Abstract
Mortiño is a member of the Ericaceae family native to the Andes that has been used by local communities for centuries. This species has shown potential in the areas of medicine, agronomy, and green technology. We used a multidisciplinary approach to review aspects related to the ecology, horticulture, composition and potential biotechnological applications of mortiño. As interest in this species grows, care must be taken to identify opportunities that justify its sustainable use while emphasizing the development of local communities. Mapping the wide variety of potential uses and the current state of conservation and utilization of this berry will help researchers to better target mortiño’s potential.
1. Introduction
Vaccinium spp. generally produce sweet berries with a very pleasant flavor [1] that are usually consumed fresh as well as in jams, drinks, and desserts. The berries of Vaccinium spp., such as blueberries (Vaccinium corymbosum) and cranberries (Vaccinium macrocarpon), are rich in phenolic acids, flavonoids, proanthocyanidins, coumarins, hydrolyzable tannins, carotenoids, and anthocyanins [2]. Moreover, they are considered “superfruits” due to their antioxidant capacity and potential health benefits [3,4]. The traditional uses and cultural values as well as the bioactive components of Vaccinium berries have promoted the cultivation and the study of potential applications of these species in pharmacy, nutrition, and health [5].
In Ecuador, three species of Vaccinium are registered, including Vaccinium distichum, Vaccinium crenatum, and Vaccinium floribundum, the last being the most common [4]. Mortiño (Vaccinium floribundum Kunth)—also known as Andean blueberry—is native to the tropical Andes of Ecuador, Colombia, and Peru [6]. The interest on mortiño as an alternative crop in tropical Andean countries benefited from the popularity of the other Vaccinium berries promoted as superfruits [4]. As a result, production and consumption of various Vaccinium berries have almost quintupled in the last 15 years [7]. However, domestication of mortiño has not been accomplished, and the use of this berry still relies on fruit harvesting from wild plants [3], which can potentially affect the conservation of the species and the balance of those ecosystems. Therefore, the analysis of new applications for mortiño should be accompanied by a holistic approach integrating ecological, horticultural, and cultural aspects associated with the growth of this plant.
The sustainable applications of mortiño in pharmacy, nutrition, and health have the potential to support the development of local communities and promote human health [8]. The objective of this work was to review the horticultural and biochemical aspects of mortiño and promote the development of new knowledge that could lead to fair and sustainable use of this species from the tropical Andes.
2. Literature Search and Systematic Analysis
The PubMed, Elsevier, NCBI, Scopus, and Google scholar databases were used to search for articles on the genus Vaccinium and—more specifically—on V. floribundum. Keywords such as polyphenols, anthocyanins, antioxidant, antimicrobial, medicinal property, in vitro propagation, ethnobotany, genetics, green technology, and pharmacological activity of mortiño were used.
Studies in areas classified as cultivation strategies, ethnobotany and ecophysiology, chemical characterization, molecular characterization, antimicrobial characterization, medicinal properties, and green technology were taken into account.
More than 200 articles on the genus Vaccinium were reviewed, including scientific articles as well as undergraduate and graduate theses, but only 65 were selected to be referenced in this review, of which 48 talked about V. floribundum. It should be noted that 17.5% of the scientific articles belonged to institutions in the United States and Europe, whose research focused mainly on the medicinal properties of mortiño [9] including chemopreventive properties [5], inhibition of adipogenesis, and inflammation [10], among other topics. On the other hand, the articles from other countries such as Ecuador focused on cultivation strategies as well as food chemistry and molecular biology of mortiño, in which European and US universities also participated. The most studied scientific areas of V. floribundum in Andean countries were ecology followed by food chemistry, green technology, and cultivation strategies (Figure 1).
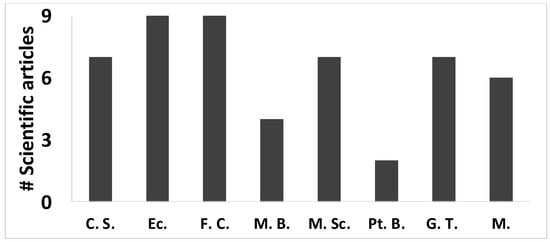
Figure 1.
Different studies on Vaccinium floribundum published around the world on topics such as cultivation strategies (C.S.), ecology (Ec), food chemistry (F.C.), molecular biology (M.B.), medicinal properties (M.Sc.), berry pretreatment (Pt. B.), green technology (G.T.), and manufacturing (M).
3. Origin, History, and Botany of Mortiño
Native to the tropical Andes of Colombia, Ecuador, and Perú [6,11], mortiño is commonly found at high altitudes on the edges of cold and humid paramos [12]. Mortiño commonly grows at temperatures between 7 and 18 °C [6], on rocky surfaces as a shrub reaching 2.5 m height, often prostrate or scandent [13]. It is one of the first species to grow back from root sprouts after fires in the paramos, playing a key ecological role in the regeneration of the ecosystem [14]. Mortiño leaves are small, coriaceous, elliptic, and ovate to ovate–lanceolate (Figure 2). The flowers are white to pink or red developed on racemes of 6–10 flowers, while the fruits are small (5–8 mm diameter) blue-black glabrous spherical berries at maturity [15] (Figure 3).
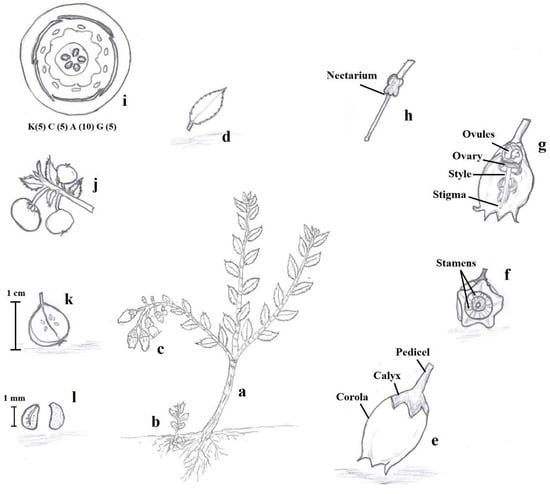
Figure 2.
