Abstract
Peony is a traditional Chinese flower with significant ornamental and medicinal value. However, there are still problems, such as serious browning, difficulties in differentiation, and rooting and low regeneration efficiency in the process of the regeneration system established, which have hindered the development of transgenic peony technology. Establishing an efficient regeneration system is considered to be an important goal among peony researchers. Here, we describe a protocol for high-frequency callus induction and establishment of peony plants using flower petals as explants. Murashige and Skoog (MS) medium supplemented with 2.0 mg/L 2,4-dichlorophenoxyacetic acid (2,4-D), 1.5 mg/L N-(phenylmethyl)-9H-purin-6-amine (6-BA), and 0.3 mg/L 1-naphthylacetic acid (NAA) was identified as the best medium for callus induction, achieving an induction rate of up to 98.52%. The highest peony proliferation rate (234%) was achieved on MS supplemented with 0.2 mg/L NAA and 3.0 mg/L 6-BA. The highest callus differentiation rate (34.81%) was achieved on MS supplemented with 2.0 mg/L 6-BA and 0.5 mg/L zeatin (ZT). The highest rooting rate was 23.33% when using 1/2 MS supplemented with 0.1 mg/L NAA and 0.05 mg/L 3-indolebutyric acid (IBA). After acclimation, the plants were transferred to pots, where they showed robust growth. We also observed the surface structures of the calluses using scanning electron microscopy and found that the differentiation characteristics of the calluses were hugely variable and that different surface structures appeared to affect bud differentiation efficiency. The efficient and rapid system for regenerating peonies using petal cultures established here will create new opportunities for the mass reproduction and genetic engineering of peony plants.
1. Introduction
The flower of the tree peony (Paeonia × suffruticosa Andr.) is a traditional Chinese flower. It is valued for its rich color, large size, exquisiteness, strong fragrance, and ornamental qualities, is widely used as a potted or cut flower, and the essential oil made from its petal components has medicinal properties, such as anti-cancer, anti-inflammatory, and anti-oxidation activities [1,2,3]. In China, the peony has been used in gardens for more than 1500 years [4,5], and, more recently, the seeds from some peony cultivars have been identified as excellent sources of α-linolenic acid, so peony seeds can be used to produce plant oil [6]. Therefore, peony appears to have broad and sustainable future market potential [7].
Traditional methods for breeding peony suffer from a low reproduction rate, slow propagation, and an extended growth cycle [8,9], which severely restrict the development of peony breeding and reproduction. Plant tissue culture is a fast and effective method for vegetative propagation of peony. Such technology has a high propagation coefficient and short cycle, and the obtained tissue culture seedlings often maintain the excellent characteristics of their parents. This method has been widely used for rapid plant propagation, plant detoxification, the selection of new varieties, and the production of artificial seeds and medicinal ingredients, resulting in the establishment of prerequisites regarding the genetic engineering of plants [10]. Therefore, the use of tissue culture technology to accelerate the breeding and reproduction processes of peony is of great significance for meeting the demand for peony seedlings and for producing and improving the quality of seedlings.
Currently, the regeneration of various peony plant tissues (e.g., underground buds, leaves, stems, petals, and flowers) has already been reported [10,11,12]. For example, there are some reports describing the use of in vitro culture and rescue of immature embryos of herbaceous peonies to induce plant regeneration [13]. He et al. [14] used two kinds of ovules and young embryos as materials to cultivate an isolated culture. After 3–4 months, the seedling rate was less than 13.5%. Xu et al. [15] used embryos of the peony as explants, and an efficient and convenient method for the direct production of seedlings from a peony embryo culture was established; the highest rate obtained for seedling production was 66.34%, with variable results across varieties. Many compositions of peony growth medium have also been tested using various explants [16]. Nevertheless, the most effective regeneration system for peony is still not adequate for production, and few reports have described attempts to develop regeneration systems for peony flower petals. Flower petals, as a kind of explant, have the characteristics of being less susceptible to viruses, allowing materials to be easily obtained, disinfected, and propagated with a high reproduction coefficient [17]. Therefore, this study investigated the effects of different plant growth regulator (PGR) combinations on the induction of calluses and the proliferation and plant regeneration of peony petals, with the hope of providing a foundation for the establishment of an efficient peony regeneration system that meets the requirements for production.
