Decay Incidence and Quality Changes of Film Packaged ‘Simeto’ Mandarins Treated with Sodium Bicarbonate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Treatments and Storage Conditions
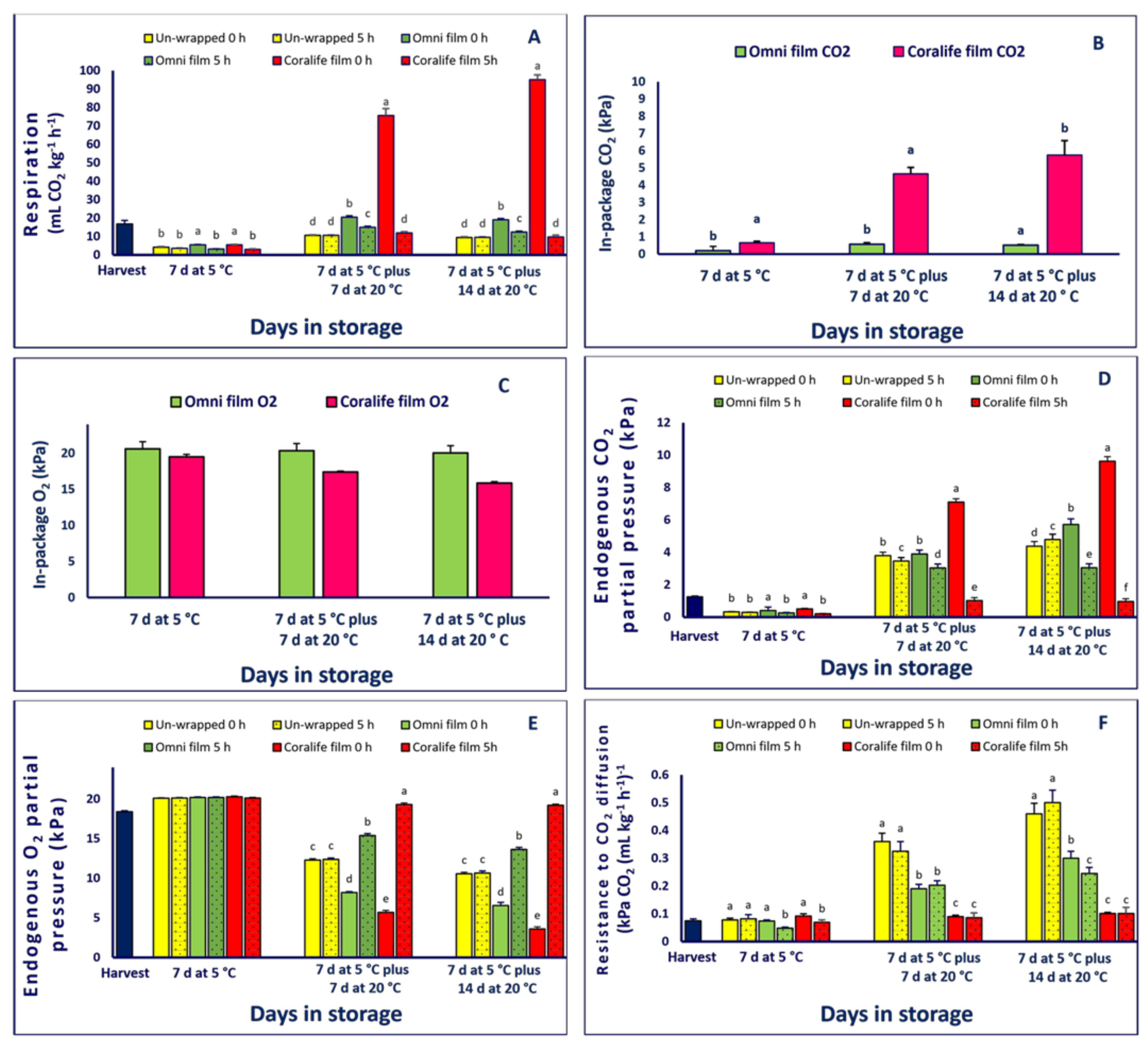
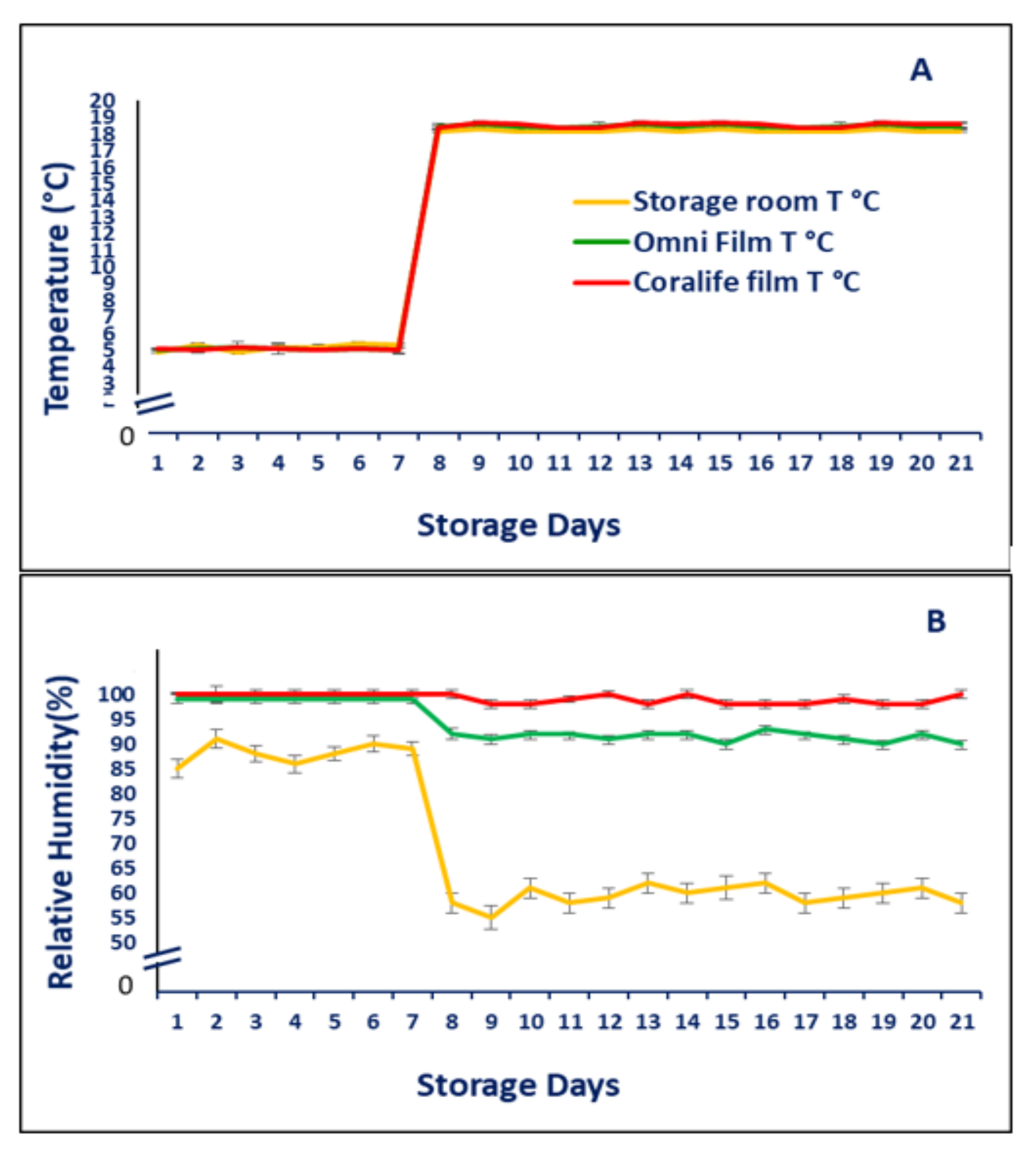
2.2. Respiratory Activity, in-Package CO2 and O2 Partial Pressure, O2epp and CO2epp, RCO2, in Package Temperature and Humidity
2.3. Decay, Weight Loss, Overall Appearance and Marketable Fruit
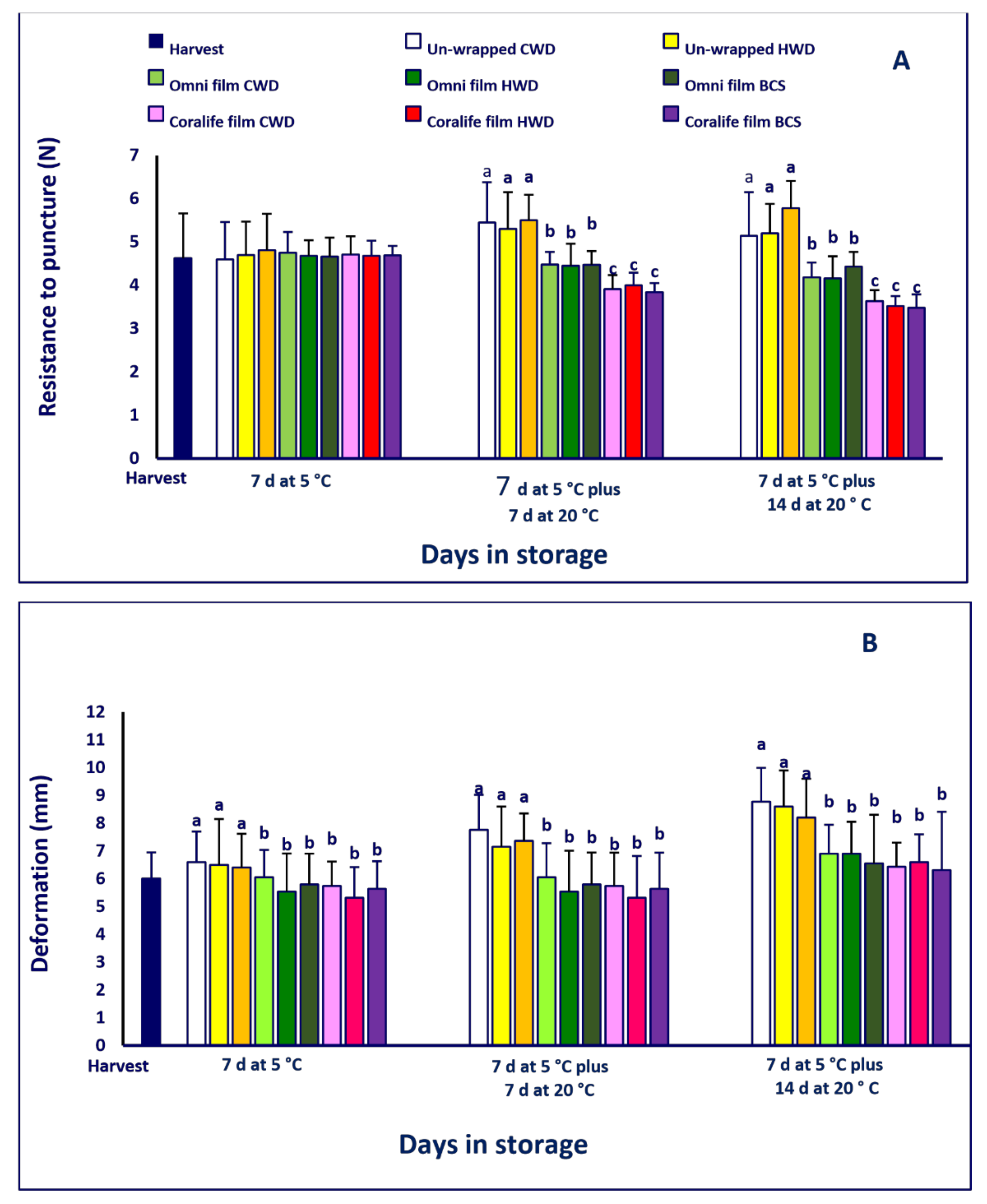
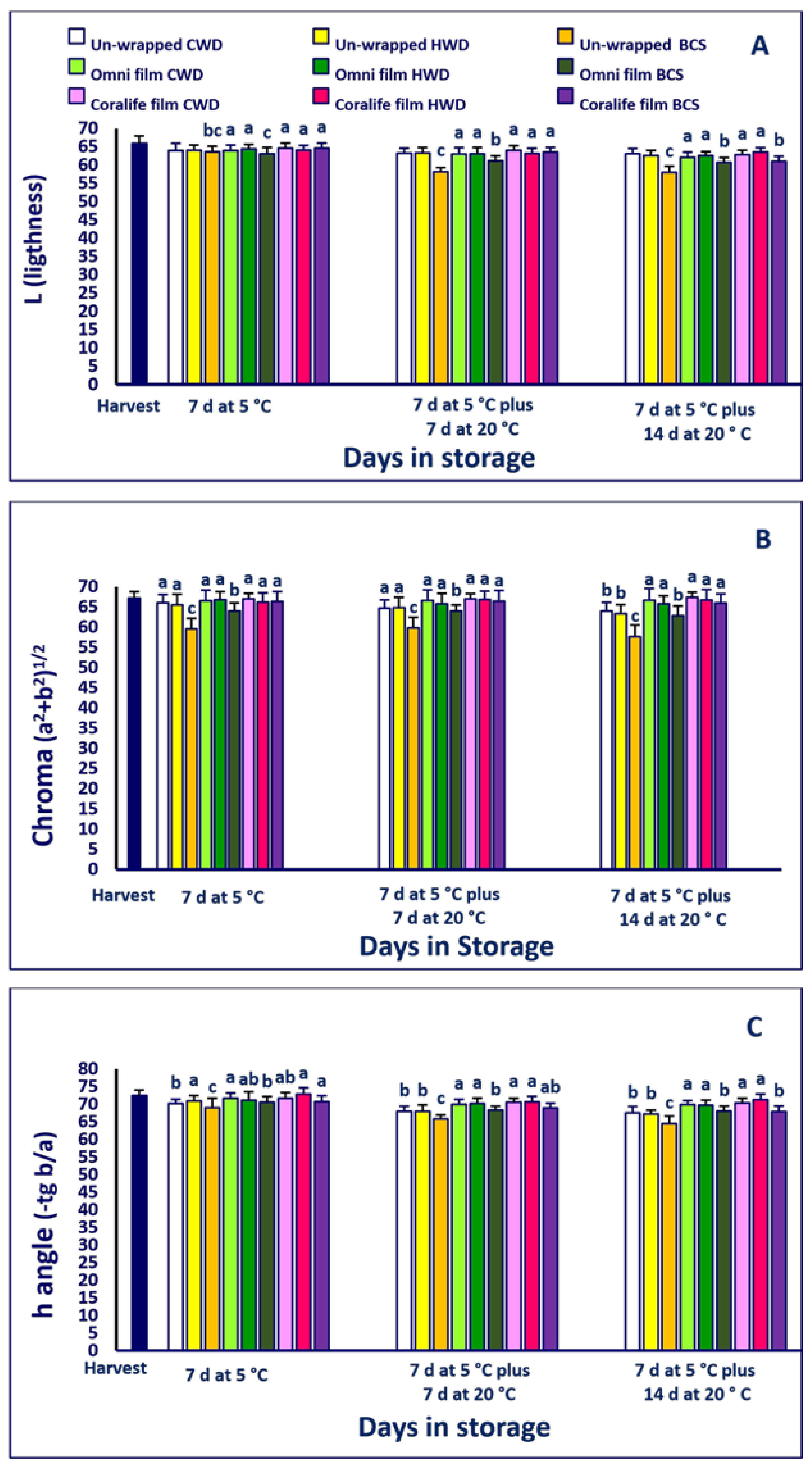
2.4. Firmness and Color
2.5. Chemical Analysis
2.6. Sensory Evaluation
2.7. Statistical Analysis
3. Results
3.1. Respiratory Activity, in-Package CO2 and O2 Partial Pressure, O2epp and CO2epp, RCO2, in Package Temperature and Humidity
3.2. Decay, Weight Loss, Overall Appearance and Marketable Fruit
3.3. Firmness and Color
3.4. Juice Chemical Composition
3.5. Sensory Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- D’Aquino, S.; Piga, A.; Agabbio, M.; McCollum, T.G. Film wrapping delays ageing of “Minneola” tangelos under shelf-life conditions. Postharvest Biol. Technol. 1998, 14, 107–116. [Google Scholar] [CrossRef]
- Strano, M.C.; Altieri, G.; Admane, N.; Genovese, F.; Di Renzo, G.C. Advance in Citrus Postharvest Management: Diseases, Cold Storage and Quality Evaluation. In Citrus Pathology; IntechOpen: London, UK, 2017; Volume 18, pp. 139–159. [Google Scholar] [CrossRef] [Green Version]
- Paul, V.; Pandey, R.; Srivastava, G.C. The fading distinctions between classical patterns of ripening in climacteric and non-climacteric fruit and the ubiquity of ethylene—An overview. J. Food Sci. Technol. 2012, 49, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joubert, J. Influence of Rind Water Content on Mandarin Citrus Fruit Quality. Ph.D. Thesis, Stellenbosch University, Stellenbosch, South Africa, 2016. [Google Scholar]
- Ahmad, M.S.; Siddiqui, M.W. Factors Affecting Postharvest Quality of Fresh Fruits. In Postharvest Quality Assurance of Fruits; Springer: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- D’Aquino, S.; Palma, A. Quality of film wrapped “Miyagawa” satsumas stored at 20–22 °C. Ital. J. Food Sci. 2004, 15, 553–568. [Google Scholar]
- D’Aquino, S.; Angioni, M.; Schirru, S.; Agabbio, M. Quality and physiological changes of film packaged “Malvasio” mandarins during long term storage. Food Sci. Technol. Lebensm.-Wiss.-Technol. 2001, 34, 206–214. [Google Scholar] [CrossRef]
- D’Aquino, S.; Suming, D.; Deng, Z.; Gentile, A.; Angioni, A.; De Pau, L.; Palma, A. A sequential treatment with sodium hypochlorite and a reduced dose of imazalil heated at 50 °C effectively control decay of individually film-wrapped lemons stored at 20 °C. Postharvest Biol. Technol. 2017, 124, 75–84. [Google Scholar] [CrossRef]