Description of Vaccinium floribundum Kunth: Semi-woody stem that can measure up to 2.5 m in height (a), Roots with their root hairs that, when they come to the surface, give rise to a seedling (b), White to lilac flowers in racemes of 6 to 10 (c), Elliptical, oval, or oval–lanceolate leathery leaves, with crenate-serrated edge, cuneate or round base, and slightly rounded acuminate apex (d), Corolla and Calyx (e), Vertical view of stamens (f), Gynoecium (g), Nectaries (h), Floral diagram of the Ericaceae (i), Round bittersweet berries of bluish to black color (j), Fruit in longitudinal section (k), Recalcitrant seeds of approximately 1 mm in size (l). Source: Herbarium of the Interpretation Center of the Protective Forest “La Prosperina” (BPP—Ar001E), of the Escuela Superior Politécnica del Litoral (ESPOL), Guayaquil—Ecuador, by Botanist Jaime Naranjo (Co-author).

Figure 3.
Mortiño plant (Vaccinium floribundum Kunth) with its floral primordia (A), A small bunch of flowers with some pigmentation (B), Serrated leaves and its ripe berries (C). Source: This article.
During its development, the fruit color transitions from green to white-pink, to pink, and finally to blue-black [16]. Fruit development after anthesis takes roughly between 60 and 100 days under natural conditions [17]. Taxonomically, V. floribundum has traditionally been classified within the Pyxothamnus section in the genus Vaccinium along with V. consanguineum and V. ovatum. However, phylogenetic analysis of tribe Vaccineae suggested Vaccinium is not monophyletic with V. floribundum, forming a clade along with V. consanguineum and V. meridionale, separate from other Vaccinium spp. [17].
Since it was first described in 1825 by Kunth as Vaccinium floribundum from the collections of Bonpland, the taxonomy of mortiño has varied little over the years. However, some common synonyms for the species have been reported, including: V. crenulatum, V. marginatum, V. ramosissimun, V. polystachium, V. mortinia, V. moritzianum, V. dasygynun, Metagonia crenulate, and M. marginata [12]. Common names for V. floribundum are many and often used ambiguously to identify other related species such as V. meridionale, Thibaudia floribunda, or Macleania rupestris. For instance, in Ecuador, mortiño is the most used vernacular name for the species; however, it is also known as uva de los Andes (grape from the Andes), manzanilla del cerro (chamomile from the hill), raspadura quemada (burnt panela), and uva de monte (mountain grape). In Peru, it is known as pushgay, uvitas, congama, and macha macha, whereas in Colombia it is also known as mortiño, agraz, agracejo, and chivaco; nonetheless, here, the name mortiño is more often associated with V. meridionale [6].
The consumption of this fruit was common in the Andean region before the arrival of the Europeans; later, it was assimilated into criollo traditions associated with the All Souls’ day, which continues to this day [6]. Currently, the species is mainly found in the wild, but it is also often present in smallholder farms [18].
4. Ecology and Genetic Diversity
Like many other neotropical Ericaceaes, V. floribundum predominates in belts of moist and cool montane forest preceding the transition towards the colder Paramo (cold and moist ecosystem typical of the high mountain in the Andes between the treeline and the snowline) between 3000 and 4500 m.a.s.l. [19,20]. On the slopes of the Rumiñahui volcano, near Quito (Ecuador), V. floribundum has commonly been observed in landscapes dominated by Calamagrostis intermedia and Carex jamesonii, although many other species, mostly dicots including other Ericaceae such as Pernettya prostrata, have been found growing in close spatial association to V. floribundum [21].
V. floribundum typically thrives in cold, nutrient-poor, moist though well-drained, shallow, and acidic soils [14]. The shrubs show remarkable adaptations to these conditions, such as shallow and almost horizontal root systems as well as profuse sprouting from roots and other vegetative tissues. These characteristics make V. floribundum one of the first species to regenerate damaged paramo ecosystems [14], drawing attention to ecosystem restoration programs in the Andes. Furthermore, V. floribundum has been reported as one of the species more often visited by a variety of bird and insect pollinators [22,23]. Adaptation to these environmental conditions is probably aided by association with specific soil microorganisms, as evidence of interactions between the V. floribundum rhizospheric microbiome and soil chemical properties has been reported [24]. Similarly, ericoid mycorrhiza forming fungi have been reported in V. floribundum roots, potentially contributing to the development of the species in nutrient-limited soils [21].
Currently, the conservation status of V. floribundum populations remains unknown. However, in Ecuador, mortiño is considered highly diverse—a typical characteristic of wild populations—and has shown both geographical and altitudinal patterns of diversification [25]. Still, concerns about the conservation of the species in scenarios of increasing demand of the berry and unsuccessful domestication efforts could pose risks, both to the species and to the paramos where it grows. For these reasons, interest in developing propagation techniques amenable for commercial cultivation has been recently growing in Colombia, Ecuador, and Peru [8].
5. Domestication Attempts
Mortiño berries have been extracted from wild populations since pre-Columbian times, when the fruit was reportedly used by the Quitu-Cara people in preparations for the celebration of their deceased [26]. After the arrival of the Spaniards, this tradition was assimilated in the emerging criollo culture in the form of “colada morada”, a traditional drink still consumed today around All Soul’s Day in Ecuador [6,27]. Coinciding with these festivities, peak production for mortiño berries occurs between October and November in Ecuador, although the fruit can be found in lower quantities throughout the year [27]. Relationships between environmental factors and flower/fruit development in mortiño have not been explored, and further research is needed to better understand the effect of climate on fruit production of V. floribundum. Other uses of mortiño by local people span from medicinal uses (allegedly as an ailment for rheumatism, fevers, colics, common colds, hangover, and liver and kidney problems), to ornamental (mainly for the color of its foliage and to form hedges), to uses as dye, fodder, or firewood [27].
In recent years, the discovery of potential health promoting properties and the growth of the market demand for berries have fueled interest in domesticating the species, a task that, to this day, remains challenging. For instance, the in vitro propagation of the species has been affected by the presence of detrimental microorganisms in the wild explants of mortiño. Those microorganisms have been particularly difficult to eliminate with simple disinfection methods, so the use of methyl N-[1H-benzimidazol-2-yl) carbamate has been proposed for disinfection of the cuttings in micropropagation studies of V. floribundum [28].
Propagation by seed is likely the main form of dispersion of the species in the wild, although it is not considered a convenient method for commercial uses. Germination of mortiño seeds has been reported to be high and fast, reaching 64 to 76% germination 40 days after seeding on tissue paper and 95% germination in agar [29]. However, development of the seedlings after germination is rather slow, at 2 cm/month or slower [13]. As in other Ericaceae species [30], light has been reported to enhance the germination of V. floribundum [31].