2. Materials and Methods
2.1. Plant Materials
Healthy 4-year-old peony plants of the type ‘Fengdanbai’ were grown in a peony nursery. Young flower petals were cut from the unfolded peony buds as explants. The unfolded buds were rinsed under running tap water for 30 min then wiped with a brush and distilled water before being transferred to a clean beaker for further sterilization. The buds were dipped in 70% ethanol for 8–10 s and then rinsed 3–5 times with sterile distilled water, followed by 20 min of sterilization with 2% NaClO; finally, the unfolded buds were rinsed 3 times with sterile distilled water. After the sterilization, all of the petals were cut from the unfolded buds and used for tissue culture inoculation.
2.2. Callus Induction and Proliferation
Prepared petals were cut into 4 mm2 samples using tweezers and a sharp scalpel before being inoculated onto a Petri dish with a diameter of 120 mm containing Murashige and Skoog (MS) [18] medium supplemented with 2,4-dichlorophenoxyacetic acid (2,4-D), 1-naphthaleneacetic acid (NAA), and N-(phenylmethyl)-9H-purin-6-amine (6-BA). To achieve an orthogonal experimental design, 9 groups were formed for growth regulators combined in different concentrations (1.0, 2.0, or 3.0 mg/L 2,4-D; 1.0, 1.5, or 2.0 mg/L 6-BA; and 0.1, 0.2, or 0.3 mg/L NAA), and the callus induction rates under different concentrations of growth regulators were determined. The control was MS medium without plant growth regulators (PGRs).
Healthy calluses (no obvious browning or vitrification; not too tight, loose, or granular) were selected for callus proliferation on the MS supplemented with different combinations of PGRs (0.1, 0.2, and 0.3 mg/L NAA; 1.0, 2.0, and 3.0 mg/L 6-BA). The control was the MS medium without PGRs. After about 30 days, the callus increment and proliferation rate were calculated with the following formulas, respectively: callus increment (g) = total weight of total callus generated on proliferation medium after 30 days—total weight of callus when transferred to proliferation medium; callus proliferation rate (%) = total weight of total callus generated on proliferation medium after 30 days/total weight of callus when transferred to proliferation medium × 100%. Thirty biological replicates were prepared for each group, with four explants in each replicate.
All of the above media contained 0.7% agar (Sigma, St. Louis, MO, USA) and 30 g/L sucrose, with the pH adjusted to 5.7, and they were autoclaved at 121 °C for 15 min. The culture flasks were placed in the dark for 10 days and then maintained under 14 h/10 h light/dark cycles using 30–50 μmol/m2/s of fluorescent light at a temperature of 24 (±2) °C.
2.3. Shoot Differentiation
Compact calluses (yellow compact granular) were sectioned into small pieces (0.5–0.75 cm) and transferred onto regeneration media to induce shoot regeneration. The differentiation media contained MS, 0.7% agar (Sigma, St. Louis, MO, USA), and 30 g/L sucrose supplemented with zeatin (ZT) at 0, 0.1, 0.5, or 1.0 mg/L and 6-BA at 0, 1.0, 2.0, or 3.0 mg/L. The pH of media was adjusted to 5.7, and they were autoclaved at 121 °C for 15 min. The MS medium without PGRs was used as a control. The callus differentiation and plant growth were carried out under the conditions of 14 h/10 h light/dark cycles using 30–50 μmol/m2/s of direct fluorescent light; the culture temperature was 25 ± 2 °C. The shoot differentiation rates of calluses in different treatment combinations with the differentiation media were calculated with the following formula: shoot differentiation rate (%) = number of shoots induced/number of calluses × 100%. Thirty biological replicates were prepared for each group, with four explants in each replicate.
