M.H.U.; Ullah, S.; Ali, A.; Ikram, M. Antagonist effects of strains of Bacillus spp. against Rhizoctonia solani for their protection against several plant diseases: Alternatives to chemical pesticides. Comptes Rendus. Biol. 2019, 342, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.B.; Li, S.D.; Ren, Q.; Xu, J.L.; Lu, X.; Sun, M.H. Biology and applications of Clonostachys rosea. J. Appl. Microbiol. 2020, 129, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhou, T.; Xie, J.; Cheng, J.; Chen, T.; Jiang, D.; Fu, Y. Mycoparasitism illuminated by genome and transcriptome sequencing of Coniothyrium minitans, an important biocontrol fungus of the plant pathogen Sclerotinia sclerotiorum. Microb. Genom. 2020, 6, e000345. [Google Scholar] [CrossRef] [PubMed]
| Biocontrol Microorganisms | Strain Name | Application Types | Application Scale | Application Manner | Application Frequency | Investigated Time | Disease Severity 2 | Disease Severity 1 | Disease Index Scale | Disease Index 2 | Disease Index 1 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fungi | |||||||||||
| Trichoderma harzianum | 1 [92] | Live organism | Greenhouse | Spray | Every three weeks | — | 12 | 65.7 | — | — | — |
| 2 [92] | Live organism | Greenhouse | Spray | Every three weeks | — | 12.7 | 65.7 | — | — | — | |
| — [86] | Live organism | Plastic house | Spray | Successive | Seven weeks | 7.7 | 95 | — | — | — | |
| — [87] | Live organism | Greenhouse | Spray | Every week | Five weeks after application | 17.2 | 30 | — | — | — | |
| — [88] | Live organism | Greenhouse | Spray | Every week | 90 days from planting | 19.3 | 100 | — | — | — | |
| Trichoderma atroviride | TRS25 [91] | Live organism | Field | Seed treatment | Once | Harvest | 40% lower than CK | — | — | — | — |
| Trichoderma viride | — [88] | Live organism | Greenhouse | Spray | Every week | 90 days from planting | 40.8 | 100 | — | — | — |
| Trichoderma hamatum | 2 [92] | Live organism | Greenhouse | Spray | Every three weeks | — | 11.7 | 65.7 | — | — | — |
| Fusarium oxysporum | FO47 [95] | Live organism | Greenhouse | Soil mix | Once | 7 days after inoculation of P. cubensis | — | — | 0–4 | 6.7 | 18.6 |
| FO47B10 [95] | Live organism | Greenhouse | Soil mix | Once | 7 days after inoculation of P. cubensis | — | — | 0–4 | 7.1 | 18.6 | |
| Y2 [94] | Fermentation supernatant | Greenhouse | Spray | Once | 7 days after application | — | — | 0–4 | 27.8 | 67.3 | |
| Pestalotiopsis microspora | Y1 [93] | Fermentation supernatant | Greenhouse | Spray | Once | — | — | — | 0–4 | 13.9 | 42.6 |
| Bacteria | |||||||||||
| Bacillus subtilis | 4 [92] | Live organism | Greenhouse | Spray | Every three weeks | — | 15.7 | 65.7 | — | — | — |
| — [87] | Live organism | Greenhouse | Spray | Every week | Five weeks after application | 17.2 | 30 | — | — | — | |
| — [101] | Live organism | Greenhouse | Spray | Every 10 days | One week after the last spray | 32.8 | 88.3 | — | — | — | |
| Bacillus pumilus | DS22 [79] | Live organism | Field | Spray | Every 10 days | 15 days after application | 4.6 | 20.2 | — | — | — |
| — [101] | Live organism | Greenhouse | Spray | Every 10 days | One week after the last spray | 35.2 | 88.3 | — | — | — | |
| Bacillus licheniformis | HS10 [79] | Live organism | Field | Spray | Every 10 days | 15 days after application | 5.5 | 20.2 | — | — | — |
| Bacillus asahii | CE8 [80] | Live organism | Field | Spray | Once | 7–12 days after application | — | — | 0–9 | 41.1 | 70.9 |
| Bacillus velezensis | HMQAU19044 [104] | Live organism | Pot experiment | Spray | Once | 7 days after application | — | — | 0–9 | 31.3 | 77.8 |
| HMQAU19044 [104] | Fermentation filtrate | Pot experiment | Spray | Once | 7 days after application | — | — | 0–9 | 15.98 | 77.8 | |
| Bacillus chitinosporus | — [105] | Metabolites | Plastic house | Spray | Every week | 35 days after application | 5.9 | 10.8 | — | — | — |
| Bacillus sp. | HP4 [79] | Live organism | Field | Spray | Every 10 days | 15 days after application | 11.6 | 20.2 | — | — | — |
| Z-X-3 [106] | Fermentation supernatant | Greenhouse | Spray | Every 6 days | — | — | — | 0–4 | 0.5 | 0.8 | |
| Z-X-10 [106] | Fermentation supernatant | Greenhouse | Spray | Every 6 days | — | — | — | 0–4 | 0.5 | 0.8 | |
| Streptomyces sp. | NO.24 [109] | — | Pot experiment | Spray | Once | 7 days after application | — | — | — | 13.8 | 38.3 |
| Pseudomonas fluorescens | 4 [92] | Live organism | Greenhouse | Spray | Every three weeks | Five weeks after application | 20.7 | 65.7 | — | — | — |
| Pf1 [107] | Filtrate product | Field | Spray | — | — | — | — | 0–5 | 8.9 | 44.6 | |
| — [86] | Live organism | Plastic house | Spray | Successive | Seven weeks | 6 | 95 | — | — | — | |
| — [87] | Live organism | Greenhouse | Spray | Every week | Five weeks after application | 16.7 | 30 | — | — | — | |
| Enterobacter cloacae | — [115] | Live organism | Greenhouse | Spray | — | — | — | — | — | 10.3 | 85.6 |
| Enterobacter sp. | DP14 [79] | Live organism | Field | Spray | Every 10 days | 15 days after application | 5.6 | 20.2 | — | — | — |
| Paenibacillus polymyxa | P1 [111] | Live organism | Field | Spray | Every 7 days | 7 days after the last application | — | — | 0–9 | 2.5 | 24.7 |
| Derxia gummosa | — [87] | Live organism | Greenhouse | Spray | Every week | Five weeks after application | 18.1 | 30 | — | — | — |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Z.; Yu, S.; Hu, Y.; Wen, Y. Biological Control of the Cucumber Downy Mildew Pathogen Pseudoperonospora cubensis. Horticulturae 2022, 8, 410. https://doi.org/10.3390/horticulturae8050410
Sun Z, Yu S, Hu Y, Wen Y. Biological Control of the Cucumber Downy Mildew Pathogen Pseudoperonospora cubensis. Horticulturae. 2022; 8(5):410. https://doi.org/10.3390/horticulturae8050410
Chicago/Turabian StyleSun, Zhanbin, Shufan Yu, Yafeng Hu, and Yanchen Wen. 2022. "Biological Control of the Cucumber Downy Mildew Pathogen Pseudoperonospora cubensis" Horticulturae 8, no. 5: 410. https://doi.org/10.3390/horticulturae8050410
APA StyleSun, Z., Yu, S., Hu, Y., & Wen, Y. (2022). Biological Control of the Cucumber Downy Mildew Pathogen Pseudoperonospora cubensis. Horticulturae, 8(5), 410. https://doi.org/10.3390/horticulturae8050410