- D’Aquino, S.; Continella, A.; Gentile, A.; Dai, S.; Deng, Z.; Palma, A. Decay control and quality of individually film wrapped lemons treated with sodium carbonate. Food Control 2020, 108, 106878. [Google Scholar] [CrossRef]
- Choudhury, M.G.F.; Khan, M.H.H.; Miaruddin, M.; Rahman, M.M. Postharvest quality and shelf-life of lemon as affected by storage temperature and relative humidity. J. Agric. Eng. 2020, 43/AE, 1–8. [Google Scholar]
- Ben-Yehoshua, S.; Rodov, V. Transpiration and water stress. In Postharvest Physiology and Pathology of Vegetables; Bartz, J.A., Brecht, J.K., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2003; pp. 111–159. [Google Scholar]
- Mir, N.; Beaudry, R.M. Modified Atmosphere Packaging. In The Commercial Storage of Fruits, Vegetables, and Florist and Nursery Stocks, Agriculture Handbook; Gross, K.C., Wang, C.Y., Saltveit, M., Eds.; U.S. Department of Agriculture, Agricultural Research Service: Washington, DC, USA, 2016; Volume 66, pp. 42–53. Available online: http://ucanr.edu/datastoreFiles/234-2927.pdf (accessed on 18 December 2021).
- Toivonen, P.M.A.; Brandenburg, J.S.; Luo, Y. Modified atmosphere packaging for fresh-cut produce. In Modified and Controlled Atmopheres for the Storage, Transportation, and Packaging of Horticultural Commodities; Elhadi, M.Y., Ed.; CRC Press: Boca Raston, FL, USA, 2009; pp. 463–489. [Google Scholar]
- Soto-Muñoz, L.; Taberner, V.; de la Fuente, B.; Jerbi, N.; Palou, L. Curative activity of postharvest GRAS salt treatments to control citrus sour rot caused by Geotrichum citri-aurantii. Int. J. Food Microbiol. 2020, 335, 108860. [Google Scholar] [CrossRef]
- Palou, L. Postharvest treatments with GRAS salts to control fresh fruit decay. Horticulturae 2018, 4, 46. [Google Scholar] [CrossRef] [Green Version]
- Kassim, A.; Workneh, T.S.; Laing, M.D. A review of the postharvest characteristics and pre-packaging treatments of citrus fruit. AIMS Agric. Food 2020, 5, 337–364. [Google Scholar] [CrossRef]
- Kahramanoğlu, İ.; Nisar, M.F.; Chen, C.; Usanmaz, S.; Chen, J.; Wan, C. Light: An alternative method for physical control of postharvest rotting caused by fungi of citrus fruit. J. Food Qual. 2020, 2020, 8821346. [Google Scholar] [CrossRef]
- Zacarias, L.; Cronje, P.J.; Palou, L. Postharvest technology of citrus fruits. In The Genus Citrus; Woodhead Publishing: Thorston, UK, 2020; pp. 421–446. [Google Scholar]
- Schirra, M.; D’Aquino, S.; Cabras, P.; Angioni, A. Control of postharvest diseases of fruit by heat and fungicides: Efficacy, residue levels, and residue persistence. A Review. J. Agric. Food Chem. 2011, 59, 8531–8542. [Google Scholar] [CrossRef] [PubMed]
- D’Aquino, S.; Palma, A. Reducing or replacing conventional postharvest fungicides with low toxicity acids and salts. In Postharvest Pathology of Fresh Horticultural Produce; Palou, L., Smilanick, J.L., Eds.; CRC Press/Taylor and Francis Group: Boca Raton, FL, USA, 2020; pp. 595–632. [Google Scholar] [CrossRef]