2.4. Root Formation and Transplantation
Healthy 4–5 cm long shoots (full green, vigorous growth, unfurled leaves) were selected and cultured for rooting on MS, woody plant (WPM) [19], 1/2 MS, or 1/4MS media supplemented with different concentrations of NAA (0, 0.05, 0.1, 0.2 mg/L), IBA (0, 0.05, 0.1, 0.2 mg/L), and sucrose (10, 15, 20, 30 g/L). The pH of the media was adjusted to 5.7, and they were autoclaved at 121 °C for 15 min. The control was WPM medium without PGRs. After about 30 days of aseptic tissue culture seedling root growth, the plants with robust growth were selected for the transplanting test. The lids of the flasks were opened and after allowing the tissue culture seedlings to domesticate for 3–7 days they were removed from the flasks and rinsed with sterile water 3 to 5 times. The agar was removed from the roots and the plants were transplanted into a culture cup filled with vermiculite. At the beginning, a protective film was placed on the culture cup, and the survival rate was calculated after 20 days. Thirty shoots for each treatment combination were selected as biological replicates.
2.5. Observation of Callus Morphology
The calluses with different morphologies at the stage of shoot differentiation were used as observation material. Firstly, the calluses were fixed with 2.5% buffered glutaraldehyde, washed with 0.05 mol/L potassium phosphate buffer (pH 6.8), stepwise dehydrated with a series of solutions of increasing ethanol concentration (30–100%), dried to the critical point in liquid CO2, and coated with palladium. Finally, the callus morphology was observed using a JEOL6100 scanning electron microscope (JEOL, TKY, JPN) according to the method of Guan et al. [18].
2.6. Data Analysis
Analysis of variance was performed using the Statistical Analysis Program (SPSS 20.0); the means were compared using Duncan’s multiple range tests (p < 0.01).
3. Results
3.1. Effects of Plant Growth Regulators (PGRs) on Callus Induction
Prepared petals were cut into 4 mm2 samples and inoculated onto a Petri dish containing MS medium supplemented with PGRs (NAA, 2,4-D, and 6-BA) (Table 1). All combinations of the three PGRs induced callus formation. Among them, the formation rate of callus in treatment 5 explants reached 98.5% (Table 1). The results of the orthogonal test were consistent in confirming that treatment 5 was the combination of media with the highest rates of callus formation and was MS supplemented with 2.0 mg/L 2,4-D, 1.5 mg/L 6-BA, and 0.3 mg/L NAA. In most cases, callus regeneration occurred at the edges of the young peony petals. We found that after the explants had been inoculated into the medium for about 10 days, the petals began to turn green. After 20 days in culture, white granular calluses began to appear at the ends of the wound surfaces (Figure 1A). After 30 days in culture, the calluses began to grow (Figure 1B).

Table 1.
Effects of different combinations of plant growth regulators (PGRs) on callus induction rate and characteristics of peony petals 30 days after explant inoculation.
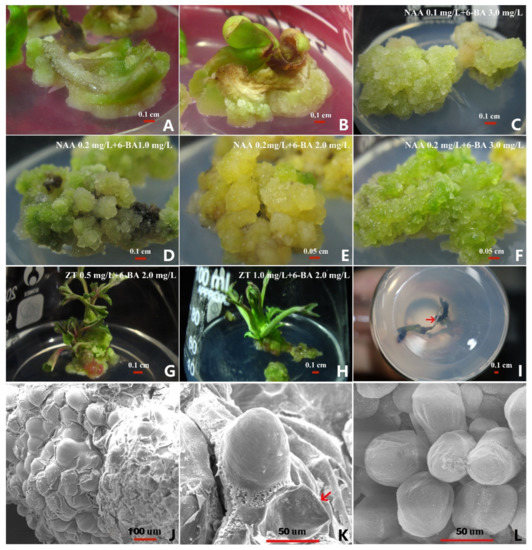
Figure 1.
