Effects of Fruit Bagging Treatment with Different Types of Bags on the Contents of Phenolics and Monoterpenes in Muscat-Flavored Table Grapes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Site
2.2. Materials and Sampling
2.3. Measurements and Methods
2.3.1. Determination of Basic Physical and Chemical Indicators
2.3.2. Measurement of Phenolic Contents in Grape Fruits
2.3.3. Extraction of Monoterpenes
2.3.4. Detection of Monoterpenes and the Qualitative and Quantitative Analysis
2.4. Data Processing and Statistical Analysis
3. Results and Analysis
3.1. Effects of Fruit Bagging Treatment with Different Types of Bags on the Basic Quality Indicators of the Three Table Grape Cultivars
3.2. Effects of Fruit Bagging Treatment with Different Types of Bags on Phenolic Contents in the Three Table Grape Cultivars
3.3. Effects of Fruit Bagging Treatment with Different Types of Bags on the Compositions and Contents of Monoterpenes in the Three Table Grape Cultivars
3.3.1. Compositions and Contents of Monoterpenes in the Three Table Grape Cultivars
3.3.2. Effects of Fruit Bagging Treatment with Different Types of Bags on the Compositions and Contents of Monoterpenes in the Three Table Grape Cultivars
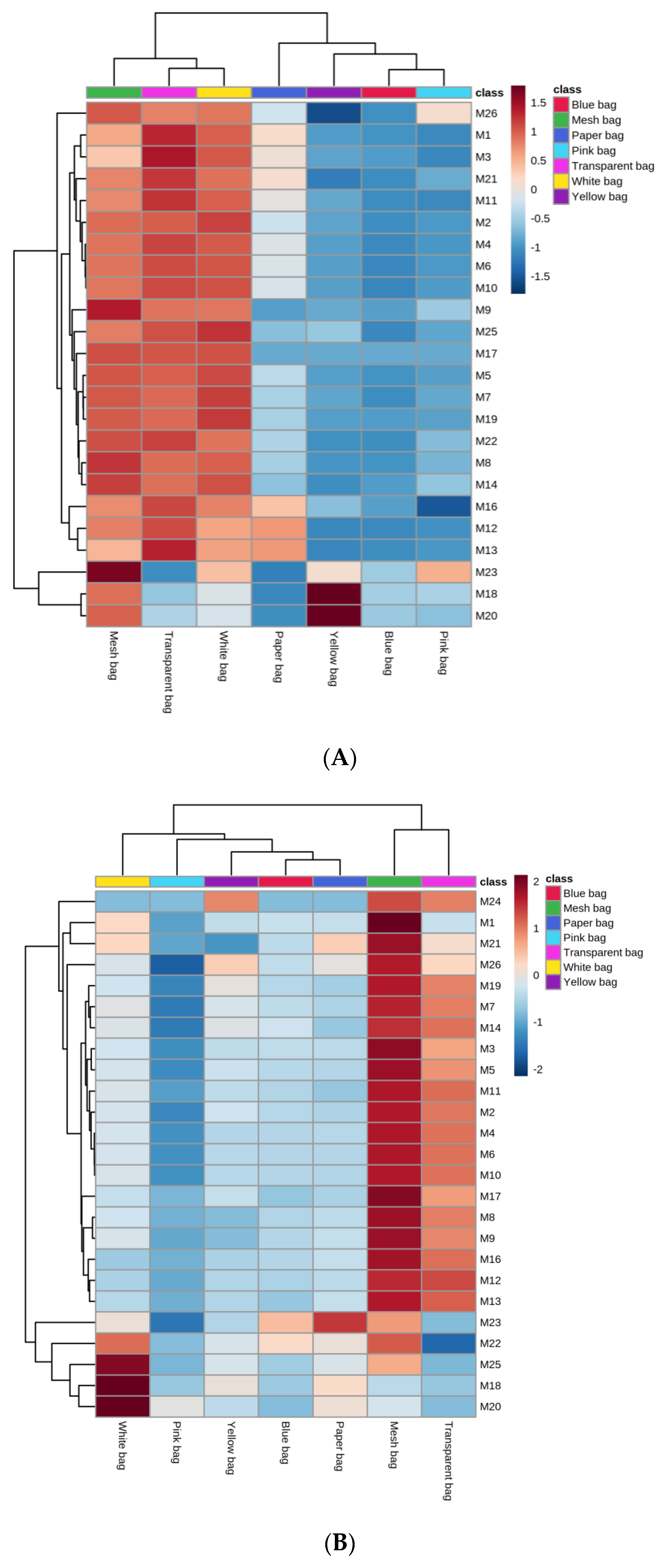
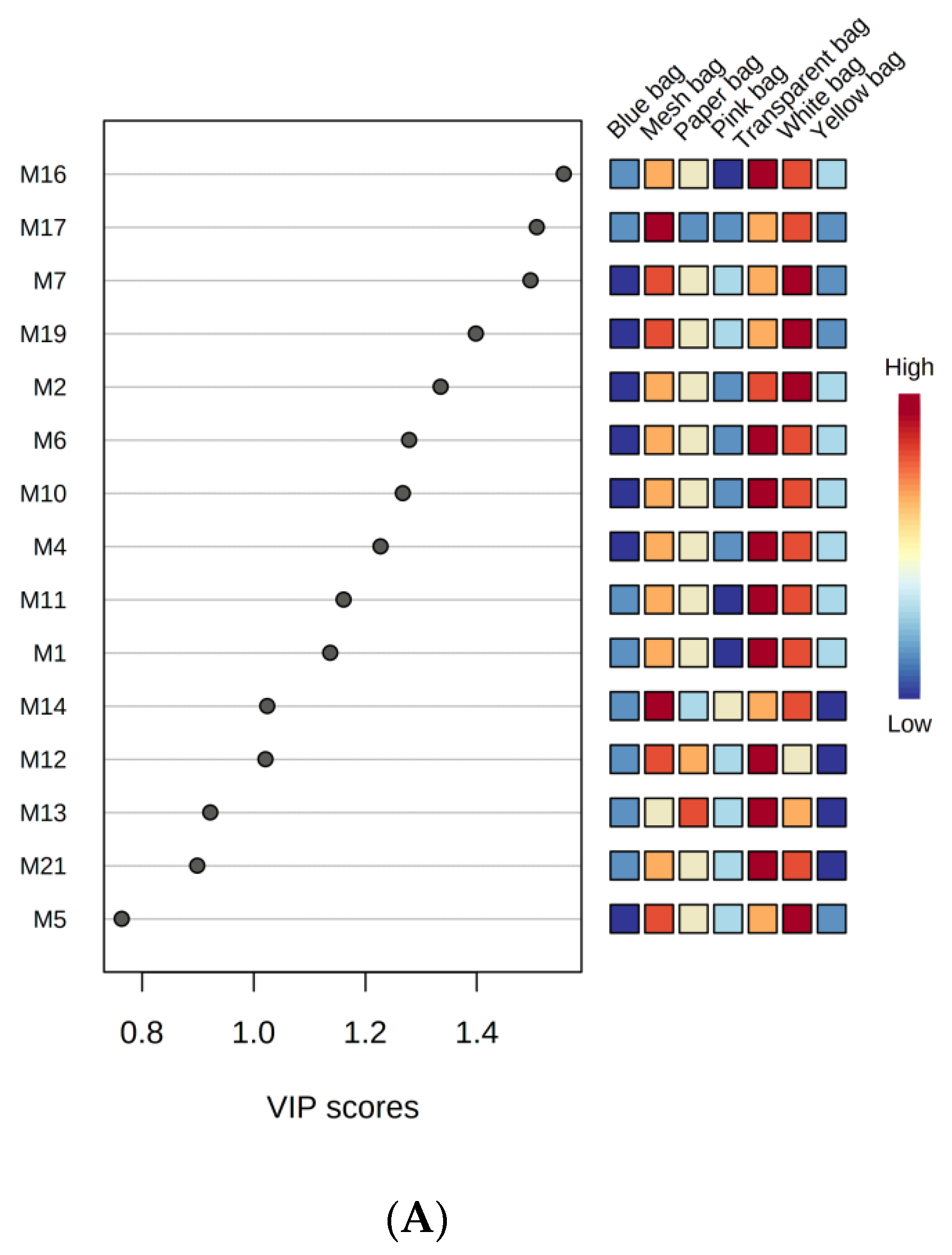
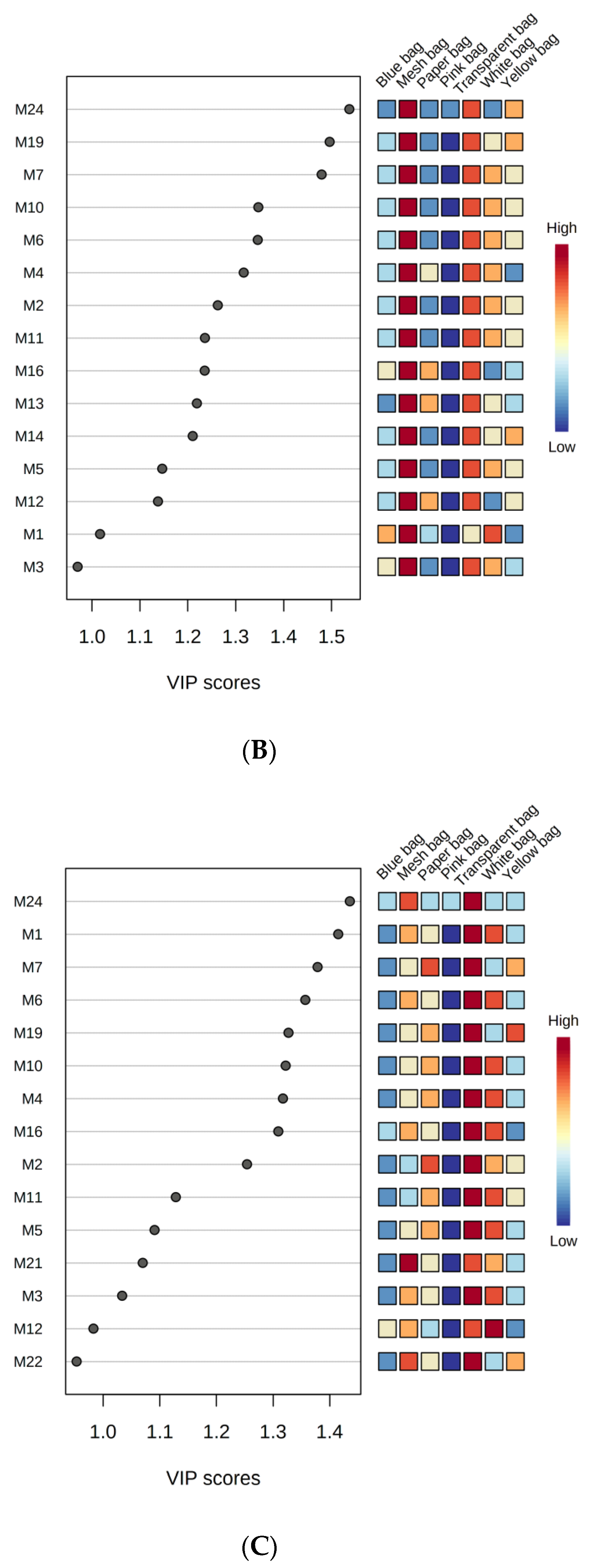
3.3.3. Least Square Discriminant Analysis of Monoterpenes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, L.; Xu, X.Q.; Wang, Y.; Vanderweide, J.; Sun, R.Z.; Cheng, G.; Pan, Q.H. Differential influence of timing and duration of bunch bagging on volatile organic compounds in Cabernet Sauvignon berries (Vitis vinifera L.). Aust. J. Grape Wine Res. 2021, 28, 75–85. [Google Scholar] [CrossRef]
- Wang, S.-M.; Gao, H.-J.; Zhang, X.-B. Research progress on the effects of bagging treatment on pear fruit. China Fruits 2002, 75, 49–52. [Google Scholar]
- Wu, W.; Lie, D.; He, S.-L. Review on the effects of bagging treatment on fruit quality. South China Fruits 2006, 35, 82–86. [Google Scholar]
- Zhang, J.; Niu, J.; Duan, Y.; Zhang, M.; Liu, J.; Li, P.; Ma, F. Photoprotection mechanism in the cv. Fuji, Raku Raku apple peel at different levels of photo oxidative sunburn. Physiol. Plant. 2015, 154, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, D.; Wang, Y.; Li, P.; Ma, F. Effects of fruit bagging on the contents of phenolic compounds in the peel and flesh of ‘Golden Delicious’, ‘Red Delicious’, and ‘Royal Gala’ apples. Sci. Hortic. 2012, 142, 68–73. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Yang, C.-X.; Liu, C.-Y.; Xu, M.; Li, S.-H.; Yang, L.; Wang, Y.-N. Effects of bagging on volatiles and polyphenols in “Wanmi” peaches during endocarp hardening and final fruit rapid growth stages. J. Food Sci. 2010, 75, 455–460. [Google Scholar] [CrossRef]
- Wang, Y.-T.; Li, X.; Li, Y.; Li, L.-L.; Zhang, S.-L. Effects of bagging on browning spot incidence and content of different forms of calcium in ‘Huangguan’ pear fruits. Acta Hortic. Sin. 2011, 38, 1507–1514. [Google Scholar]
- Wang, J.-Y.; Feng, J.; Hou, X.-D.; Tao, J.-M. Effects of bagging treatments with different materials on aroma components and their biosynthetic gene expression in ‘Shine Muscat’ grape berry. J. Fruit Sci. 2017, 34, 1–11. [Google Scholar]