Wild populations of mortiño in Ecuador showed high diversity, suggesting random crossing of this species [25]. Such high diversity, while ecologically advantageous, could be difficult to manage in commercial plantations where some degree of homogeneity in the plant biology is desirable. Nevertheless, being a diploid [32,33], high diversity of mortiño, derived from adaptations to different environmental conditions [16], might provide a rich gene pool for launching selection and breeding programs to develop locally adapted materials [25,34].
Most commercial Vaccinium berries are clonally propagated either by stem cuttings or using in vitro techniques [26]. Both methods have also been tested for the propagation of mortiño, with varying degrees of success. Studies have tested both cuttings and micro cuttings on different substrates and rooting agents, with very low success [26]. Differences in the degree of rooting have been reported between young (25 to 47% rooting) and semi-hard (8.1 to 24% rooting) wood cuttings from V. floribundum; in both cases, rooting was still considered very low [16]. On the other hand, better rooting was reported when using lignocellulosic residues such as rice husks and pine sawdust in contrast to more compact substrates such as peat or sand. However, rooting still remained below 50%, which was inefficient to support commercial propagation [26].
Similarly, in vitro propagation has been assayed with moderate success in mortiño. The in vitro establishment of axillary buds required high initial concentrations of cytokinins (TZR) and the acclimatization showed a low yield [31]. A complete regeneration of mortiño plants through axillary bud culture has been reported using 6-(γ,γ-Dimethylallylamino) purine (2iP) instead of TZR to successfully induce sprouting [35]. Survival rate after transfer to a peat plus vermiculite substrate was above 75% in all the treatments; additionally, the average growth rate in the 14 weeks after transfer to the substrate ranged between 1.67 and 2.16 cm/week. However, after a second transfer to paramo soil, the overall survival rate fell to 46.6% due to fungal infection, while growth was arrested in some of the plants [35]. Studies that follow the fate of these plants after establishment under field conditions are still needed to assess the feasibility of domesticating mortiño from micropropagated plants. The increased availability of micropropagated plants could allow horticultural evaluation of mortiño production systems to begin, an activity that can benefit from the current knowledge attained from similar Vaccinium berries, such as blueberries.
In nature, the rhizosphere of Vaccinium spp. has coexisted with specialized fungal communities such as ericoid mycorrhizal fungi and dark septate endophytes [36], including Leotia lubrica, Leotia viscosa, Arbutus menziesii, Arctostaphylos uva-ursi, Rhizoscyphus ericae, and Acephala applanata, among others [32,37]. These symbiotic fungi can play a fundamental role in the adaptation and acclimatization of mortiño seedlings obtained from in vitro culture since this fungal species can mitigate different types of stress by promoting a greater tolerance to harmful elements and facilitate access to nutrients [33,38]. Researching symbiont candidates to improve the establishment of mortiño plants in substrates and field conditions remains an underexplored field of research.
6. Chemical Composition of Mortiño
The berries from Vaccinium spp. are known for their substantial amounts of sugars, polyphenols, vitamins B and C, minerals, and anthocyanins [39]. Table 1 shows the bromatological parameters and Table 2 describes works on mortiño phytochemistry.

Table 1.
Physical and bromatological parameters of the Vaccinium floribundum berry.

Table 2.
Bioactive compounds from Vaccinium floribundum.
V. floribundum berries have shown high amounts of polyphenols, with levels reaching up to 7254.62 ± 10.86 mg GAE/100 g [44]. The most representative phenolic acids and flavonols reported in mortiño were quercetin-3-O-arabinofuranoside, chlorogenic acid, and quercetin-3-O-galactoside [5].
In comparative studies, other superfruits such as Prunus serotina showed lower levels of polyphenols than those found in mortiño, with reported concentrations of 1494 ± 385 and 2167 ± 835 mg GAE/100 g for each species, respectively [43]. Similarly, a higher content of phenolic acids and flavonols was observed in V. floribundum (41.6 ± 10.2 mg/100 g FW) when compared to V. myrtillus (13.7 ± 0.2 mg/100 g FW) [5]. In another study, the content of total soluble phenolic of mortiño (882 ± 38 mg GAE/100 g FW) was almost double that of guava (462 mg GAE/100 g FW) and plum (440 mg GAE/100 g FW); furthermore, it was four times higher than the values observed in strawberry (238 mg GAE/100 g FW) but less than half the content in the Andean blackberry (2167 mg GAE/100 g FW) [41].
Anthocyanins are other important components in V. floribundum berries, accounting for up to 67% of their total phenolic compounds [41]. Delphinidin-3-arabinose and cyanidin-3-arabinose have been reported as the most abundant anthocyanins in mortiño [10]. Values from 376.2 ± 49.9 [5] to 1095.39 mg/100 g FW [3] of anthocyanin content in blueberry have been reported, exceeding that observed in V. myrtillus (568.8 ± 8.8 mg/100 g FW) [5]. However, sample processing can reduce the levels of anthocyanins, as commercial mortiño powder showed lower levels of these bioactive compounds compared to fresh berries [10]. On the other hand, the levels of proanthocyanidins observed in V. floribundum (5.2 mg/g dry weight epicatechin equivalent) were higher than those in Aristotelia chilensis berries (4.0 mg/g DW EE) but lower than the values observed in V. myrtillus (13.7 mg/g DW EE) [10].
Lastly, it is also very important to know the content of P, Mn, Se, and I of mortiño berries. Unfortunately, to date there are no studies that provide this information. Further research is needed to assess the mineral content of mortiño berries.
7. Factors Influencing the Chemical Composition and Genotype of Mortiño
The variability observed in the chemical composition and genotype of V. floribundum has been related to climatic and geographic influences [3,12]. A geographic pattern has been associated with the genetic diversity of V. floribundum [12]; in addition, the influence of geoclimatic factors on the genetic and chemical composition of mortiño has been proposed [3]. These observations differ from the negligible effect of the growing location on the fruit composition observed in other Vaccinium species [47,48]. Further research is needed to understand the factors that influence the nutraceutical quality of the mortiño berries.
It has been observed that the levels of polyphenols and other bioactive compounds can vary significantly according to the geographical location of the mortiño plants, as well as the growing conditions influenced by radiation, altitude, and temperature [3]. In the same way, fruit development and ripening along with the processing of the samples for analysis can cause the observed variations in the levels of polyphenols, ranging from 100 to 5000 [44] or even up to 7254.62 [44] mg GAE/100 g.