(A) A callus from petals (white, compact, granular). (B) A growing callus (white, compact, granular). (C–F) The proliferation of calluses on different media (the characteristics of the calluses were light-yellow, loose, granular; yellow-green, loose, granular; yellow, compact, clumpy; light-green, loose, granular). (G,H) Shoot formation from calluses on different media. (I) Root formation of plants (the red arrows represent the growing root). (J,K) The surface of a callus on a medium of MS + 1.0 mg/L 6-BA (the protrusions had a loose arrangement and the bottom of the protrusions was cracked; the red arrow indicates the concave protrusion surface). (L) The surface of a callus on a medium of MS + 0.5 mg/L ZT + 2.0 mg/L 6-BA (the protrusions were closely arranged).
3.2. Effects of PGRs on Callus Proliferation and Shoot Formation
Healthy calluses were selected for the assessment of callus proliferation on MS supplemented with plant growth regulators (Table 2). All the calluses could proliferate on the media; different media showed different proliferation rates. Among the different treatment combinations, the highest proliferation rates were those of treatments 3 to 6, at 203, 207, 205, and 234%, respectively (Figure 1C–F). Treatment 9 had the lowest proliferation rate. It can be seen that the MS medium with 0.2 mg/L NAA and 3.0 mg/L 6-BA (treatment 6) had the highest overall rate and can be considered the best for callus proliferation (Figure 1F). The highest and lowest callus proliferation rates were 234 and 165%, respectively.

Table 2.
Effects of different concentrations of growth regulators on callus proliferation 30 days after being transferred to the proliferation medium.
Healthy calluses were selected for shoot induction and transferred to the differentiation media (Table 3). After 4 weeks, calluses with multiple shoot buds were subcultured. As shown in Table 3, the induction effect of the combination of two PGRs was better than that of a single PGR, and when the concentration of 6-BA was constant, the calluses treated with 0.5 mg/L ZT had the highest bud differentiation rate, which reached 34.8% (Figure 1G,H).

Table 3.
The effects of combinations of zeatin (ZT) and 6-BA on shoot differentiation from flower petals after 4 weeks.
3.3. Rooting Induction in Peony Plants
Healthy, 4–5 cm-long shoots were selected and cultured for rooting on media supplemented with various combinations of PGRs. The results showed that the average rooting rate with the 1/2 MS and WPM medium was higher than that with MS medium (Table 4). Treatment 8 (0.1 mg/L NAA, 0.05 mg/L IBA, and 30.0 g/L sucrose in 1/2 MS) had the highest rooting rate, reaching 23.3%, but the difference from treatments 3–7 was not significant (Figure 1I). It can be seen that the effect of using the 1/2 MS and WPM medium combined with PGRs to induce rooting in tissue-cultured peony microplants is better than that when using MS medium combined with PGRs.

Table 4.
Rooting rate of tissue-cultured peony microplants with different combinations of media.
3.4. Scanning Electron Microscope Observations of Peony Calluses
In order to reveal the corresponding changes in cells during the differentiation of peony calluses, we observed the surface structures of the calluses with the highest and lowest shoot differentiation rates (Table 3). In the treatment with the lowest shoot differentiation rate (MS + 1.0 mg/L 6-BA), the same piece of callus had two different structures: smooth surface protrusions and cracked protrusions (Figure 1J). There were relatively few protrusions, the bottom of the protrusions was cracked, and some cells around the protrusions were even concave (Figure 1K, red arrow). However, in the treatment with the highest shoot differentiation rate (MS + 0.5 mg/L ZT + 2.0 mg/L 6-BA), the surfaces of the calluses had many protrusions—significantly more than the treatment group with the lowest shoot differentiation rate—and these protrusions were tightly arranged; only a low proportion of the protrusions were sunken on the surface (Figure 1L).