- Feng, F.; Li, M.; Ma, F.; Cheng, L. The effects of bagging and debagging on external fruit quality, metabolites, and the expression of anthocyanin biosynthetic genes in ‘Jonagold’ apple (Malus domestica Borkh.). Sci. Hortic. 2014, 165, 123–131. [Google Scholar] [CrossRef]
- Sun, R.-Z.; Guo, C.; Qiang, L.; Zhu, Y.-R.; Xue, Z.; Yu, W.; He, Y.-N.; Li, S.-Y.; Lei, H.; Wu, C.; et al. Comparative physiological, metabolomic, and transcriptomic analyses reveal developmental stage-dependent effects of cluster bagging on phenolic metabolism in Cabernet Sauvignon grape berries. BMC Plant Biol. 2019, 19, 59. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.-G.; Wang, H.-Y.; Wang, M.; Sun, J.-S.; Liu, Y.-F.; Schrader, L. Effect of bagging on microenvironments of apple fruits. Acta Ecol. Sin. 2005, 25, 1082–1087. [Google Scholar]
- Mateo, J.J.; Jimenez, M. Monoterpenes in grape juice and wines. J. Chromatogr. A 2000, 881, 557–567. [Google Scholar] [CrossRef]
- Ruiz-Garcia, L.; Hellin, P.; Flores, P.; Fenoll, J. Prediction of Muscat aroma in table grape by analysis of rose oxide. Food Chem. 2014, 154, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, Y.; Wu, B.; Fang, J.; Li, S. Volatile compounds evolution of three table grapes with different flavour during and after maturation. Food Chem. 2011, 128, 823–830. [Google Scholar] [CrossRef]
- Gunata, Y.Z.; Bayonove, C.L.; Baumes, R.L.; Cordonnier, R.E. The aroma of grapes—localization and evolution of free and bound fractions of some grape aroma components Cv muscat during 1st development and maturation. J. Sci. Food Agric. 1985, 36, 857–862. [Google Scholar] [CrossRef]
- Luan, F.; Wüst, M. Differential incorporation of 1-deoxy-D-xylulose into (3S)-linalool and geraniol in grape berry exocarp and mesocarp. Phytochemistry 2002, 60, 451–459. [Google Scholar] [CrossRef]
- Park, S.K.; Morrison, J.C.; Adams, D.O.; Noble, A.C. Distribution of free and glycosidically bound monoterpenes in the skin and mesocarp of Muscat of Alexandria grapes during development. J. Agric. Food Chem. 1991, 39, 514–518. [Google Scholar] [CrossRef]
- Yue, X.F.; Ma, X.; Tang, Y.L.; Wang, Y.; Wu, B.W.; Jiao, X.L.; Zhang, Z.W.; Ju, Y.L. Effect of cluster zone leaf removal on monoterpene profiles of sauvignon blanc grapes and wines. Food Res. Int. 2020, 131, 109028. [Google Scholar] [CrossRef]
- Falcao, L.D.; Revel, G.D.; Perello, M.C.; Moutsiou, A.; Zanus, M.C.; Bordignon-Luiz, M.T. A survey of seasonal temperatures and vineyard altitude influences on 2-methoxy-3-isobutylpyrazine, C13-norisoprenoids, and the sensory profile of Brazilian Cabernet Sauvignon wines. J. Agric. Food Chem. 2007, 55, 3605–3612. [Google Scholar] [CrossRef] [Green Version]
- Kalua, C.M.; Boss, P.K. Evolution of volatile compounds during the development of Cabernet Sauvignon grapes (Vitis vinifera L.). J. Agric. Food Chem. 2009, 57, 3818–3830. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Zhang, G.-J.; Sun, L.; Zhao, Y.; Yan, A.-L.; Wang, H.-L.; Ren, J.-C.; Xu, H.-Y. Effects of two trellis systems on viticultural characteristics and fruit quality of three table grape cultivars. Sci. Agric. Sin. 2019, 52, 1150–1163. [Google Scholar]
- Zhang, J. Effects of Different Dwarf Training Patterns on Volatile Flavor Compound of Cabernet Gernischt. Master’s Thesis, Ningxia University, Yinchuan, China, 2018. [Google Scholar]