- Strano, M.C.; Timpanaro, N.; Allegra, M.; Foti, P.; Pangallo, S.; Romeo, F.V. Effect of ozonated water combined with sodium bicarbonate on microbial load and shelf life of cold stored Clementine (Citrus clementina Hort. ex Tan.). Sci. Hortic. 2021, 276, 109775. [Google Scholar] [CrossRef]
- Timpanaro, N.; Strano, M.C.; Allegra, M.; Foti, P.; Granuzzo, G.; Carboni, C.; Romeo, F.V. Assessing the Effect of ozonated water on microbial load and quality of Nocellara Etnea Table Olives. Ozone Sci. Eng. 2021, 43, 571–578. [Google Scholar] [CrossRef]
- Ben-Yehoshua, S.; Porat, R. Heat treatments to reduce decay. In Environmentally Friendly Technologies for Agricultural Produce Quality; Ben-Yehoshua, S., Ed.; CRC Press/Taylor & Francis Group: Boca Raton, FL, USA, 2005; pp. 11–42. [Google Scholar]
- Smilanick, J.L.; Margosan, D.A.; Mlikota Gabler, F.; Usall, J.; Michael, I.F. Control of citrus green mold by carbonate and bicarbonate salts and the influence of commercial postharvest practices on their efficacy. Plant Dis. 1999, 83, 139–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schirra, M.; D’Aquino, S.; Palma, A.; Angioni, A.; Cabras, P. Factors affecting the synergy of thiabendazole, sodium bicarbonate, and heat to control postharvest green mold of citrus fruit. J. Agric. Food Chem. 2008, 56, 10793–10798. [Google Scholar] [CrossRef]
- Trout, S.A.; Hall, E.G.; Robertson, R.N.; Hackney, F.M.V.; Sykes, S.M. Studies in the metabolism in apples. 1, Preliminary investigation on internal gas composition and its relation to changes in stored ‘Granny Smith’ apples. Austral. J. Expt. Biol. Med. Sci. 1942, 20, 219–223. [Google Scholar] [CrossRef]
- McLaren, K. Food Colorimetry. In Developments in Food Colors; Walford, J.C., Ed.; Applied Science Publishers: London, UK, 1980; Volume 1, pp. 27–45. [Google Scholar]
- Yuan, J.P.; Chen, F. Simultaneous separation and determination of sugars, ascorbic acid and furanic compounds by HPLC-dual detection. Food Chem. 1999, 64, 423–427. [Google Scholar] [CrossRef]
- Chinnici, F.; Spinabell, U.; Riponi, C.; Amati, A. Optimization of the determination of organic acids and sugars in fruit juices by ion-exclusion liquid chromatography. J. Food Compos. Anal. 2005, 18, 121–131. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic–phosphotungstic acid reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar]
- Bonded, V.; Brand-Williams, W.; Berset, C. Kinetics and mechanism of antioxidant activity using the DPPH free radical method. Lebensm. Wiss. Technol. 1997, 30, 609–615. [Google Scholar] [CrossRef]
- Schirra, M.; Mulas, M.; Fadda, A.; Cauli, E. Cold quarantine responses of blood oranges to postharvest hot water and hot air treatments. Postharvest Biol. Technol. 2004, 31, 191–200. [Google Scholar] [CrossRef]