Only few studies have attempted to characterize the effect of the genotype on the composition of V. floribundum. Some studies on other Vaccinium species showed that biometric and nutritional parameters were not affected by the cultivar or the cultivation altitude. Still, the fresh mass and volume of the fruit, as well as the crude protein and crude fat, varied significantly between cultivars, although their dry mass and energy content were similar in the samples tested in a previous report [47]. Furthermore, the levels of bioactive compounds were affected by the genotype, and the manipulation of varieties through traditional breeding or advanced biotechnological methods has been suggested as a tool to modify the patterns and composition of Vaccinium species [49]. Another study showed that the antioxidant capacity of different Vaccinium species ranged from 13.9 to 45.9 μmol TE/g of fresh berries and 63.2 to 282.3 μmol TE/g of dry matter, and rose as fruit ripeness increased at harvest. Anthocyanins and the total phenolic contents also increased as the fruit ripened, but the growing location did not affect the levels of these bioactive compounds in the berries [48].
8. Biological Activities of Mortiño
Due to the high amounts of polyphenols, anthocyanins, and antioxidants, mortiño has shown various bioactive properties. Table 3 shows some of the antimicrobial and medicinal properties of mortiño. Several studies have shown the antimicrobial capacity of Vaccinium spp. For example, V. corymbosum extracts inhibited the growth of various pathogens such as Salmonella spp. [50] and Listeria monocytogenes [51], while V. macrocarpon prevented the growth of Bacillus cereus and Micrococcus luteus [52]. Similarly, two studies have reported the antimicrobial capacity of mortiño. In one study, mortiño extracts obtained from lyophilized fruits or leaves in 70% ethanol inhibited the growth of 12 species of human pathogens, with inhibition halos ranging from 4.3 ± 0.3 to 39.7 ± 0.2 mm. The reported inhibition halos were greater than those observed when the antibiotic ampicillin was used [3]. Similarly, aqueous pulp and peel extracts of mortiño inhibited the growth of Streptococcus mutans ATCC35668 [46].

Table 3.
Medicinal properties of Vaccinium floribundum.
Various medicinal properties have been attributed to V. floribundum, including potential applications in managing the symptoms of diabetes [9] and protection against oxidative stress [45]. Additionally, anti-inflammatory properties of mortiño have been suggested, as phenolic extracts of this species decreased the production of inflammatory mediators such as nitric oxide (NO), prostaglandin E2, and cycloxygenase-2 in lipopolysaccharide-stimulated RAW 264.7 macrophages [10]. Similarly, mortiño has been proposed as an inhibitor of adipogenesis, since proanthocyanidin-rich extracts of V. floribundum significantly prevented lipid accumulation in adipocytes and increased the expression of the preadipocyte factor 1 (Pref-1) by 4% in preadipocytes, a value higher than the 2.2% reached by A. chilensis but similar to the 5.9% achieved by epigallocatechin gallate used as a positive control (5.9%) [10]. Likewise, proanthocyanidins from mortiño successfully inhibited the enzymes α-glucosidase and α-amylase in vitro, with a 50% inhibition concentrations (IC50) of 35 and 25 μg/mL, respectively, suggesting a potential use of this berry for diabetes therapy [9].
The berries of V. floribundum have also shown a high antioxidant capacity ranging from 0.339 ± 0.01 g/mL to 0.69 ± 0.03 g/mL [8,10], comparable to that of V. myrtillus (0.42 ± 0.01 g/mL) [5]. Through the Trolox Equivalent Antioxidant Capacity test, 250.01 ± 2.0 μmol TEq/g FW was reported in mortiño, which was higher than the 1.52 ± 3.1 μmol TEq/g FW observed in the berries of Rubus glaucus [45]. Due to its high antioxidant capacity, mortiño has the potential to protect human cells against oxidative stress. Crude extracts of V. floribundum attenuated the damage to Human Dermal Fibroblasts (HDFa) caused by oxidative stress, providing better protection than Rubus glaucus extracts [45]. However, no chemopreventive activity of mortiño was observed in mutagenicity and genotoxicity tests against 4-nitroquinoline-1-oxide (4-NQO) using SOS Chromotests [5]. Further research is needed to assess the potential antioxidant and radical scavenging applications of V. floribundum. In vivo studies, either in clinics or other controlled environments, are still needed to confirm the health benefits of mortiño.
9. Other Uses of Mortiño
There is a growing interest on plant antioxidants for green technology applications. For this reason, mortiño has been used in the synthesis of nanoparticles and solar cells.
The synthesis of nanoparticles commonly requires the use of toxic chemicals that serve as reducing agents. As a result of its high antioxidant capacity, mortiño has the potential to replace hazardous molecules for green production of nanoparticles. Mortiño extracts with high antioxidant capacity have been used for the green synthesis of graphene and functionalization of this material, with silver nanoparticles yielding a highly efficient photocatalyst [53]. Similarly, mortiño extracts were applied as a reducing and stabilizing agent for multicomponent nanoparticles (MCNPs) [54] and zero-valent iron nanoparticles (nZVIs) for bioremediation studies [40,55]. The resulting MCNPs showed >99% removal efficiency of toxic metals in water [54], whereas the zVINs removed at least 80% of total petroleum hydrocarbons (TPH) in contaminated water and soil [55]. The researchers indicated that the efficient formation and stabilization of the nZVIs was probably related to the -OH and -COOH groups from the berry polyphenols.
Additionally, mortiño extracts have also served as sensitizers for dye-sensitized solar cells, yielding efficiencies between 0.18 and 0.26% [56]. Table 4 shows some of the applications of mortiño.

Table 4.
Other applications of Vaccinium floribundum.
Mortiño has also been used in the wine industry [59,60], baking [61], and even in the production of mortiño gummies [62]. The bioactive compounds of mortiño have attracted much interest in various sectors, which is why several methods have been proposed for the conservation and long-term storage of this berry, including short exposure to UV-C [63] and drying pretreatments [64] (Table 5). In a study, it was shown that after a storage period of 21 days, UV-C-treated (12.5 kJ m−2) mortiño retained 90% of the original anthocyanin levels compared to 76.85% of the untreated berries. However, the concentration of polyphenols was similar in both UV-C-treated and untreated berries [63]. Surprisingly, dry mortiño retained 93% of the anthocyanins and all the polyphenols in a storage period of 8 weeks [64]. However, other food processing technologies have been detrimental for the bioactive compounds of mortiño. Compared to lyophilization, heating in a sand bath to obtain a commercial mortiño powder yielded significantly lower levels of bioactive compounds such as anthocyanins (2.3 ± 0.6 vs. 11.1 ± 0.5%), proanthocyanidins (4.6 ± 0.3 vs. 5.3 ± 0.5%), total phenols (495.6 ± 9.1 vs. 524.4 ± 4.5 mg/g), and antioxidant capacity (3.3 ± 0.1 vs. 8.3 ± 0.4 mmol/g Trolox equivalents estimated by Oxygen Radical Absorbance Capacity (ORAC)) [10] (Table 5). In general, the processing of berries can degrade the anthocyanins naturally present in the fruits [65] (Table 3). Therefore, the pre-treatment of mortiño using UV-C represents a non-chemical approach to complement the treatment for low temperature storage, especially to maintain the anthocyanin concentration. Table 5 shows some of the preservation studies carried out on mortiño.