- Ji, X.-H.; Wang, B.-L.; Wang, X.-D.; Shi, X.-B.; Liu, P.-P.; Liu, F.-Z.; Wang, H.-B. Effects of different color paper bags on aroma development of Kyoho grape berries. J. Integr. Agric. 2019, 18, 70–82. [Google Scholar] [CrossRef]
- Jiang, X.-F. Effects of Different Bags on Fruit Quality and Aroma Components in Grape cv. ‘Maserlan’. Master’s Thesis, Gansu Agricultural University, Lanzhou, China, 2018. [Google Scholar]
- Zhang, G.-J.; Wang, X.-Y.; Sun, L.; Yan, A.-L.; Wang, H.-L.; Ren, J.-C.; Xu, H.-Y. Grapevine vigor control theory and coping strategy for grape growing under mainland monsoon type climate. Sino Overseas Grapevine Wine 2016, 3, 30–33. [Google Scholar]
- Yang, F.-C.; Wu, J.; Cheng, J.-H.; Xu, K.; Chen, J.-W. Studies on extraction and physical-chemical properties of anthocyanin from red globe grape peel. J. Fruit Sci. 2007, 24, 287–292. [Google Scholar]
- Ramchandani, A.G.; Chettiyar, R.S.; Pakhale, S. Evaluation of antioxidant and anti-initiating activities of crude polyphenolic extracts from seedless and seeded Indian grapes. Food Chem. 2010, 119, 298–305. [Google Scholar] [CrossRef]
- Dai, H.; Qin, C.; Ding, L. Effects of salicylic acid on the contents of total flavonoids and resveratrol and related enzyme activities in ‘Cabernet Sauvignon’. J. China Agric. Univ. 2016, 21, 37–42. [Google Scholar]
- Wu, Y.-W.; Pan, Q.-H.; Qu, W.-J.; Duan, C.-Q. Comparison of volatile profiles of nine litchi (Litchi chinensis Sonn.) cultivars from Southern China. J. Agric. Food Chem. 2009, 57, 9676–9681. [Google Scholar] [CrossRef]
- Wu, Y.-W.; Zhu, B.-Q.; Tu, C.; Duan, C.-Q.; Pan, Q.-H. Generation of volatile compounds in litchi wine during winemaking and short-term bottle storage. J. Agric. Food Chem. 2011, 59, 4923–4931. [Google Scholar] [CrossRef]
- Sun, L.; Zhu, B.-Q.; Zhang, X.-Y.; Zhang, G.-J.; Yan, A.-L.; Wang, H.-L.; Wang, X.-Y.; Xu, H.-Y. The accumulation profiles of terpene metabolites in three Muscat table grape cultivars through HS-SPME-GCMS. Sci. Data 2020, 7, 5. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Crisosto, G.M. Understanding American and Chinese consumer acceptance of ‘Red Globe’ table grapes. Postharvest Biol. Technol. 2002, 24, 155162. [Google Scholar] [CrossRef] [Green Version]
- Jayasena, V.; Cameron, I. Brix/acid ratio as a predictor of consumer acceptability of Crimson Seedless table grapes. J. Food Qual. 2008, 31, 736–750. [Google Scholar] [CrossRef]
- Kong, J.-J.; Cao, P.; Wu, X.; Yuan, Y.-Z.; Yu, P.-J.; Tao, S.-T.; Zhang, S.-L. Effects of light quality on fruit quality and absorption of mineral elements in ‘DangshanSuli’ pear fruit development. Acta Hortic. Sin. 2018, 45, 1173–1184. [Google Scholar]
- Verreynne, J.S.; Rabe, E.; Theron, K.I. Effect of bearing position on fruit quality of mandarin types. S. Afr. J. Plant Soil 2004, 21, 1–7. [Google Scholar] [CrossRef]
- Wang, H.-B.; Zhang, K.-K.; Ji, X.-H.; Wang, X.-D.; Shi, X.-B.; Wang, B.-L.; Zheng, X.-C.; Liu, F.-Z. Effects of different color paper bags on volatile constituents of Kyoho grape berries. Chin. J. Appl. Ecol. 2017, 28, 1274–1280. [Google Scholar]