4. Discussion
Tissue cultures can be used to effectively overcome the problems of the long cycle and low breeding rate encountered when using traditional peony breeding methods [19,20]. The selection of explants is the most important step in the process of plant tissue culture. Different explants have varying differentiation capabilities and induction results. There are many types of explants available for tissue cultures of peonies. At present, most research focuses on buds, stem tips, embryos, and leaves [21,22,23,24]. Compared with other explants, not only do petals have fewer viruses and a lower pollution rate but their use for tissue culture also has the characteristics of easy extraction, easy disinfection, and a high reproduction coefficient [25]. In this study, petals were used as explants, and the callus induction rate on the medium of MS + 2.0 mg/L 2,4-D+1.5 mg/L 6-BA + 0.3 mg/L NAA could reach as high as 98.52%, which is higher than the induction rates with other explants, such as leaves, stems, and auxiliary buds [26]. For example, Li et al. [27] used stem and single bud stem as materials to study the optimal conditions for callus induction, and the highest callus induction rates only reached 66.7 and 62.5%, respectively. It can be seen that petals are ideal explants for inducing peony calluses. In addition, with the gradual proliferation of calluses, the original petals gradually turned brown, and the calluses became transparent and green under conditions of light (Figure 1A,B). The formation process is similar to that of other explant calluses [10]. It is worth mentioning that in our research we also found that the age of the used petals was also related to the formation of calluses. The more tender the petals, the easier it was to induce callus formation, which is consistent with the results of previous studies [28,29].
Peony callus differentiation has always been a bottleneck restricting the establishment of peony regeneration systems, and most peony calluses are difficult to differentiate. Du et al. [30] transferred ‘Fengdan’ filament calluses to a differentiation medium and only a low rate of differentiation was achieved; in another study, petiole callus was used to induce differentiation, and the differentiation rate was also low, most of the differentiation rates below 10% [16]. In this study, petal-induced calluses were used for shoot differentiation on a medium supplemented with different concentrations of 6-BA and ZT, and the highest differentiation rate we achieved was 34.81%. The results show (Table 3) that using either 6-BA or ZT alone can cause callus bud differentiation, but the differentiation rate with ZT alone is significantly higher than that with 6-BA alone, and the effect of adding both at the same time is the best. These results indicated that ZT could accelerate the division and differentiation of meristem in the presence of 6-BA, and similar results have been reported elsewhere. In the process of callus induction and shoot differentiation in Lewis iris, the best medium was MS + 1.0 mg/L 6-BA + 1.0 mg/L ZT, and the average differentiation rate was as high as 86.7% [3,31]. This shows that ZT and 6-BA can work together to accelerate the division and accelerate the differentiation of meristems. Some studies have also suggested that when using a variety of cytokinin combinations, including thidiazuron, forchlorfenuron, ZT, and kinetin, the frequency of bud organogenesis is very high [32]. Whether this would achieve better results in the study of peony shoot differentiation still needs further research.
In addition to the components of the media, the structural characteristics of calluses also significantly affect shoot differentiation efficiency [33,34,35,36]. In this study, the surface structure of the bud-differentiated calluses was visually observed using scanning electron microscopy. There was a big difference between the surface structures of the calluses with a poor bud differentiation rate and the calluses with a high differentiation rate (Figure 1J–L). There were two structures on the surfaces of the calluses with a low differentiation rate: smooth protrusions and cracks (Figure 1J), and the roots of the smooth protrusions generally appeared to be cracked (Figure 1K). These structures may greatly restrict the development of shoot primordia. Even if the culture conditions are appropriate, a high bud differentiation rate cannot be obtained. The surface structures of the calluses with a high differentiation rate presented completely smooth protrusions that were tightly arranged (Figure 1L). Therefore, when selecting calluses for further differentiation and culture, apart from removing the browned parts and choosing healthy calluses, calluses with different structures should also be treated separately. Selection of calluses with obvious protrusions that are densely arranged for further processing can greatly promote shoot differentiation efficiency [37,38]. This experiment provides a basis for further screening of suitable calluses for subculture. In addition, it was found in this study that although the differentiation rate of calluses transferred into differentiation medium was improved compared with previous studies, some of them still could not differentiate normally. During the development process, callus malformation and browning would occur and the ability to further differentiate would be lost. However, in callus that can differentiate into buds, there are many imbalanced phenomena of root and bud development; some can only differentiate into buds but cannot induce rooting when transferred into rooting medium and finally cannot form complete plantlets. This may be due to imbalance in the internal physiological state of tissues caused by excessive exogenous growth regulators, the lack of accumulation of key metabolites in the body and the early differentiation of buds, and ultimately the failure of plant morphogenesis [39,40].