Table 5.
Berry quality preservation studies.
10. Future Prospect of Mortiño
Today, berries are common cash crops of global demand due to the excellent quality of the fruits and their potential health benefits and have historically been used for medicinal purposes [9]. Local communities in Ecuador have traditionally used the plant to treat various medical conditions, including diabetes and inflammation. In addition to the pharmaceutical potential, the cosmetic potential of mortiño has also been proposed due to the intense color of the pigments in this berry [9].
With the advancement in food processing technologies, commercial mortiño is now available in the markets in different presentations: powder, capsules, and wines, known as Wine of the Andes [1]. These are all indicators of the growing popularity of this plant. Even so, mortiño has not been marketed yet as a functional drink, and uses of the by-products from this plant have rarely been proposed. To make a functional drink from mortiño, it is necessary to investigate its nutraceutical and quality parameters such as color, minerals, phytochemicals, and antioxidant capacity as well as its biological activities, such as the inhibitory effects of α-glucosidase and lipase in the final product, with due scientific rigor [66]. Similarly, mortiño by-products could be used to provide added value to cereals and other nutritional snacks, minimizing the cost of waste management for the agro-industrial area [67]. Both consumers and the scientific community expect more exciting results from superfruit products in the upcoming years [4].
Mortiño pigments showed the potential to replace the toxic chemicals commonly used as reducing agents for the synthesis of nanoparticles for the production of solar cells [53] and removal of hydrocarbons [55], among others. Therefore, applications of V. floribundum in green nanotechnology should continue to be explored.
Experts indicate that the demand for berries of the various domesticated Vaccinium species will continue to grow in the near future [68]. However, mortiño is still generated exclusively through traditional reproduction. Methods such as hybridization and controlled selection as well as in vitro reproduction are still needed. Nevertheless, studies on genetic markers continue to fill the genomic databases of Vaccinium and results that will contribute to improve efficiency of mortiño multiplication are beginning to emerge [25]. Even so, more genetic studies are still needed.
11. Conclusions
Superfruits that have exceptional nutrients and a high content of phytochemicals—particularly phenolic acids and flavonoids—with remarkable antioxidant activity and enormous nutraceutical potential, such as mortiño, are being increasingly used. Research has shown that many of the active principles in both berries and leaves of mortiño are highly desirable for the nutraceutical, food, wine, and pharmaceutical industry, as well as for green technology processes in the synthesis of nanoparticles for the sensitization of solar cells and the elimination of petroleum hydrocarbons or heavy metals. Despite its great potential and ethnobotanical history among indigenous communities, mortiño remains a wild species vulnerable to extinction due to the destruction of its habitat. Therefore, the achievement of an effective in vitro reproduction methodology should also be prioritized to promote the conservation and sustainable use of this natural resource.
Author Contributions
S.A.L.-C.: Review of the databases, structured the sections of the article, wrote the article, created and elaborated the figures. F.L.-T.: Review and correction of the article. P.M.-S.: Review and correction of the article. J.R.: Review and correction of the article. J.N.-M.: Botanized the mortiño and made the drawings of its parts. L.S.-M.: English spelling and grammar correction. E.C.-M.: Writing a section of the article, revised and corrected. J.M.C.-C.: Issued ideas and made very important corrections to the article. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Flemish Inter-University Council (VLIR-UOS) grant VLIR Network Ecuador.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors have no conflict of interest to report.
References
- Ortiz, J.; Marín-Arroyo, M.-R.; Noriega-Domínguez, M.-J.; Navarro, M.; Arozarena, I. Color, Phenolics, and Antioxidant Activity of Blackberry (Rubus Glaucus Benth.), Blueberry (Vaccinium Floribundum Kunth.), and Apple Wines from Ecuador. J. Food Sci. 2013, 78, C985–C993. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Sun, Z.; Zeng, Y.; Luo, M.; Yang, J. Molecular Mechanism and Health Role of Functional Ingredients in Blueberry for Chronic Disease in Human Beings. Int. J. Mol. Sci. 2018, 19, 2785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llivisaca, S.; Manzano, P.; Ruales, J.; Flores, J.; Mendoza, J.; Peralta, E.; Cevallos-Cevallos, J.M. Chemical, Antimicrobial, and Molecular Characterization of Mortiño (Vaccinium floribundum Kunth) Fruits and Leaves. Food Sci. Nutr. 2018, 6, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Alasalvar, C.; Shahidi, F. Superfruits: Phytochemicals, Antioxidant Efficacies, and Health Effects—A Comprehensive Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1580–1604. [Google Scholar] [CrossRef] [PubMed]
- Prencipe, F.P.; Bruni, R.; Guerrini, A.; Rossi, D.; Benvenuti, S.; Pellati, F. Metabolite Profiling of Polyphenols in Vaccinium Berries and Determination of Their Chemopreventive Properties. J. Pharm. Biomed. Anal. 2014, 89, 257–267. [Google Scholar] [CrossRef]
- Coba, P.; Coronel, D.; Verdugo, K.; Paredes, M.; Yugsi, E.; Huachi, L. Ethnobotanical Study of the Mortiño (Vaccinium fLoribundum) as Ancestral and Potentially Functional Meal. La Granja 2012, 16, 5. [Google Scholar] [CrossRef]
- FAO. FAOSTAT. Available online: http://www.fao.org/faostat/es/#home (accessed on 19 August 2019).