- Ben-Yehoshua, S.; Cameron, A. Exchange determination of water vapor, carbon dioxide, oxygen, ethylene and other gases of fruits and vegetables. In Gases in Plant and Microbial Cells; Linskens, H.F., Jackson, J.F., Eds.; Modern Methods of Plant Analysis, New Series 9; Springer: New York, NY, USA, 1989; pp. 178–193. [Google Scholar]
- Hagenmeier, R.; Shaw, P. Permeability of shellac coatings to gases and water vapor. J. Agric. Food Chem. 1991, 39, 825–829. [Google Scholar] [CrossRef]
- Kader, A.A.; Saltveit, M.E. Respiration and gas exchange. In Postharvest Physiology and Pathology of Vegetables; Bartz, J.A., Brecht, J.K., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2003; pp. 1–29. [Google Scholar]
- Ben-Yehoshua, S.; Burg, S.P.; Young, R. Resistance of citrus fruit to mass transport of water vapour and other gases. Plant Physiol. 1985, 79, 1048. [Google Scholar] [CrossRef] [Green Version]
- Piga, A.; D’Aquino, S.; Agabbio, M. Evolution of respiration rate, internal CO2 or O2 and resistance to gas diffusion of anaerobic exposed and waxed ‘Miho’ satsuma fruit during market life. Adv. Hortic. Sci. 1998, 12, 132–137. [Google Scholar]
- D’Aquino, S.; Palma, A.; Agabbio, M. Evolution of physiological and qualitative parameters in ‘Okitsu’ satsumas stored at 20 °C under different hygrometric conditions. Acta Hortic. 2003, 599, 737–743. [Google Scholar] [CrossRef]
- Davis, P.L.; Chase, W.G.; Cubbedge, R.H. Factors affecting internal oxygen and carbon dioxide concentration of citrus fruit. HortScience 1967, 2, 168169. [Google Scholar]
- Ben-Yehoshua, S.; Kobiler, I.; Shapiro, B. Some physiological effect of delaying deterioration of citrus fruits by individual seal packaging in high density polyethylene film. J. Am. Soc. Hortic. Sci. 1979, 104, 868–872. [Google Scholar]
- Purvis, A.C. Effects of film thickness and storage temperature on water loss and internal quality of seal-packaged grapefruit. J. Am. Soc. Hortic. Sci. 1983, 108, 562. [Google Scholar]
- Palou, L. Penicillium digitatum, Penicillium italicum (Green mold, blue mold). In Postharvest Decay Control Strategy; Silvia, B.B., Ed.; Academic Press: London, UK, 2014; pp. 45–1201. [Google Scholar]
- Plaza, P.; Usall, J.; Teixidó, N.; Viñas, I. Effect of water activity and temperature on competitive abilities of common postharvest citrus fungi. Int. J. Food Microbiol. 2004, 90, 75–82. [Google Scholar] [CrossRef]
- Smilanick, J.L.; Brown, G.E.; Eckert, J.W. The biology and control of postharvest diseases. In Fresh Citrus Fruits; Wardowski, W.F., Miller, W.M., Hall, D.J., Grierson, W., Eds.; Florida Science Source: Ocala, FL, USA, 2006; pp. 339–396. [Google Scholar]