- Sharma, R.R.; Pal, R.K.; Asrey, R.; Sagar, V.R.; Dhiman, M.R.; Rana, M.R. Pre-harvest fruit bagging influences fruit color and quality of apple cv. Delicious. Agric. Sci. 2013, 4, 443–448. [Google Scholar] [CrossRef] [Green Version]
- Shang, J.-B.; Tian, S.-F.; Ji, X.; Wang, D.; Liu, H.; Zhang, N. Effects of fruit bagging on the quality of grapes under protected cultivation. Nor. Hortic. 2014, 1, 42–45. [Google Scholar]
- Cheng, J.-H.; Wei, L.-Z.; Lei, M.; Zheng, T.; Wu, J. Influences of different light filter film bags on berry quality in ‘Red Globe’. J. Fruit Sci. 2015, 32, 87–93. [Google Scholar]
- Song, J.; Forney, C.F. Flavour volatile production and regulation in fruit. Can. J. Plant Sci. 2008, 88, 537–550. [Google Scholar] [CrossRef]
- Wang, H.-L.; Wang, X.-Y.; Yan, A.-L.; Sun, L.; Zhang, G.-J.; Ren, J.-C.; Xu, H.-Y. The accumulation of monoterpenes and the expression of its biosynthesis related genes in ‘AishenMeigui’ grape berries cultivated in different trellis systems during Ripening Stage. Sci. Agric. Sin. 2019, 52, 1136–1149. [Google Scholar]
- Wen, Y.-Q.; Zhong, G.-Y.; Gao, Y.; Lan, Y.-B.; Duan, C.-Q.; Pan, Q.-H. Using the combined analysis of transcripts and metabolites to propose key genes for differential terpene accumulation across two regions. BMC Plant Biol. 2015, 8, 1226. [Google Scholar] [CrossRef] [Green Version]
- Pino, J.A.; Mesa, J. Contribution of volatile compounds to mango (Mangifera indica L.) aroma. Flavour Fragr. J. 2006, 21, 207–213. [Google Scholar] [CrossRef]
- Fenoll, J.; Manso, A.; Hellin, P.; Ruiz, L.; Flores, P. Changes in the aromatic composition of the Vitis vinifera grape Muscat Hamburg during ripening. Food Chem. 2009, 114, 420–428. [Google Scholar] [CrossRef]

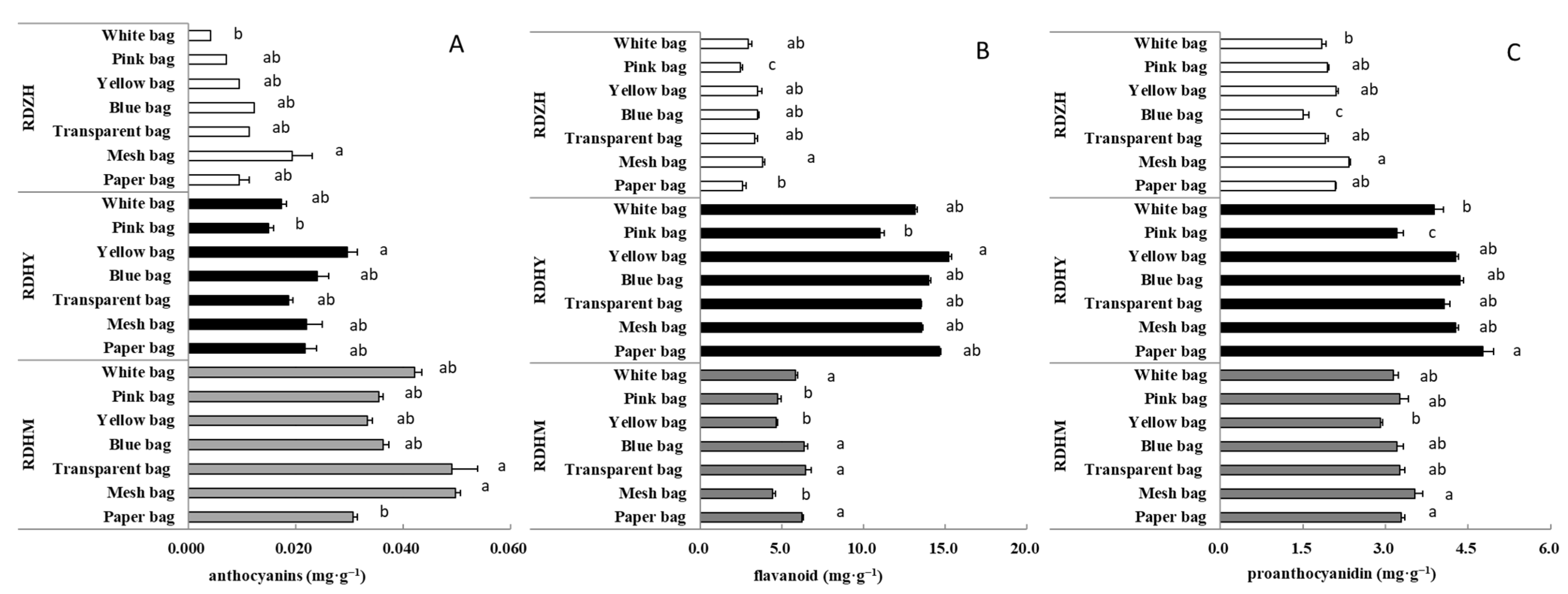
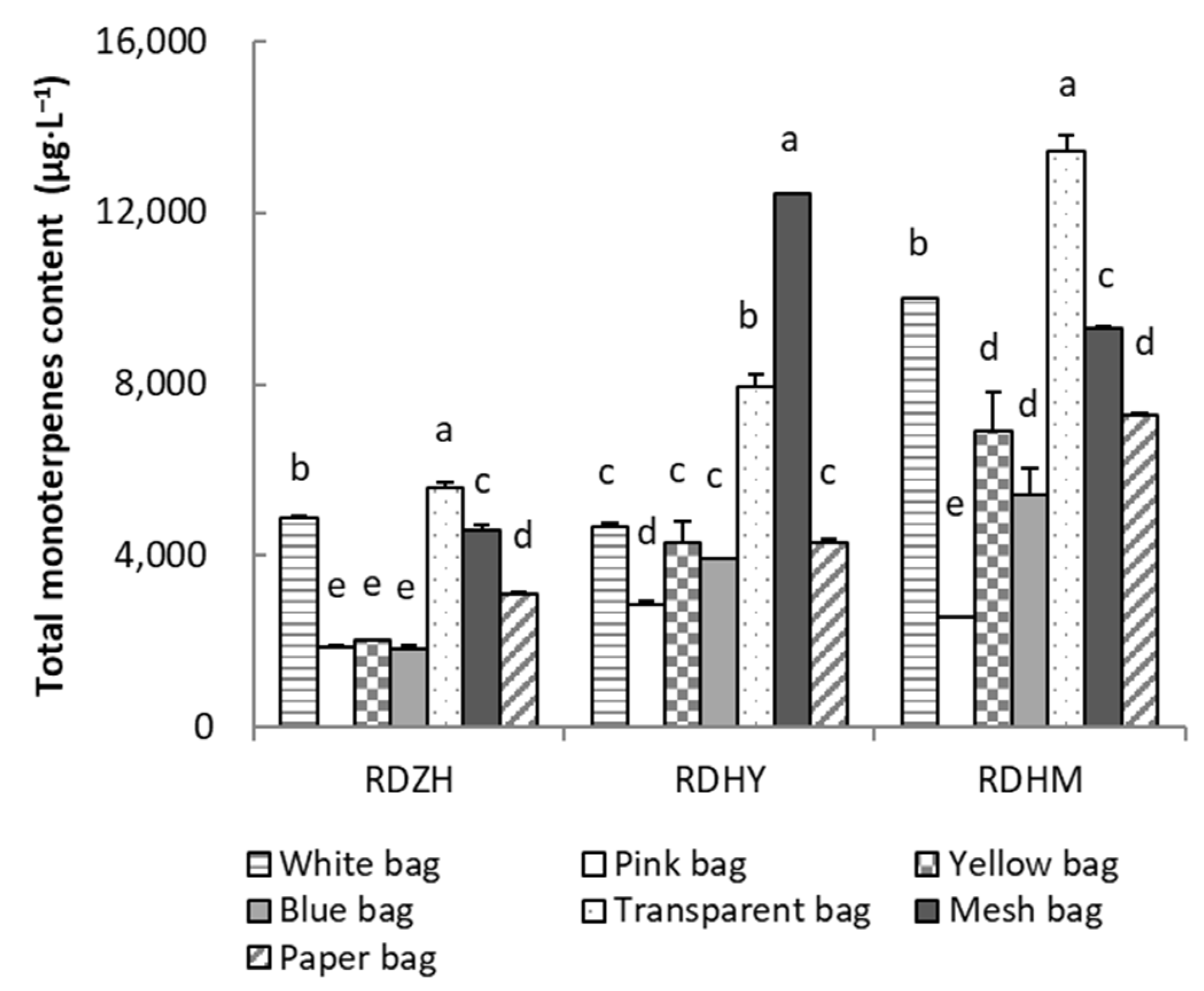

| Cultivar | Bag Type | Cluster Weight/g | Total Soluble Solids/° Brix | Titratable Acidity/(%) | TSS/TA |
|---|---|---|---|---|---|
| RDZH | White bag | 595 ± 74 a | 15.6 ± 0.1 a | 0.312 ± 0.002 a | 49.8 ± 0.3 b |
| Pink bag | 558 ± 73 a | 14.0 ± 0.1 c | 0.271 ± 0.002 a | 51.6 ± 0.4 b | |
| Yellow bag | 614 ± 97 a | 14.8 ± 0.1 ab | 0.322 ± 0.013 a | 46.0 ± 1.5 c | |
| Blue bag | 643 ± 80 a | 15.1 ± 0.1 a | 0.299 ± 0.001 a | 50.5 ± 0.3 b | |
| Transparent bag | 600 ± 26 a | 14.3 ± 0.0 b | 0.307 ± 0.001 a | 46.4 ± 0.2 c | |
| Mesh bag | 514 ± 85 a | 14.8 ± 0.0 ab | 0.270 ± 0.003 a | 54.8 ± 0.3 a | |
| Paper bag | 692 ± 104 a | 13.8 ± 0.0 c | 0.270 ± 0.001 a | 51.3 ± 0.2 b | |
| RDHY | White bag | 354 ± 55 a | 20.7 ± 0.0 d | 0.395 ± 0.0.02 a | 52.3 ± 0.2 b |
| Pink bag | 404 ± 59 a | 21.3 ± 0.1 c | 0.386 ± 0.006 b | 55.5 ± 0.3 b | |
| Yellow bag | 375 ± 21 a | 21.8 ± 0.1 b | 0.360 ± 0.001 d | 60.5 ± 0.4 a | |
| Blue bag | 381 ± 42 a | 19.2 ± 0.1 e | 0.360 ± 0.004 d | 53.2 ± 0.6 b | |
| Transparent bag | 375 ± 21 a | 23.0 ± 0.1 a | 0.377 ± 0.002 c | 60.9 ± 0.2 a | |
| Mesh bag | 380 ± 37 a | 21.0 ± 0.2 c | 0.381 ± 0.001 bc | 55.1 ± 0.3 b | |
| Paper bag | 387 ± 55 a | 18.7 ± 0.1 f | 0.357 ± 0.003 d | 52.4 ± 0.5 b | |
| RDHM | White bag | 666 ± 59 a | 16.9 ± 0.6 a | 0.562 ± 0.002 b | 29.5 ± 1.6 bc |
| Pink bag | 667 ± 142 a | 15.5 ± 0.2 b | 0.505 ± 0.009 bc | 31.0 ± 0.3 b | |
| Yellow bag | 592 ± 14 a | 15.9 ± 0.6 ab | 0.447 ± 0.001 e | 35.8 ± 1.5 a | |
| Blue bag | 597 ± 90 a | 15.9 ± 0.8 ab | 0.496 ± 0.001 c | 30.1 ± 1.9 bc | |