- Abreu, O.A.; Barreto, G.; Prieto, S. Vaccinium (Ericaceae): Ethnobotany and Pharmacological Potentials. Emir. J. Food Agric. 2014, 26, 577–591. [Google Scholar] [CrossRef]
- Schreckinger, M.; Lila, M.; Yousef, G.; Gonzalez, E. Inhibition of α-Glucosidase and α-Amylase by Vaccinium floribundum and Aristotelia Chilensis Proanthocyanidins. ACS Symp. Ser. 2012, 1109, 71–82. [Google Scholar]
- Schreckinger, M.; Wang, J.; Yousef, G.; Lila, M.; Gonzalez, E. Antioxidant Capacity and in Vitro Inhibition of Adipogenesis and Inflammation by Phenolic Extracts of Vaccinium floribundum and Aristotelia Chilensis. J. Agric. Food Chem. 2010, 58, 8966–8976. [Google Scholar] [CrossRef]
- Schreckinger, M.; Lotton, J.; Lila, M.; Gonzalez, E. Berries from South America: A Comprehensive Review on Chemistry, Health Potential, and Commercialization. J. Med. Food 2010, 13, 233–246. [Google Scholar] [CrossRef]
- Cobo, M.; Gutiérrez, B.; Torres, A.; Torres, M. Preliminary Analysis of the Genetic Diversity and Population Structure of Mortiño (Vaccinium floribundum Kunth). Biochem. Syst. Ecol. 2016, 64, 14–21. [Google Scholar] [CrossRef]
- Pedraza, P. Vaccinium floribundum. Available online: http://tropical.theferns.info/viewtropical.php?id=Vaccinium+floribundum (accessed on 15 January 2021).
- Ramsay, P.; Oxley, E. Fire Temperatures and Postfire Plant Community Dynamics in Ecuadorian Grass Páramo. Vegetatio 1996, 124, 129–144. [Google Scholar] [CrossRef]
- Pedraza, P. Taxon Details—Vaccinium floribundum Kunth. Available online: http://sweetgum.nybg.org/science/projects/ericaceae/taxon-details/?irn=113261 (accessed on 22 January 2021).
- Arteaga Dalgo, M.; Andrade Cuvi, M.J.; Moreno Guerrero, C. Relación Del Desarrollo Del Color Con El Contenido de Antocianinas y Clorofila En Diferentes Grados de Madurez de Mortiño (Vaccinium floribundum). Enfoque UTE 2014, 5, 14–28. [Google Scholar] [CrossRef] [Green Version]
- Mendoza, F. Fenología Floral Del Mortiño (Vaccinium floribundum Kunth) Acorde a La Escala BBCH En El Páramo Andino Del Atacazo, Ecuador. Available online: https://rraae.cedia.edu.ec/Record/UDLA_1155233eaaba390cb211e874da1511b6 (accessed on 19 March 2022).
- Magnitskiy, S.; Ligarreto, G.; Lancheros, H. Rooting of Two Types of Cuttings of Fruit Crops Vaccinium floribundum Kunth and Disterigma alaternoides (Kunth) Niedenzu (Ericaceae). Agron. Colomb. 2011, 29, 197–203. [Google Scholar]
- Kron, K.A.; Powell, E.A.; Luteyn, J.L. Phylogenetic Relationships within the Blueberry Tribe (Vaccinieae, Ericaceae) Based on Sequence Data from MatK and Nuclear Ribosomal ITS Regions, with Comments on the Placement of Satyria. Am. J. Bot. 2002, 89, 327–336. [Google Scholar] [CrossRef]
- Popenoe, W. Economic Fruit-Bearing Plants of Ecuador; Smithsonian Institution: Washington, DC, USA, 1924. [Google Scholar]
- Hidalgo, M.; Vásquez, W. Caracterización Morfológica de Microorganismos, Físico-Química Del Suelo y Arvenses Presentes En El Hábitat de Crecimiento Del Mortiño (Vaccinium floribundum Kunth) En El Páramo Del Volcán Rumiñahui, Pichincha; Universidad de las Américas: Quito, Ecuador, 2016. [Google Scholar]
- Pelayo, R.; Soriano, P.; Márquez, N.; Navarro, L. Phenological Patterns and Pollination Network Structure in a Venezuelan Páramo: A Community-Scale Perspective on Plant-Animal Interactions. Plant Ecol. Divers. 2019, 12, 607–618. [Google Scholar] [CrossRef]
- Torres, M.; Pinos, A. Exploring the Microbiome Composition of the Rhizosphere Associated with the Wild Andean Blueberry (Vaccinium floribundum, Kunth) in the Highlands of Ecuador. Master’s Thesis, Universidad San Francisco de Quito, Colegio de Posgrados, Quito, Ecuador, 2020. [Google Scholar]
- Setaro, S.; Kottke, I.; Oberwinkler, F. Anatomy and Ultrastructure of Mycorrhizal Associations of Neotropical Ericaceae. Mycol. Prog. 2006, 5, 243–254. [Google Scholar] [CrossRef]
- Vega, P.; Cobo, M.; Argudo, A.; Gutierrez, B.; Rowntree, J.; Torres, M. Characterizing the Genetic Diversity of the Andean Blueberry (Vaccinium floribundum Kunth.) Across the Ecuadorian Highlands. PLoS ONE 2020, 15, e0243420. [Google Scholar] [CrossRef]
- Vásquez, P.; Osejo, J. Análisis Histórico Comparativo de La Forma de Preparación de Los Platos Más Destacados de La Gastronomía Ecuatoriana; Universidad de las Américas: Quito, Ecuador, 2016. [Google Scholar]
- Muñoz, V.; Caviedes, M. Determinación de Métodos Para Producción de Mortiño (Vaccinium floribundum Kunth), Con Fines de Propagación y Producción Comercial; Universidad San Francisco de Quito—USFQ: Quito, Ecuador, 2011. [Google Scholar]