- Palou, L.; Smilanick, J.L.; Usall, J.; Viñas, I. Control of postharvest blue and green molds of oranges by hot water, sodium carbonate, and sodium bicarbonate. Plant Dis. 2001, 85, 371–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palou, L.; Usall, J.; Muñoz, J.A.; Smilanick, J.L.; Viñas, I. Hot water, sodium carbonate, and sodium bicarbonate for the control of postharvest green and blue molds of clementine mandarin. Postharvest Biol. Technol. 2002, 24, 93–96. [Google Scholar] [CrossRef]
- D’Aquino, S.; Angioni, A.; Suming, D.; Palma, A.; Schirra, M. Residue levels, persistance and effectiveness of imazalil against a resistant strain of Penicillium digitatum when applied in combination with heat and sodium bicarbonate. Commun. Appl. Biol. Sci. Ghent. Univ. 2013, 78, 139–149. [Google Scholar]
- Palou, L.; Smilanick, J.L.; Droby, S. Alternatives to conventional fungicides for the control of citrus postharvest green and blue moulds. Stewart Postharvest Rev. 2008, 4, 1–16. [Google Scholar] [CrossRef]
- Shewfelt, R.L. Measuring quality and maturity. In Postharvest Handling: A System Approach; Florkowski, W., Shewfelt, R., Prussia, S., Brueckner, B., Eds.; Elsevier: London, UK, 2014; pp. 387–410. [Google Scholar]
- Tietel, Z.; Plotto, A.; Fallik, E.; Lewinsohn, E.; Porat, R. Taste and aroma of fresh and stored mandarins. J. Sci. Food Agric. 2010, 91, 14–23. [Google Scholar] [CrossRef]
- Li, Y.; Golding, J.B.; Arcot, J.; Wills, R.B.H. Continuous exposure to ethylene in the storage environment adversely affects ‘Afourer’ mandarin fruit quality. Food Chem. 2018, 242, 585–590. [Google Scholar] [CrossRef]
- Hagenmaier, R.D. The flavor of mandarins hybrids with different coatings. Postharvest Biol. Technol. 2002, 24, 79–87. [Google Scholar] [CrossRef]
- Goldenberg, L.; Yaniv, Y.; Choi, H.J.; Doron-Faigenboin, A.; Carmi, N.; Porat, R. Elucidating the biochemical factors governing off-flavor perception in mandarins. Postharvest Biol. Technol. 2016, 120, 167–179. [Google Scholar] [CrossRef]
- Ummarat, N.; Arpaia, M.L.; Obenland, D. Physiological, biochemical and sensory characterization of the response to waxing and storage of two mandarin varieties differing in postharvest ethanol accumulation. Postharvest Biol. Technol. 2015, 109, 82–96. [Google Scholar] [CrossRef]
- Zou, Z.; Xi, W.; Hu, Y.; Nie, C.; Zhou, Z. Antioxidant activity of Citrus fruit. Food Chem. 2016, 196, 885–896. [Google Scholar] [CrossRef]
- Xu, G.; Liu, D.; Ma, Y.; Ye, Q. Phenolic Compounds and Bioactive Agents in Citrus Fruits. In Phytochemicals in Citrus—Applications in Functional Foods; Ye, X., Ed.; CRC Press: London, UK, 2018; pp. 169–210. [Google Scholar]
- Strano, M.C.; Di Silvestro, S.; Allegra, M.; Russo, G.; Caruso, M. Effect of cold storage on the postharvest quality of different Tarocco sweet orange clonal selections. Sci. Hortic. 2021, 285, 110167. [Google Scholar] [CrossRef]