| Transparent bag | 601 ± 52 a | 15.5 ± 0.2 b | 0.466 ± 0.006 d | 33.6 ± 0.3 a | |
| Mesh bag | 644 ± 107 a | 17.3 ± 0.3 a | 0.634 ± 0.005 a | 27.4 ± 0.6 c | |
| Paper bag | 706 ± 97 a | 16.0 ± 0.7 ab | 0.508 ± 0.001 bc | 31.6 ± 1.7 b | |
| p-values | Cultivar | <0.001 | <0.001 | <0.001 | <0.001 |
| Treatment | 0.547 | <0.001 | <0.001 | <0.001 | |
| Cultivar × Treatment | 0.591 | <0.001 | <0.001 | <0.001 |
| Code | Compound | Cultivar | Type of Bags | Cultivar p Value | Treatment p Value | Treatment × Cultivar p Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| White Bag | Pink Bag | Yellow Bag | Blue Bag | Transparent Bag | Mesh Bag | Paper Bag | ||||||
| M1 | β-Myrcene | RDZH | 289.51 ± 0.28 b | 76.89 ± 3.10 e | 84.73 ± 0.84 e | 81.21 ± 3.51 e | 356.47 ± 11.65 a | 219.73 ± 1.30 c | 169.38 ± 1.93 d | <0.001 | <0.001 | <0.001 |
| RDHY | 234.83 ± 8.28 a | 100.46 ± 4.86 b | 160.64 ± 22.52 ab | 163.84 ± 3.95 a | 502.35 ± 21.37 a | 815.37 ± 8.74 a | 162.01 ± 4.84 ab | |||||
| RDHM | 1232.81 ± 3.35 b | 225.14 ± 2.41 g | 558.30 ± 22.70 e | 457.62 ± 18.14 f | 1538.13 ± 51.74 a | 998.11 ± 1.79 c | 828.12 ± 51.22 d | |||||
| M2 | Limonene | RDZH | 25.72 ± 0.74 a | 5.37 ± 0.02 d | 5.74 ± 0.15 c | 4.98 ± 0.10 e | 22.94 ± 0.93 a | 21.58 ± 0.55 a | 9.10 ± 0.09 b | <0.001 | <0.001 | <0.001 |
| RDHY | 14.47 ± 0.07 c | 6.93 ± 0.17 d | 14.28 ± 2.38 c | 12.28 ± 0.45 c | 33.21 ± 0.82 b | 50.62 ± 1.35 a | 11.84 ± 0.06 c | |||||
| RDHM | 61.36 ± 0.79 a | 22.15 ± 0.01 c | 52.30 ± 2.05 ab | 28.21 ± 0.37 b | 138.64 ± 5.32 a | 42.97 ± 8.24 ab | 61.91 ± 1.11 a | |||||
| M3 | Phellandrene | RDZH | 4.38 ± 0.04 b | 1.81 ± 0.03 c | 1.98 ± 0.07 bc | 1.93 ± 0.06 bc | 5.14 ± 0.14 a | 3.29 ± 0.24 b | 2.93 ± 0.03 bc | <0.001 | <0.001 | <0.001 |
| RDHY | 3.45 ± 0.05 c | 2.08 ± 0.27 d | 3.24 ± 0.32 c | 3.22 ± 0.00 c | 5.63 ± 0.15 b | 10.44 ± 0.15 a | 3.16 ± 0.01 c | |||||
| RDHM | 9.92 ± 0.07 b | 3.57 ± 0.03 f | 6.99 ± 0.28 d | 5.49 ± 0.11 e | 15.38 ± 0.56 a | 8.99 ± 0.11 c | 8.54 ± 0.30 c | |||||
| M4 | β-trans-Ocimene | RDZH | 114.96 ± 1.71 a | 29.84 ± 1.09 d | 31.25 ± 1.39 d | 27.56 ± 1.54 d | 124.59 ± 4.50 a | 103.83 ± 2.19 b | 53.45 ± 0.26 c | <0.001 | <0.001 | <0.001 |
| RDHY | 70.66 ± 0.14 b | 34.95 ± 0.72 c | 60.21 ± 10.42 bc | 58.69 ± 0.95 bc | 171.69 ± 5.13 b | 266.60 ± 4.90 a | 59.39 ± 0.90 bc | |||||
| RDHM | 289.22 ± 0.70 b | 77.17 ± 0.06 d | 198.41 ± 12.47 b | 147.79 ± 16.54 c | 518.90 ± 15.56 a | 247.31 ± 1.34 b | 248.07 ± 8.29 b | |||||
| M5 | γ-Terpinen | RDZH | 4.91 ± 0.02 a | 2.09 ± 0.02 b | 2.08 ± 0.04 b | 2.00 ± 0.02 b | 4.65 ± 0.10 a | 4.78 ± 0.25 a | 2.64 ± 0.01 b | <0.001 | <0.001 | <0.001 |
| RDHY | 3.49 ± 0.03 c | 1.84 ± 0.26 d | 3.33 ± 0.35 c | 3.02 ± 0.06 c | 6.49 ± 0.09 b | 11.94 ± 1.11 a | 2.94 ± 0.03 c | |||||
| RDHM | 9.21 ± 0.04 b | 3.85 ± 0.00 d | 7.87 ± 0.32 b | 5.50 ± 0.50 c | 18.91 ± 0.87 a | 8.44 ± 0.19 b | 8.94 ± 0.24 b | |||||
| M6 | β-cis-Ocimene | RDZH | 304.88 ± 1.67 a | 73.78 ± 2.79 c | 76.99 ± 2.09 c | 66.65 ± 2.89 c | 316.65 ± 12.28 a | 269.59 ± 7.22 a | 132.39 ± 0.95 b | <0.001 | <0.001 | <0.001 |
| RDHY | 178.49 ± 0.86 b | 86.59 ± 1.22 c | 156.88 ± 26.79 b | 147.94 ± 3.83 b | 444.09 ± 11.32 b | 691.05 ± 8.78 a | 147.80 ± 2.01 b | |||||