5. Conclusions
In this study, the results show that the optimal medium for the induction of calluses using ‘Fengdanbai’ petals is MS supplemented with 2.0 mg/L 2,4-D, 1.5 mg/L 6-BA, and 0.3 mg/L NAA, whereby the induction rate reached 98.5%. The addition of NAA and 6-BA can promote the proliferation of calluses, and the best medium for callus proliferation was MS supplemented with 0.2 mg/L NAA and 3.0 mg/L BA; the highest proliferation rate was 234%, and the differentiation rate was 34.8%. The optimal medium for callus differentiation is MS supplemented with 2.0 mg/L 6-BA and 0.5 mg/L ZT. In screening to improve shoot differentiation efficiency, the calluses that should be selected are those with obvious protrusions on the surface structure that have a close arrangement which are associated with a high differentiation efficiency. The best combination of media found for the promotion of high rates of callus rooting was 1/2 MS supplemented with 0.1 mg/L NAA and 0.05 mg/L IBA.
Author Contributions
X.Z. and C.Y. (Chao Yu) planned and designed the research. X.C., H.Y., C.Y. (Chengyang Ye), W.J., Z.X., S.Y., H.W. and S.J. performed the experiments, conducted the fieldwork, and analyzed the data. X.Z. and X.C. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program of China, grant number 2019YFD1001500, and the Talent Project of Jiyang College of Zhejiang A&F University, grant numbers RQ2020B04, RQ1911B05.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All datasets generated for this study are included in the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, E.Q.; Wang, Z.Y.; Pang, J.J.; Ji, H.L.; Han, H. Thoughts on the development of my country’s peony industry. Jiangsu Agric. Sci. 2020, 48, 24–28. [Google Scholar]
- Li, T. The application status and development prospects of peony. Mod. Hortic. 2017, 16, 11. [Google Scholar]
- Zhu, X.T.; Wang, Y.; Wu, Q.; Zhang, J.L.; Zhu, K.Y. Efficient induction of callus and plant regeneration from Paeonia sufruticosa Andr. J. Nuclear Agric. Sci. 2015, 29, 56–62. [Google Scholar]
- Zhang, J.J.; Wang, L.S.; Shu, Q.Y.; Liu, Z.A.; Li, C.H.; Zhang, J.; Wei, X.L.; Tian, D.K. Comparison of anthocyanins in non-blotches and blotches of the petals of Xibei tree peony. Sci. Hortic. 2007, 114, 104–111. [Google Scholar] [CrossRef]
- Han, X.Y.; Wang, L.S.; Liu, Z.A.; Jan, D.R.; Shu, Q.Y. Characterization of sequence-related amplified polymorphism markers analysis of tree peony bud sports. Sci. Hortic. 2008, 115, 261–267. [Google Scholar] [CrossRef]
- Li, S.S.; Yuan, R.Y.; Chen, L.G.; Wang, L.S.; Hao, X.H.; Wang, L.J. Systematic qualitative and quantitative assessment of fatty acids in the seeds of 60 tree peony (Paeonia section Moutan DC.) cultivars by GC-MS. Food Chem. 2015, 173, 133–140. [Google Scholar] [CrossRef]
- Picerno, P.; Mencherini, T.; Sansone, F.; Del Gaudio, P.; Granata, I.; Porta, A. Screening of a polar extract of Paeonia rockii: Composition and antioxidant and antifungal activities. J. Ethnopharmacol. 2011, 138, 705–712. [Google Scholar] [CrossRef]