- Llivisaca, S.; Juan, C.; Mendoza, J.; Piña, F.; Peralta, E.; Sanchez, E.; Flores, J. Issue Disinfection Evaluation and In-Vitro Propagation Protocol for the Estabishment Of Mortiño (Vaccinium floribundum Kunth.). Plant Tissue Cult. Biotechnol. 2020, 30, 167–177. [Google Scholar] [CrossRef]
- Cardozo, H.; González, D.; Guzmán, R.; Lancheros, O.; Mesa, I.; Pacheco, A.; Pérez, A.; Ramos, A.; Torres, E.; Zúñiga, T. Especies Útiles En La Región Andina de Colombia; Pérez, A., Córdoba, L., Eds.; Imprenta Nacional de Colombia: Bogotá, Colombia, 2009; Volume 2, ISBN 978-958-97749-2-2. [Google Scholar]
- Barney, D. Effects of Light, Surface Sterilization, and Fungicides on the Germination of Black Huckleberry Seeds. Small Fruits Rev. 2010, 2, 73–80. [Google Scholar] [CrossRef]
- Torres, M.; Trujillo, D.; Arahana, V. Cultivo In Vitro del Mortiño (Vaccinium floribundum Kunth). ACI Av. en Ciencias e Ing. 2010, 2, B9–B15. [Google Scholar] [CrossRef]
- Lukešová, T.; Kohout, P.; Větrovský, T.; Vohník, M. The Potential of Dark Septate Endophytes to Form Root Symbioses with Ectomycorrhizal and Ericoid Mycorrhizal Middle European Forest Plants. PLoS ONE 2015, 10, e0124752. [Google Scholar] [CrossRef] [Green Version]
- Casarrubia, S.; Martino, E.; Daghino, S.; Kohler, A.; Morin, E.; Khouja, H.R.; Murat, C.; Barry, K.W.; Lindquist, E.A.; Martin, F.M.; et al. Modulation of Plant and Fungal Gene Expression Upon Cd Exposure and Symbiosis in Ericoid Mycorrhizal Vaccinium myrtillus. Front. Microbiol. 2020, 11, 341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyrene, P.; Vorsa, N.; Ballington, J. Polyploidy and Sexual Polyploidization in the Genus Vaccinium. Euphytica 2003, 133, 27–36. [Google Scholar] [CrossRef]
- Cobo, M.; Gutiérrez, B.; Torres, M. Regeneration of Mortiño (Vaccinium floribundum Kunth) Plants Through Axillary Bud Culture. Vitr. Cell. Dev. Biol.—Plant 2018, 54, 112–116. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, X.; Liu, C.; Bai, L.; Zhao, M.; Li, L. Diversity and Characteristics of Colonization of Root-Associated Fungi of Vaccinium uliginosum. Sci. Rep. 2018, 8, 15283. [Google Scholar] [CrossRef]
- Kühdorf, K.; Münzenberger, B.; Begerow, D.; Gómez, J.; Hüttl, R. Leotia Cf. Lubrica Forms Arbutoid Mycorrhiza with Comarostaphylis Arbutoides (Ericaceae). Mycorrhiza 2015, 25, 109–120. [Google Scholar] [CrossRef] [Green Version]
- Kosola, K.R.; Workmaster, B.A.A.; Spada, P.A. Inoculation of Cranberry (Vaccinium macrocarpon) with the Ericoid Mycorrhizal Fungus Rhizoscyphus Ericae Increases Nitrate Influx. New Phytol. 2007, 176, 184–196. [Google Scholar] [CrossRef]
- Efferth, T.; Banerjee, M.; Paul, N.; Abdelfatah, S.; Arend, J.; Elhassan, G.; Hamdoun, S.; Hamm, R.; Hong, C.; Kadioglu, O.; et al. Biopiracy of Natural Products and Good Bioprospecting Practice. Phytomedicine 2016, 23, 166–173. [Google Scholar] [CrossRef] [Green Version]
- Torrenegra Alarcón, M.E.; Villalobos Lagares, O.L.; Castellar Abello, E.A.; León Méndez, G.; Granados Conde, C.; Pajaro, N.P.; Caro Soto, M.S. Evaluation of the Antioxidant Activity of Pulps from Rubus glaucus B., Vaccinium floribundum K. and Beta Vulgaris L. Rev. Cuba. Plantas Med. 2016, 21, 1–8. [Google Scholar]
- Vasco, C.; Riihinen, K.; Ruales, J.; Kamal-Eldin, A. Chemical Composition and Phenolic Compound Profile of Mortiño (Vaccinium floribundum Kunth). J. Agric. Food Chem. 2009, 57, 8274–8281. [Google Scholar] [CrossRef] [PubMed]
- Estrella, E. El Pan de America: Etnohistoria de Los Alimentos Aborigenes En El Ecuador. Publicaciones del C.S.I.C 1988, 29, 390. [Google Scholar]
- Murgueitio, E.; Debut, A.; Landivar, J.; Cumbal, L. Synthesis of Iron Nanoparticles Through Extracts of Native Fruits of Ecuador, as Capuli (Prunus serotina) and Mortiño (Vaccinium floribundum). Biol. Med. 2016, 8, 1. [Google Scholar] [CrossRef]
- Llerena, W.; Samaniego, I.; Angós, I.; Brito, B.; Ortiz, B.; Carrillo, W. Biocompounds Content Prediction in Ecuadorian Fruits Using a Mathematical Model. Foods 2019, 8, 284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alarcón-Barrera, K.S.; Armijos-Montesinos, D.S.; García-Tenesaca, M.; Iturralde, G.; Jaramilo-Vivanco, T.; Granda-Albuja, M.G.; Giampieri, F.; Alvarez-Suarez, J.M. Wild Andean Blackberry (Rubus glaucus Benth) and Andean Blueberry (Vaccinium floribundum Kunth) from the Highlands of Ecuador: Nutritional Composition and Protective Effect on Human Dermal Fibroblasts Against Cytotoxic Oxidative Damage. J. Berry Res. 2018, 8, 223–236. [Google Scholar] [CrossRef]
- Reyes, I.; Villacres, C.; Santacruz, S.; Castro, M.; Chávez, M.; Armas, A. Antibacterial and Antioxidant Effect of Ecuadorian Red Fruits on Streptococcus Mutans: In Vitro Study. Available online: https://www.scielo.sa.cr/scielo.php?pid=S1659-07752019000200023&script=sci_arttext (accessed on 28 November 2019).