| RDHM | 736.44 ± 4.87 b | 195.07 ± 0.18 d | 517.19 ± 32.10 b | 367.11 ± 40.19 c | 1380.25 ± 41.72 a | 638.25 ± 1.87 b | 635.52 ± 19.41 b | |||||
| M7 | Terpinolen | RDZH | 31.97 ± 0.56 a | 5.96 ± 0.05 d | 5.80 ± 0.20 d | 4.93 ± 0.01 d | 26.19 ± 1.53 c | 28.47 ± 0.95 b | 8.20 ± 0.17 d | <0.001 | <0.001 | <0.001 |
| RDHY | 15.56 ± 0.09 c | 5.18 ± 0.20 d | 14.71 ± 2.55 c | 12.28 ± 0.88 c | 35.92 ± 0.71 b | 58.75 ± 1.99 a | 11.08 ± 0.17 c | |||||
| RDHM | 61.68 ± 0.85 b | 25.54 ± 0.00 d | 69.51 ± 4.02 b | 34.09 ± 3.96 c | 189.01 ± 9.36 a | 63.64 ± 0.49 b | 73.30 ± 1.39 b | |||||
| M8 | cis-Rose oxide | RDZH | 3.65 ± 0.01 a | 3.42 ± 0.00 b | 3.39 ± 0.00 b | 3.39 ± 0.00 b | 3.64 ± 0.01 a | 3.68 ± 0.00 a | 3.46 ± 0.00 a | <0.001 | <0.001 | <0.001 |
| RDHY | 3.96 ± 0.00 c | 3.61 ± 0.01 e | 3.67 ± 0.08 e | 3.84 ± 0.01 e | 4.73 ± 0.01 b | 5.32 ± 0.06 a | 3.89 ± 0.01 d | |||||
| RDHM | 3.47 ± 0.03 a | 3.27 ± 0.00 c | 3.31 ± 0.00 b | 3.29 ± 0.01 c | 3.40 ± 0.01 a | 3.40 ± 0.00 a | 3.33 ± 0.00 a | |||||
| M9 | trans-Rose oxide | RDZH | 3.34 ± 0.01 a | 3.29 ± 0.00 a | 3.28 ± 0.00 b | 3.27 ± 0.00 b | 3.34 ± 0.00 a | 3.36 ± 0.00 a | 3.27 ± 0.00 b | <0.001 | <0.001 | <0.001 |
| RDHY | 3.45 ± 0.00 c | 3.33 ± 0.01 f | 3.35 ± 0.02 f | 3.40 ± 0.00 e | 3.61 ± 0.00 b | 3.76 ± 0.02 a | 3.42 ± 0.00 d | |||||
| RDHM | 3.26 ± 0.00 a | 3.26 ± 0.00 a | 3.26 ± 0.00 a | 3.26 ± 0.00 a | 3.26 ± 0.00 a | 3.26 ± 0.00 a | 3.26 ± 0.00 a | |||||
| M10 | Allo-ocimene | RDZH | 96.53 ± 0.27 a | 23.66 ± 1.13 c | 24.34 ± 0.98 c | 21.04 ± 1.01 c | 99.30 ± 3.72 a | 83.22 ± 2.08 a | 41.36 ± 0.37 b | <0.001 | <0.001 | <0.001 |
| RDHY | 56.56 ± 0.48 c | 26.47 ± 0.76 d | 47.93 ± 8.45 c | 45.63 ± 1.22 c | 137.47 ± 3.73 b | 211.96 ± 3.62 a | 45.21 ± 1.02 c | |||||
| RDHM | 250.94 ± 2.28 b | 71.04 ± 0.05 e | 194.90 ± 16.88 c | 130.80 ± 14.13 d | 486.07 ± 15.13 a | 217.58 ± 1.49 c | 228.47 ± 7.20 c | |||||
| M11 | (E,Z)-Allo-ocimene | RDZH | 16.95 ± 0.09 b | 4.43 ± 0.21 e | 5.25 ± 0.04 e | 4.52 ± 0.25 e | 19.84 ± 0.91 a | 14.40 ± 0.09 c | 8.71 ± 0.17 d | <0.001 | <0.001 | <0.001 |
| RDHY | 10.90 ± 0.29 c | 5.93 ± 0.14 e | 9.65 ± 1.58 d | 8.81 ± 0.09 d | 24.99 ± 0.57 b | 36.96 ± 0.77 a | 7.70 ± 0.15 d | |||||
| RDHM | 41.19 ± 0.69 b | 15.85 ± 0.57 d | 37.17 ± 2.97 b | 26.03 ± 2.74 c | 86.13 ± 3.32 a | 36.64 ± 0.08 b | 39.39 ± 1.14 b | |||||
| M12 | cis-Furan linalool oxide | RDZH | 15.89 ± 0.06 d | 6.03 ± 0.06 e | 5.75 ± 0.05 e | 5.78 ± 0.05 e | 21.98 ± 0.68 a | 18.36 ± 0.37 b | 16.79 ± 0.36 c | <0.001 | <0.001 | <0.001 |
| RDHY | 25.54 ± 0.15 c | 19.46 ± 0.41 d | 26.82 ± 3.07 c | 25.77 ± 0.17 c | 82.06 ± 2.45 b | 93.47 ± 0.03 a | 27.80 ± 0.44 c | |||||
| RDHM | 39.33 ± 0.19 a | 7.88 ± 0.03 c | 15.15 ± 0.66 bc | 15.93 ± 1.52 bc | 31.91 ± 0.86 b | 20.24 ± 0.14 b | 15.50 ± 0.04 bc | |||||
| M13 | trans-Furan linalool oxide | RDZH | 11.30 ± 0.17 b | 4.66 ± 0.03 d | 4.31 ± 0.02 f | 4.47 ± 0.03 e | 16.92 ± 0.21 a | 10.39 ± 0.09 c | 11.61 ± 0.14 b | <0.001 | <0.001 | <0.001 |
| RDHY | 11.30 ± 0.25 bc | 8.26 ± 0.08 d | 11.11 ± 0.92 bc | 9.65 ± 0.19 c | 33.56 ± 0.99 b | 46.16 ± 0.37 a | 12.22 ± 0.02 bc | |||||
| RDHM | 19.35 ± 0.38 a | 4.41 ± 0.00 d | 6.81 ± 0.24 d | 7.46 ± 0.57 cd | 15.99 ± 0.29 b | 11.10 ± 0.06 c | 7.38 ± 0.05 cd | |||||
| M14 | Nerol oxide | RDZH | 50.06 ± 0.15 a | 18.61 ± 0.21 ab | 14.33 ± 0.28 b | 15.32 ± 0.42 b | 45.23 ± 1.65 a | 53.26 ± 2.29 a | 18.47 ± 0.11 b | <0.001 | <0.001 | <0.001 |