- Baldwin, E.A.; Bai, J.; Plotto, A.; Ritenour, M.A. Citrus fruit quality assessment; producer and consumer perspectives. Stewart Postharvest Rev. 2014, 10, 408. [Google Scholar]
- Mditshwa, A.; Magwaza, L.S.; Tesfay, S.Z.; Opara, U.L. Postharvest factors affecting vitamin C content of citrus fruits: A review. Sci. Hortic. 2017, 218, 95–104. [Google Scholar] [CrossRef]
- Nagy, S. Vitamin C contents of citrus fruit and their products: A review. J. Agric. Food Chem. 1980, 28, 8–18. [Google Scholar] [CrossRef]
- Qiu, S.; Wang, J. Effects of storage temperature and time on internal quality of satsuma (Citrus unshiu marc.) by means of E-nose and E-tongue based on two-way MANOVA analysis and random forest. Innov. Food Sci. Emerg. Technol. 2015, 31, 139–150. [Google Scholar] [CrossRef]
- Baldwin, E.A.; Nisperos-Cariedo, M.O.; Shaw, P.E.; Burns, J.K. Effect of coatings and prolonged storage conditions on fresh orange flavor volatiles, degrees Brix, and ascorbic acid levels. J. Agric. Food Chem. 1995, 43, 1321–1331. [Google Scholar] [CrossRef]
- Bassal, M.; El-Hamahmy, M. Hot water dip and preconditioning treatments to reduce chilling injury and maintain postharvest quality of Navel and Valencia oranges during cold quarantine. Postharvest Biol. Technol. 2011, 60, 186–191. [Google Scholar] [CrossRef]
- Erkan, M.; Pekmezci, M.; Wang, C.Y. Hot water and curing treatments reduce chilling injury and maintain post-harvest quality of ‘Valencia’ oranges. Int. J. Food Sci. Technol. 2005, 40, 91–96. [Google Scholar] [CrossRef]
- Mohamed, M.A.A.; Abdel-Hafeez, A.A.; Mehaisen, S.M.A. Response of ‘Valencia’ orange and ‘Marsh’ seedless grapefruit to postharvest hot water dips and storage temperature. Ann. Agric. Sci. 2002, 40, 2247–2264. [Google Scholar]
- Yang, Y.T.; Song, S.C.; Kim, S.H.; Kim, J.Y.; Koh, J.S. Cold storage characteristics of early variety of Citrus unshius produced in Cheju with various treatments. J. Korean Soc. Agric. Chem. Biotechnol. 1997, 40, 117–122. [Google Scholar]
- Rapisarda, P.; Lo Bianco, M.; Pannuzzo, P.; Timpanaro, N. Effect of Cold Storage on Vitamin C, Phenolics and Antioxidant Activity of Five Orange Genotypes (Citrus sinensis (L.) Osbeck). Postharvest Biol. Technol. 2008, 49, 348–354. [Google Scholar] [CrossRef]

| Film | O2 Permeance 1 (μmol s−1 m−2 kPa−1) | CO2 Permeance (μmol s−1 m−2 kPa−1) | Water Vapor Transmission Rate (μg s−1 m−2) |
|---|---|---|---|
| Omni | 0.145 (ASTM D-1434) | 1.048 (ASTM 1434) | 8000 (ASTM E-96) |
| Coralene SWAF 400 | 0.011 (ASTM D-3985) | 0.044 2 | 81.2 (ASTM E-96) |
| Coralife SWAF 400 3 | 0.181 (ASTM D-3985) | 0.175 (COV-E68) | 83.4 (ASTM F 1249-90) |
| Film | Expected in-Package CO2 Partial Pressure (kPa) | Expected in-Package O2 Partial | ||
|---|---|---|---|---|
| Pressure (kPa) | ||||
| Storage at 5 °C | Storage at 20 °C | Storage at 5 °C | Storage at 20 °C | |