- Correia, S.; Gonçalves, B.; Aires, A.; Silva, A.; Ferreira, L.; Carvalho, R.; Fernandes, H.; Freitas, C.; Carnide, V.; Silva, A.P. Effect of Harvest Year and Altitude on Nutritional and Biometric Characteristics of Blueberry Cultivars. J. Chem. 2016, 2016, 8648609. [Google Scholar] [CrossRef]
- Prior, R.L.; Cao, G.; Martin, A.; Sofic, E.; McEwen, J.; O’Brien, C.; Lischner, N.; Ehlenfeldt, M.; Kalt, W.; Krewer, G.; et al. Antioxidant Capacity As Influenced by Total Phenolic and Anthocyanin Content, Maturity, and Variety of Vaccinium Species. J. Agric. Food Chem. 1998, 46, 2686–2693. [Google Scholar] [CrossRef]
- Scalzo, J.; Politi, A.; Pellegrini, N.; Mezzetti, B.; Battino, M. Plant Genotype Affects Total Antioxidant Capacity and Phenolic Contents in Fruit. Nutrition 2005, 21, 207–213. [Google Scholar] [CrossRef]
- Pervin, M.; Hasnat, M.A.; Lim, B.O. Antibacterial and Antioxidant Activities of Vaccinium corymbosum L. Leaf Extract. Asian Pac. J. Trop. Dis. 2013, 3, 444–453. [Google Scholar] [CrossRef]
- Shen, X.; Sun, X.; Xie, Q.; Liu, H.; Zhao, Y.; Pan, Y.; Hwang, C.-A.; Wu, V.C.H. Antimicrobial Effect of Blueberry (Vaccinium corymbosum L.) Extracts Against the Growth of Listeria monocytogenes and Salmonella enteritidis. Food Control 2014, 35, 159–165. [Google Scholar] [CrossRef]
- Viskelis, P.; Rubinskiene, M.; Jasutiene, I.; Šarkinas, A.; Daubaras, R.; Česoniene, L. Anthocyanins, Antioxidative, and Antimicrobial Properties of American Cranberry (Vaccinium macrocarpon Ait) and Their Press Cakes. J. Food Sci. 2009, 74, C157–C161. [Google Scholar] [CrossRef] [PubMed]
- Vizuete, K.S.; Kumar, B.; Vaca, A.V.; Debut, A.; Cumbal, L. Mortiño (Vaccinium floribundum Kunth) Berry Assisted Green Synthesis and Photocatalytic Performance of Silver–Graphene Nanocomposite. J. Photochem. Photobiol. A Chem. 2016, 329, 273–279. [Google Scholar] [CrossRef]
- Abril, M.; Ruiz, H.; Cumbal, L.H. Biosynthesis of Multicomponent Nanoparticles with Extract of Mortiño (Vaccinium floribundum Kunth) Berry: Application on Heavy Metals Removal from Water and Immobilization in Soils. J. Nanotechnol. 2018, 2018, 9504807. [Google Scholar] [CrossRef] [Green Version]
- Murgueitio, E.; Cumbal, L.; Abril, M.; Izquierdo, A.; Debut, A.; Tinoco, O. Green Synthesis of Iron Nanoparticles: Application on the Removal of Petroleum Oil from Contaminated Water and Soils. J. Nanotechnol. 2018, 2018, 4184769. [Google Scholar] [CrossRef] [Green Version]
- Taco-Ugsha, M.A.; Santacruz, C.P.; Espinoza-Montero, P.J. Natural Dyes from Mortiño (Vaccinium floribundum) as Sensitizers in Solar Cells. Energies 2020, 13, 785. [Google Scholar] [CrossRef] [Green Version]
- Kumar, B.; Vizuete, K.S.; Sharma, V.; Debut, A.; Cumbal, L. Ecofriendly Synthesis of Monodispersed Silver Nanoparticles Using Andean Mortiño Berry as Reductant and Its Photocatalytic Activity. Vacuum 2019, 160, 272–278. [Google Scholar] [CrossRef]
- Ramirez-Perez, J.; Maria, C.; Santacruz, C.P. Impact of Solvents on the Extraction and Purification of Vegetable Dyes onto the Efficiency for Dye-Sensitized Solar Cells. Renew. Wind. Water Sol. 2019, 6, 1–15. [Google Scholar] [CrossRef]
- Albán Martínez, D.P.; Marcalla Montaguano, W.R. Estudio de Pre-Factibilidad Para La Producción Tecnificada de Vino de Mortiño (Vaccinium floribundum Kunth) En El Cantón Sigchos Comunidad Quinticusig Asociacion de Vinicultores Período 2012–2013. Bachelor’s Thesis, Universidad Técnica de Cotopaxi/UTE, Latacunga, Ecuador, 2013. [Google Scholar]
- Pastuña, G. Estudio de Factibilidad Para La Creación de Una Microempresa Dedicada a La Producción y Comercialización de Vino a Base de Mortiño, Ubicada En La Provincia de Cotopaxi, Cantón Sigchos, Año 2019. 2019. Available online: http://www.dspace.cordillera.edu.ec:8080/xmlui/handle/123456789/5166 (accessed on 24 November 2021).
- Ceron, J. Determinación de La Vida Útil Del Pan de Mortiño. 2018. Available online: http://repositorio.ute.edu.ec/xmlui/handle/123456789/18131 (accessed on 24 November 2021).
- Vicente, S.; Argüello, Y. Elaboración de Gomitas de Mortiño (Vaccinium floribundum). Bachelor’s Thesis, Universidad Técnica de Cotopaxi/UTE, Latacunga, Ecuador, 2016. [Google Scholar]
- Andrade-Cuvi, M.J.; Moreno, C.; Zaro, M.J.; Vicente, A.R.; Concellón, A. Improvement of the Antioxidant Properties and Postharvest Life of Three Exotic Andean Fruits by UV-C Treatment. J. Food Qual. 2017, 2017, 4278795. [Google Scholar] [CrossRef] [Green Version]
- García, A.; Ruales, J. Study the Effect of Pre-Treatment of Drying ‘Mortiño’ (Vaccinium floribundum Kunth) with Reference to Drying Rate and Total Content of Soluble Polyphenols and Anthocyanins. Rev. Politécnica 2018, 40, 47–57. [Google Scholar]
- Yue, X.; Xu, Z. Changes of Anthocyanins, Anthocyanidins, and Antioxidant Activity in Bilberry Extract During Dry Heating. J. Food Sci. 2008, 73, C494–C499. [Google Scholar] [CrossRef]
- Guijarro, M.; Andrade, M.; Bravo, J.; Ramos, L.; Vernaza, M. Andean Blueberry (Vaccinium floribundum) Bread: Physicochemical Properties and Bioaccessibility of Antioxidants. Food Sci. Technol. 2019, 39, 56–62. [Google Scholar] [CrossRef] [Green Version]
- Ziemlewska, A.; Zagórska-Dziok, M.; Nizioł-Łukaszewska, Z. Assessment of Cytotoxicity and Antioxidant Properties of Berry Leaves as By-Products with Potential Application in Cosmetic and Pharmaceutical Products. Sci. Rep. 2021, 11, 3240. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Hancock, J. Vaccinium. In Wild Crop Relatives: Genomic and Breeding Resources: Temperate Fruits; Springer: Berlin/Heidelberg, Germany, 2011; pp. 197–221. ISBN 9783642143878. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).