- Hall, A.J.; Catley, J.L.; Walton, E.F. The effect of forcing temperature on peony shoot and flower development. Sci. Hortic. 2007, 113, 188–195. [Google Scholar] [CrossRef]
- Silva Jaime, A.; Teixeira, D.A.; Shen, M.; Yu, X.N. Tissue culture and micropropagation of tree peony (Paeonia suffruticosa Andr.). J. Crop. Sci. Biotechnol. 2012, 15, 159–168. [Google Scholar] [CrossRef]
- Zhu, X.T.; Li, X.Q.; Ding, W.J.; Jin, S.H.; Wang, Y. Callus induction and plant regeneration from leaves of peony. Hortic. Environ. Biotechnol. 2018, 59, 575–582. [Google Scholar] [CrossRef]
- Gabryszewska, E. Regeneration and growth of peony (Paeonia spp.) in vitro. Acta Agrobot. 2013, 57, 5–19. [Google Scholar] [CrossRef][Green Version]
- Ding, Y.; He, S.; Silva, J.A.T.D.; Li, G.; Tanaka, M. Effects of a new light source (cold cathode fluorescent lamps) on the growth of tree peony plantlets in vitro. Sci. Hortic. 2010, 125, 167–169. [Google Scholar] [CrossRef]
- Zhao, D.Q.; Xue, Y.F.; Shi, M.; Tao, J. Rescue and in vitro, culture of herbaceous peony immature embryos by organogenesis. Sci. Hortic. 2017, 217, 123–129. [Google Scholar] [CrossRef]
- He, G.M.; Cheng, F.Y.; Li, P. Preliminary studies on culture in vitro of ovule and immature embryo of two tree-peony cultivars. Acta Hortic. Sin. 2006, 33, 185. [Google Scholar]
- Xu, L.; Cheng, F.Y.; Zhong, Y. Study on rapid seedling-raising technology of tree peony embryo culture. Bull. Bot. Res. 2017, 37, 690–699. [Google Scholar]
- Wang, J.E.; Gong, Z.H.; Li, X.F. Optimization on techniques of callus induction and differentiation of Paeonia suffruticosa. Acta Agric. Boreali-Occident. Sin. 2008, 17, 282–286. [Google Scholar]
- Wei, M.M.; Wang, J.M.; Muhammad, I.; Hong, B. In vitro culture and plant regeneration of chimeric petals of chrysanthemum flower color. J. Beijing For. Univ. 2014, 36, 107–112. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- McCown, B.H. From gene manipulation to forest establishment: Shoot cultures of woody plants can be a central tool. Tappi J. 1985, 68, 116–119. [Google Scholar]
- Guan, Z.J.; Guo, B.; Huo, Y.L.; Guan, Z.P.; Dai, J.K.; Wei, Y.H. Short communication: Organogenesis and somatic embryogenesis in callus derived from hbsag-transgenic tomato mutant. Can. J. Plant Sci. 2012, 92, 747–756. [Google Scholar] [CrossRef]
- Yin, L.Q.; Tian, L.; Chen, M.M.; Zhang, Y.C.; Sun, Y.; Cai, Y.M. Research advance of Paeonia suffruticosa embryo culture. Mol. Plant Breed. 2019, 17, 3016–3023. [Google Scholar]
- Lian, X.F.; Li, Y.Y.; Zhang, W.Q.; Guo, L.L.; Zhang, Y.F.; Hou, X.G. Establishment of embryo culture system of Fengdan peony and analysis of methylation variation of first generation malformed seedlings. Henan Agric. Sci. 2020, 550, 116–125. [Google Scholar]
- Jia, W.Q.; Liu, H. Micropropagation of dwarf tree peony from lateral buds. J. Appl. Sci. 2014, 14, 2189–2193. [Google Scholar] [CrossRef][Green Version]