| Omni | 0.23 | 1.05 | 19.3 | 13.4 |
| Coralife SWAF 400 | 1.38 | 6.29 | 19.7 | 14.9 |
| Storage Period | pH | Citric Acid (g L−1) | TSS (%) | Vitamin C (mg L−1) | ||||
|---|---|---|---|---|---|---|---|---|
| Harvest | 3.46 ± 0.072 | 12.0 ± 0.017 | 11.63 ± 0.16 | 321.2 ± 6.9 | ||||
| 7 d at 5 °C | ||||||||
| Unwrapped | 3.53 ± 0.028 b 1 | 11.0 ± 0.29 a | 11.58 ± 0.20 b | 323 ± 2.4 a | ||||
| Omni film | 3.52 ± 0.012 b | 10.5 ± 0.20 b | 11.82 ± 0.13 a | 321 ± 3.2 a | ||||
| Coralife film | 3.64 ± 0.029 a | 9.40 ± 0.27 c | 11.27 ± 0.17 c | 324 ± 3.8 a | ||||
| 7 d at 5 °C plus 7 d at 20 °C | ||||||||
| Unwrapped | 3.63 ± 0.054 b | 10 ± 0.17 a | 11.98 ± 0.24 a | 326 ± 6.3 a | ||||
| Omni film | 3.61 ± 0.019 b | 9.6 ± 0.01 b | 11.67 ± 0.12 b | 319 ± 3.7 a | ||||
| Coralife film | 3.81 ± 0.016 a | 8.6 ± 0.15 c | 11.23 ± 0.17 c | 325 ± 4.4 a | ||||
| 7 d at 5 °C plus 14 d at 20 °C | ||||||||
| Unwrapped | 3.70 ± 0.055 b | 9.6 ± 0.39 a | 12.2 ± 0.21 a | 334 ± 11.1 a | ||||
| Omni film | 3.77 ± 0.037 b | 9.1 ± 0.37 b | 12.1 ± 0.24 a | 329 ± 9.2 a | ||||
| Coralife film | 3.89 ± 0.028 a | 7.8 ± 0.34 c | 11.2 ± 0.22 b | 322 ± 9.4 a | ||||
| A N O V A | ||||||||
| Source | df | F-ratio | F-ratio | F-ratio | F-ratio | |||
| Period (P) | 3 | 7.37 *** | 645 *** | 9.56 *** | 1.67 ns | |||
| Treatment (T) | 2 | 0.61 ns | 0.37 ns | 0.15 ns | 0.68 ns | |||
| Film (F) | 2 | 3.70 * | 0.182 *** | 72.5 *** | 0.43 ns | |||
| P × T | 6 | 0.68 ns | 0.38 ns | 0.96 ns | 0.14 ns | |||
| P × F | 6 | 0.63 ns | 20.8 *** | 14.2 *** | 0.16 ns | |||
| T × F | 4 | 0.53 ns | 0.38 ns | 0.17 ns | 0.11 ns | |||
| P × T × F | 12 | 0.52 ns | 0.12 ns | 0.18 ns | 0.28 ns | |||
| Storage Period | Total Phenols (mg L−1) | TEAC (mmol Trolox eq. L−1) | Ethanol (mg L−1) | Acetaldehyde (mg L−1) | |
|---|---|---|---|---|---|
| Harvest | 520.6 ± 19.7 | 25.2 ± 0.09 | 39.2 ± 2.53 | 2.8 ± 0.30 | |
| 7 d at 5 °C | |||||
| Unwrapped | 529.2 ± 26.6 a 1 | 24.8 ± 0.83 b | 42.8 ± 2.68 b | 3.23 ± 0.47 b | |
| Omni film | 526.6 ± 18.2 a | 25.4 ± 0.62 a,b | 42.3 ± 2.34 b | 3.63 ± 0.40 b | |
| Coralife film | 523.5 ± 22.7 a | 25.9 ± 0.76 a | 47.4 ± 5.59 a | 4.35 ± 0.70 a | |
| 7 d at 5 °C plus 7 d at 20 °C | |||||
| Unwrapped | 538.4 ± 27.2 a | 24.2 ± 0.54 a | 62.4 ± 11.5 b | 4.43 ± 0.77 b | |
| Omni film | 532.3±9.61 a | 24.8 ± 0.75 a | 66.0 ± 5.96 b | 4.35 ± 0.54 b | |
| Coralife film | 526.6±28.6 a | 23.6 ± 0.98 a | 75.2 ± 4.74 a | 7.78 ± 0.99 a | |
| 7 d at 5 °C plus 14 d at 20 °C | |||||
| Unwrapped | 568.7 ± 22.3 a | 23.9 ± 2.98 a | 80.8 ± 6.46 b | 7.58 ± 0.68 b | |
| Omni film | 565.4 ± 12.1 a | 22.1 ± 1.28 a | 69.3 ± 26.2 b | 6.90 ± 0.77 b | |
| Coralife film | 532.6 ± 32.2 a | 20.3 ± 2.12 a | 102.8 ± 11.4 a | 9.18 ± 1.21 a | |
| A N O V A | |||||
| Source | df | F-ratio | F-ratio | F-ratio | F-ratio |
| Period (P) | 3 | 15.9 *** | 18.1 *** | 114.6 *** | 236.1 *** |