| RDHY | 31.09 ± 0.21 b | 12.97 ± 0.01 c | 32.39 ± 4.50 b | 28.36 ± 0.86 b | 67.52 ± 3.15 b | 91.02 ± 2.61 a | 21.90 ± 0.12 b | |||||
| RDHM | 41.78 ± 0.39 b | 18.90 ± 0.01 be | 32.37 ± 1.99 c | 23.35 ± 1.61 d | 67.55 ± 3.25 a | 34.74 ± 0.31 c | 32.22 ± 0.55 c | |||||
| M15 | Citronellal | RDZH | nd | nd | nd | nd | nd | nd | nd | \ | \ | \ |
| RDHY | nd | nd | nd | nd | nd | nd | nd | |||||
| RDHM | tr | tr | tr | tr | tr | 86.25 ± 0.72 | tr | |||||
| M16 | Linalool | RDZH | 1762.30 ± 1.90 b | 216.97 ± 1.07 g | 474.2 ± 1.10 e | 377.73 ± 9.25 f | 2393.30 ± 34.43 a | 1682.54 ± 30.35 c | 1205.12 ± 1.11 d | <0.001 | <0.001 | <0.001 |
| RDHY | 1298.49 ± 1.77 c | 1074.63 ± 3.15 c | 1415.71 ± 206.33 c | 1455.78 ± 7.22 c | 4130.05 ± 117.28 b | 6503.62 ± 23.10 a | 1587.67 ± 35.39 c | |||||
| RDHM | 6119.10 ± 11.03 b | 916.68 ± 3.07 c | 2880.75 ± 477.51 bc | 3001.95 ± 417.51 bc | 6235.79 ± 95.99 a | 4446.00 ± 15.01 bc | 3308.75 ± 94.21 bc | |||||
| M17 | 4-Terpineol | RDZH | 1.77 ± 0.00 a | tr | tr | tr | 1.73 ± 0.01 a | 1.78 ± 0.02 a | tr | <0.001 | <0.001 | <0.001 |
| RDHY | 1.70 ± 0.01 c | 1.50 ± 0.01 e | 1.70 ± 0.05 c | 1.56 ± 0.01 d | 2.22 ± 0.04 b | 2.97 ± 0.01 a | 1.62 ± 0.01 c | |||||
| RDHM | 2.49 ± 0.01 a | 1.67 ± 0.01 c | 2.49 ± 0.20 a | 1.87 ± 0.07 b | 4.15 ± 0.18 a | 2.35 ± 0.02 a | 2.35 ± 0.04 a | |||||
| M18 | Neral | RDZH | 3.02 ± 0.07 b | 2.94 ± 0.03 b | 3.34 ± 0.12 a | 2.94 ± 0.04 b | 2.92 ± 0.03 b | 3.19 ± 0.10 ab | 2.88 ± 0.05 b | <0.001 | 0.002 | 0.115 |
| RDHY | 2.85 ± 0.02 a | 2.81 ± 0.08 a | 2.80 ± 0.05 a | 2.78 ± 0.05 a | 2.78 ± 0.05 a | 2.82 ± 0.08 a | 2.84 ± 0.08 a | |||||
| RDHM | 2.87 ± 0.07 a | 2.91 ± 0.10 a | 3.14 ± 0.15 a | 2.86 ± 0.07 a | 3.03 ± 0.11 a | 3.04 ± 0.03 a | 2.88 ± 0.03 a | |||||
| M19 | α-Terpineol | RDZH | 78.80 ± 0.47 a | 15.24 ± 0.29 b | 14.97 ± 0.47 b | 14.84 ± 0.08 b | 64.10 ± 3.54 a | 67.72 ± 3.09 a | 21.49 ± 1.32 ab | <0.001 | <0.001 | <0.001 |
| RDHY | 41.00 ± 0.89 c | 17.17 ± 0.80 f | 50.68 ± 6.14 c | 35.07 ± 0.99 d | 106.46 ± 5.58 b | 189.84 ± 0.47 a | 31.45 ± 0.15 e | |||||
| RDHM | 156.97 ± 0.02 b | 68.73 ± 0.01 d | 212.25 ± 35.57 b | 97.82 ± 9.03 c | 480.07 ± 26.69 a | 182.72 ± 1.31 b | 199.46 ± 1.20 b | |||||
| M20 | Geranial | RDZH | 3.90 ± 0.03 c | 3.69 ± 0.02 e | 4.91 ± 0.04 a | 3.72 ± 0.00 e | 3.78 ± 0.02 d | 4.47 ± 0.04 b | 3.51 ± 0.01 f | <0.001 | <0.001 | <0.001 |
| RDHY | 3.33 ± 0.02 a | 3.05 ± 0.02 b | 3.01 ± 0.03 b | 2.97 ± 0.02 b | 2.97 ± 0.01 b | 3.04 ± 0.01 b | 3.07 ± 0.01 b | |||||
| RDHM | 3.36 ± 0.02 b | 3.42 ± 0.01 ab | 4.56 ± 0.28 a | 3.45 ± 0.08 ab | 4.22 ± 0.03 a | 4.44 ± 0.09 a | 3.74 ± 0.00 a | |||||
| M21 | β-Citronellol | RDZH | 30.38 ± 0.06 b | 12.90 ± 0.10 e | 10.64 ± 0.04 f | 11.32 ± 0.42 f | 35.68 ± 0.43 a | 28.60 ± 0.68 c | 20.54 ± 0.37 d | <0.001 | <0.001 | <0.001 |
| RDHY | 75.53 ± 1.19 ab | 57.75 ± 1.23 b | 56.67 ± 6.43 b | 65.77 ± 0.81 b | 74.25 ± 3.74 ab | 105.02 ± 1.84 a | 76.98 ± 1.52 ab | |||||
| RDHM | 10.22 ± 0.36 a | 3.81 ± 0.04 c | 8.97 ± 1.26 a | 5.23 ± 0.60 b | 14.33 ± 0.41 a | 15.10 ± 0.09 a | 9.04 ± 0.00 a | |||||
| M22 | Nerol | RDZH | 85.60 ± 0.46 b | 43.90 ± 0.16 d | 37.79 ± 0.17 d | 37.72 ± 1.82 d | 96.57 ± 2.43 a | 93.31 ± 2.43 ab | 48.96 ± 1.09 c | <0.001 | <0.001 | <0.001 |