- Chen, Y.P.; Ding, L.; Zhao, M.G. Induction of healing with different explants of purple spotted tree Paeonia research on wounded tissue. J. Northwest Norm. Univ. 2001, 37, 66–69. [Google Scholar]
- Zhu, X.T.; Wang, Y.; Peng, Z.H. Effect of flower buds size of Paeonia suffruticosa on induction rate of anther tissue culture. Hunan Agric. Sci. 2010, 6, 102–104. [Google Scholar]
- Liu, H.C.; Jia, W.Q.; Xu, X.B. Adventitious bud regeneration of 6 peony varieties research on force difference. Guangdong Agric. Sci. 2011, 38, 35–36. [Google Scholar]
- Li, B.P.; Liu, X.L.; Fan, X.F. Peony “Yujiyanli” stem tip culture. J. For. Sci. Technol. 2017, 4, 63–67. [Google Scholar]
- Wen, S.S.; Chen, L.; Tian, R.N. Micropropagation of tree peony (Paeonia sect. Moutan): A review. Plant Cell Tissue Organ Cult. 2020, 141, 1–14. [Google Scholar] [CrossRef]
- Shi, X.L.; He, S.L.; Jia, W.Q. Preliminary study on rapid propagation technique of terminal bud of Peony “Dahuhong”. J. Henan Inst. Sci. Technol. 2021, 49, 1–5. [Google Scholar]
- Mao, H.Y.; Li, X.H.; Liu, Z.G. Tissue culture of young petals of ground cover chrysanthemum. J. Shenyang Agric. Univ. 2005, 1, 68–71. [Google Scholar]
- George, E.F.; Hall, M.A.; De Klerk, G.J. Plant Propagation by Tissue Culture: Vol. 1. The Background Hardcover; Springer: Dordecht, The Netherlands, 2007. [Google Scholar]
- Du, Y.M.; Zhong, Y.; Shang, H.Q.; Cheng, F.Y. Callus induction and differentiation from the filament of Paeonia ostii “Fengdan”. Plant Res. 2020, 40, 514–522. [Google Scholar]
- Wu, Y.Y.; Mao, J.P.; Zhou, Q.Q. Callus induction and bud differentiation in Louisiana iris tissue culture. Zhejiang Agric. Sci. 2009, 1, 86–89. [Google Scholar]
- Du, Y.M.; Cheng, F.Y.; Zhong, Y. Induction of direct somatic embryogenesis and shoot organogenesis and histological study in tree peony (Paeonia sect. Moutan). Plant Cell Tissue Organ Cult. 2020, 141, 557–570. [Google Scholar] [CrossRef]
- Zhu, X.T.; Wang, Y.; Peng, Z.H. Observation of peony callus by scanning electron microscope. For. Sci. Res. 2011, 24, 609–612. [Google Scholar]
- Jing, R.Y.; Huo, K.; Li, Z.H. Study on the difference between embryogenic and non-embryogenic callus of Cinnamomum camphora. J. Cent. South Univ. For. Technol. 2020, 40, 70–78. [Google Scholar]
- Zhao, D.C.; Liu, B.H.; Shu, X.G. Scanning electron microscope observation of budding callus formation in early-fruiting walnut. Econ. For. Res. 2019, 37, 52–56. [Google Scholar]
- Nakamura, T.; Maeda, E. A scanning electron microscope study on Japonica type rice callus cultures, with emphasis on plantlet initiation. Jpn. J. Crop. Sci. 2008, 58, 395–403. [Google Scholar] [CrossRef]
- Cui, K.R.; Dai, R.L. Molecular Biology of Plant Somatic Embryogenesis; Science Press: Beijing, China, 2000; pp. 48–57. [Google Scholar]
- Liu, B.J.; Liu, G.F. Studies on Embryogenic Callus Induction and Plant Regeneration of Anthurium andreanum. J. Trop. Subtrop. Bot. 2018, 26, 407–414. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).