| Treatment (T) | 2 | 0.01 ns | 0.10 ns | 0.16 ns | 0.82 ns |
| Film (F) | 2 | 0.44 ns | 0.58 ns | 13.9 *** | 51.2 *** |
| P × T | 6 | 0.01 ns | 0.10 ns | 0.86 ns | 0.16 ns |
| P × F | 6 | 0.09 ns | 1.04 ns | 5.11 ** | 11.8 *** |
| T × F | 4 | 0.003 ns | 0.03 ns | 0.66 ns | 0.28 ns |
| P × T × F | 12 | 0.002 ns | 0.12 ns | 0.42 ns | 0.11 ns |
| Storage Period | Crunchiness | Off-Flavor | Off-odor | Acceptability | |
|---|---|---|---|---|---|
| Harvest | 9 ± 0 | 1 ± 0 | 1 ± 0 | 9 ± 0 | |
| 7 d at 5 °C | |||||
| Unwrapped | 6.5 ± 0.74 c 1 | 2.2 ± 0.59 a | 1.3 ± 0.46 b | 5.9 ± 0.83 c | |
| Omni film | 7.2 ± 0.86 b | 1.5 ± 0.51 b | 1.8 ± 0.68 a | 7.0 ± 0.92 b | |
| Coralife film | 8.9 ± 0.26 a | 2.6 ± 0.63 a | 1.7 ± 0.46 a | 8.0 ± 0.65 a | |
| 7 d at 5 °C plus 7 d at 20 °C | |||||
| Unwrapped | 5.0 ± 0.76 c | 2.7 ± 0.88 a | 2.8 ± 0.68 b | 4.5 ± 1.40 c | |
| Omni film | 6.5 ± 0.51 b | 1.7 ± 0.46 b | 3.0 ± 0.76 b | 5.7 ± 0.61 b | |
| Coralife film | 8.5 ± 0.64 a | 2.8 ± 0.67 a | 3.9 ± 0.70 a | 7.6 ± 0.76 a | |
| 7 d at 5 °C plus 14 d at 20 °C | |||||
| Unwrapped | 1.9 ± 0.83 c | 4.3 ± 0.90 a | 3.2 ± 0.80 b | 2.9 ± 0.91 c | |
| Omni film | 5.3 ± 0.72 b | 2.8 ± 0.73 b | 3.8 ± 0.86 a | 5.4 ± 0.63 b | |
| Coralife film | 8.1 ± 0.70 a | 4.9 ± 0.88 a | 3.9 ± 0.80 a | 6.9 ± 0.91 a | |
| A N O V A | |||||
| Source | df | F-ratio | F-ratio | F-ratio | F-ratio |
| Period (P) | 3 | 116 *** | 131 *** | 184 *** | 239 *** |
| Treatment (T) | 2 | 0.72 ns | 0.27 ns | 0.99 ns | 0.91 ns |
| Film (F) | 2 | 394 *** | 37.8 *** | 14.3 *** | 113 *** |
| PxT | 6 | 0.12 ns | 0.17 ns | 1.08 ns | 0.64 ns |
| PxF | 6 | 24.3 *** | 6.00 *** | 3.52 ** | 16.2 *** |
| TxF | 4 | 0.24 ns | 0.56 ns | 0.77 ns | 0.21 ns |
| PxTxF | 12 | 0.65 ns | 1.19 ns | 0.86 ns | 0.80 ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Aquino, S.; Strano, M.C.; Gentile, A.; Palma, A. Decay Incidence and Quality Changes of Film Packaged ‘Simeto’ Mandarins Treated with Sodium Bicarbonate. Horticulturae 2022, 8, 354. https://doi.org/10.3390/horticulturae8050354
D’Aquino S, Strano MC, Gentile A, Palma A. Decay Incidence and Quality Changes of Film Packaged ‘Simeto’ Mandarins Treated with Sodium Bicarbonate. Horticulturae. 2022; 8(5):354. https://doi.org/10.3390/horticulturae8050354
Chicago/Turabian StyleD’Aquino, Salvatore, Maria Concetta Strano, Alessandra Gentile, and Amedeo Palma. 2022. "Decay Incidence and Quality Changes of Film Packaged ‘Simeto’ Mandarins Treated with Sodium Bicarbonate" Horticulturae 8, no. 5: 354. https://doi.org/10.3390/horticulturae8050354
APA StyleD’Aquino, S., Strano, M. C., Gentile, A., & Palma, A. (2022). Decay Incidence and Quality Changes of Film Packaged ‘Simeto’ Mandarins Treated with Sodium Bicarbonate. Horticulturae, 8(5), 354. https://doi.org/10.3390/horticulturae8050354