| RDHY | 100.32 ± 1.42 a | 72.64 ± 2.06 b | 81.21 ± 8.18 b | 86.08 ± 0.92 b | 62.70 ± 2.76 c | 102.40 ± 0.90 a | 83.52 ± 2.03 b | |||||
| RDHM | 66.07 ± 0.54 c | 46.30 ± 0.31 e | 117.83 ± 16.91 b | 54.95 ± 4.61 d | 167.17 ± 7.33 a | 138.46 ± 0.56 b | 90.92 ± 0.45 b | |||||
| M23 | cis-Isogeraniol | RDZH | 0.52 ± 0.00 ab | 0.53 ± 0.00 ab | 0.50 ± 0.03 ab | 0.46 ± 0.00 b | 0.42 ± 0.01 c | 0.64 ± 0.06 a | 0.41 ± 0.00 c | <0.001 | <0.001 | <0.001 |
| RDHY | 1.02 ± 0.00 c | 0.70 ± 0.07 d | 0.90 ± 0.05 d | 1.14 ± 0.03 b | 0.84 ± 0.09 d | 1.23 ± 0.05 b | 1.46 ± 0.09 a | |||||
| RDHM | 0.49 ± 0.01 a | 0.53 ± 0.02 a | 0.83 ± 0.22 a | 0.44 ± 0.01 b | 0.91 ± 0.02 a | 1.13 ± 0.01 a | 0.79 ± 0.02 a | |||||
| M24 | trans-Isogeraniol | RDZH | nd | nd | nd | nd | nd | nd | nd | <0.001 | <0.001 | <0.001 |
| RDHY | tr | tr | 0.55 ± 0.02 b | tr | 0.58 ± 0.02 b | 0.82 ± 0.05 a | tr | |||||
| RDHM | tr | tr | tr | tr | 0.95 ± 0.01 a | 0.71 ± 0.00 b | tr | |||||
| M25 | Geraniol | RDZH | 1479.47 ± 11.82 a | 960.55 ± 2.19 b | 1020.42 ± 3.25 b | 918.98 ± 57.23 b | 1428.81 ± 35.10 a | 1356.98 ± 33.83 a | 1005.08 ± 27.40 b | <0.001 | <0.001 | <0.001 |
| RDHY | 1152.08 ± 22.92 a | 457.63 ± 14.53 b | 576.93 ± 60.73 b | 497.57 ± 5.74 b | 461.10 ± 23.04 b | 751.39 ± 12.20 a | 579.61 ± 16.96 b | |||||
| RDHM | 787.40 ± 6.12 b | 799.42 ± 9.01 b | 1818.86 ± 269.13 a | 948.40 ± 98.76 b | 1938.07 ± 65.03 a | 1927.52 ± 4.34 a | 1371.84 ± 8.79 a | |||||
| M26 | Geranic acid | RDZH | 485.05 ± 1.76 a 1352.11 ± 53.42 b | 361.99 ± 20.49 a839.78 ± 81.15 c | 184.12 ± 7.55 d 1586.83 ± 110.41 b | 229.83 ± 7.17 c 1265.26 ± 13.28 b | 474.83 ± 27.11 a | 520.57 ± 28.02 a | 317.48 ± 1.66 b | <0.001 | <0.001 | <0.001 |
| RDHY | 1537.73 ± 86.14 b | 2365.66 ± 64.53 a | 1405.63 ± 17.42 b | |||||||||
| RDHM | 45.46 ± 1.35 b | 68.84 ± 7.05 b | 147.77 ± 30.49 a | 47.65 ± 8.64 b | 105.64 ± 0.36 a | 157.80 ± 2.19 a | 115.27 ± 0.31 a | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.-Y.; Wang, H.-L.; Zhang, G.-J.; Yan, A.-L.; Ren, J.-C.; Liu, Z.-H.; Xu, H.-Y.; Sun, L. Effects of Fruit Bagging Treatment with Different Types of Bags on the Contents of Phenolics and Monoterpenes in Muscat-Flavored Table Grapes. Horticulturae 2022, 8, 411. https://doi.org/10.3390/horticulturae8050411
Wang X-Y, Wang H-L, Zhang G-J, Yan A-L, Ren J-C, Liu Z-H, Xu H-Y, Sun L. Effects of Fruit Bagging Treatment with Different Types of Bags on the Contents of Phenolics and Monoterpenes in Muscat-Flavored Table Grapes. Horticulturae. 2022; 8(5):411. https://doi.org/10.3390/horticulturae8050411
Chicago/Turabian StyleWang, Xiao-Yue, Hui-Ling Wang, Guo-Jun Zhang, Ai-Ling Yan, Jian-Cheng Ren, Zhen-Hua Liu, Hai-Ying Xu, and Lei Sun. 2022. "Effects of Fruit Bagging Treatment with Different Types of Bags on the Contents of Phenolics and Monoterpenes in Muscat-Flavored Table Grapes" Horticulturae 8, no. 5: 411. https://doi.org/10.3390/horticulturae8050411
APA StyleWang, X.-Y., Wang, H.-L., Zhang, G.-J., Yan, A.-L., Ren, J.-C., Liu, Z.-H., Xu, H.-Y., & Sun, L. (2022). Effects of Fruit Bagging Treatment with Different Types of Bags on the Contents of Phenolics and Monoterpenes in Muscat-Flavored Table Grapes. Horticulturae, 8(5), 411. https://doi.org/10.3390/horticulturae8050411






