Effect of Genotype and Harvest Date on Fruit Quality, Bioactive Compounds, and Antioxidant Capacity of Strawberry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental site and Plant Materials
2.2. Fruit Color and Firmness Assessment
2.3. Total Soluble Solid, Titratable Acid, and Ascorbic Acid Content Determination
2.4. Total Anthocyanin, Phenolic Content, and Antioxidant Capacity Assay
2.5. Amino Acid Content Measurement
2.6. Statistical Analysis
3. Results
3.1. Source of Variation (F Value) in Quality Traits of Strawberry
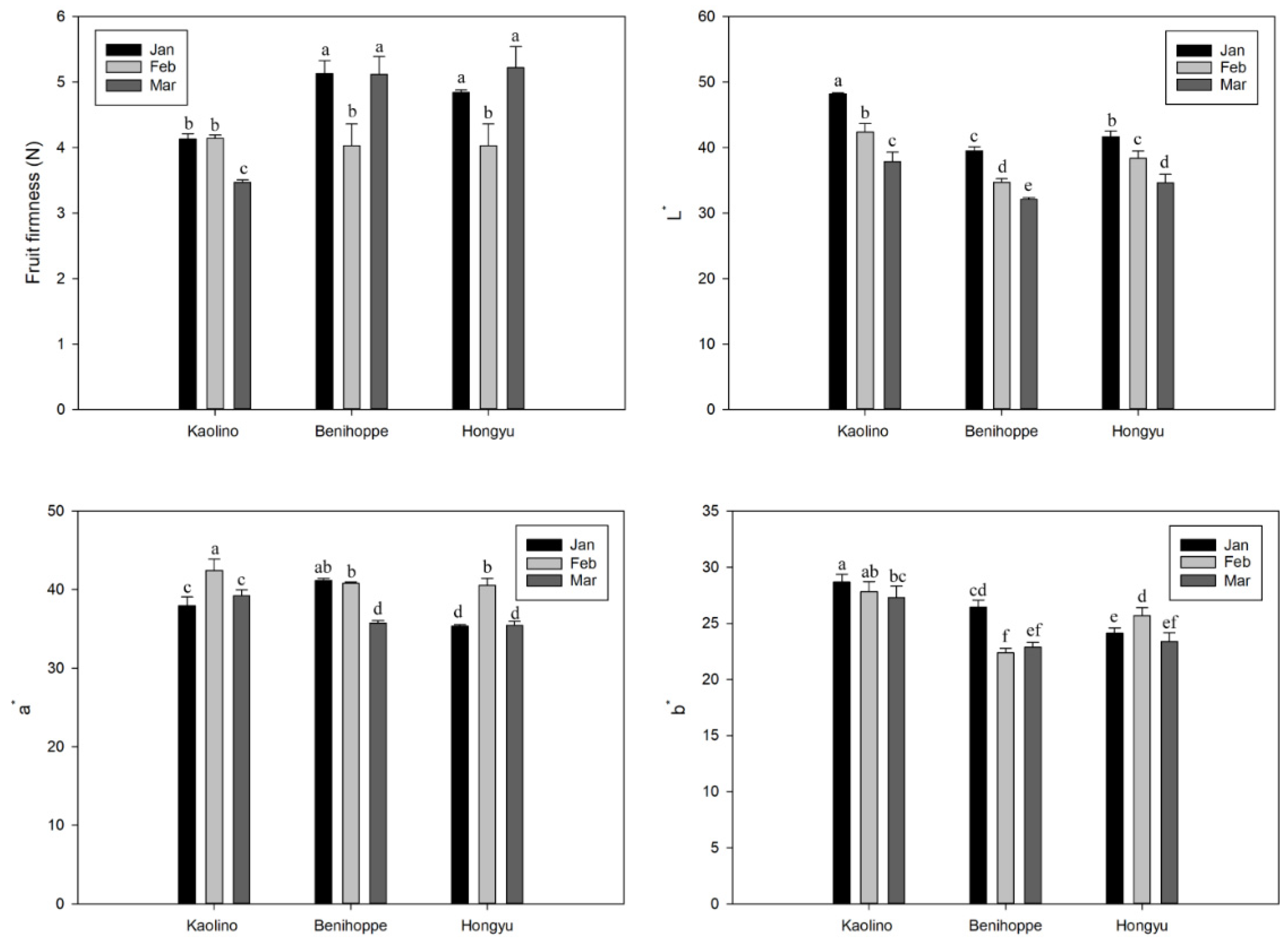
3.2. Fruit Firmness and Surface Color
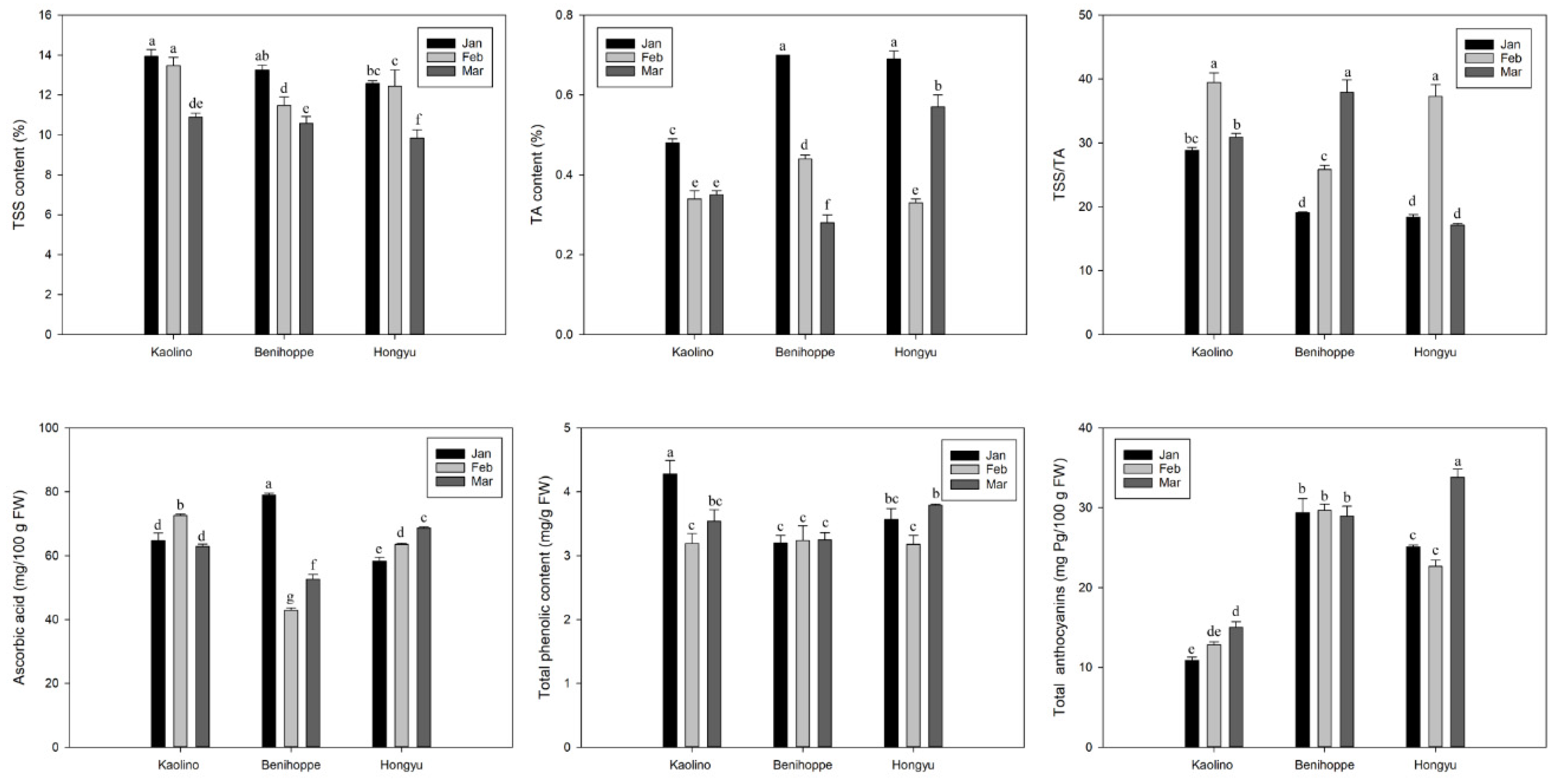
3.3. TSS, TA, TSS/TA Ratio, AsA, Total Phenolics, and Anthocyanins
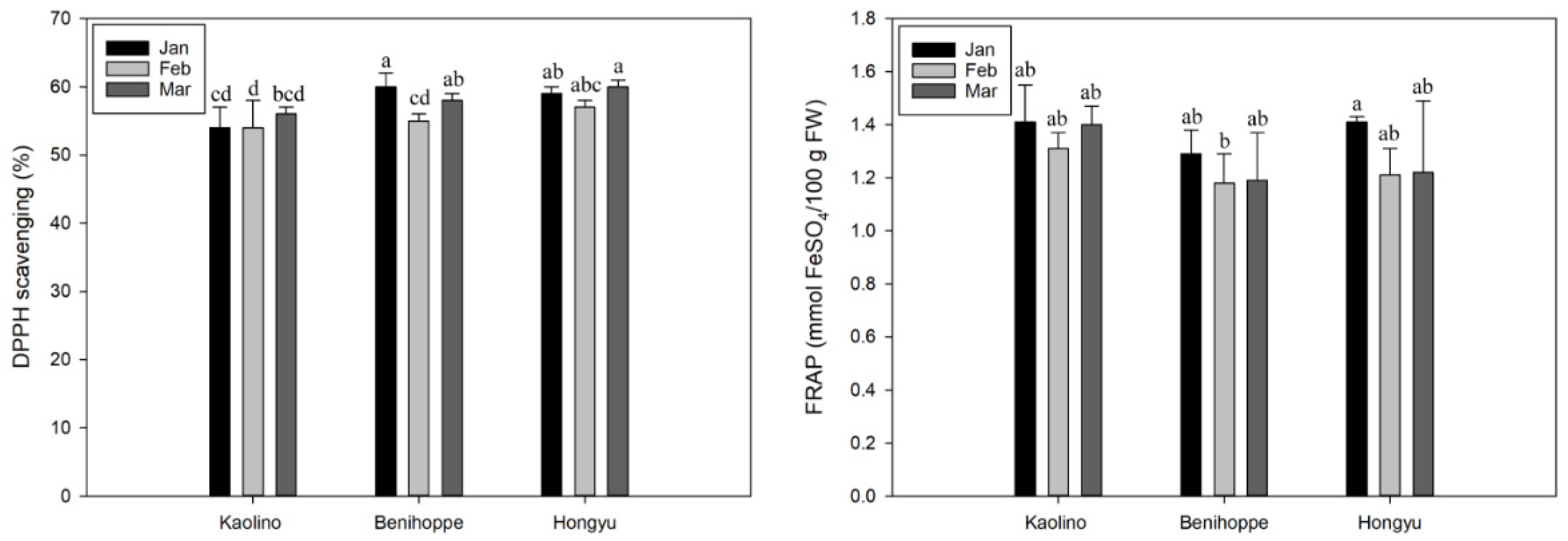
3.4. Antioxidant Capacity
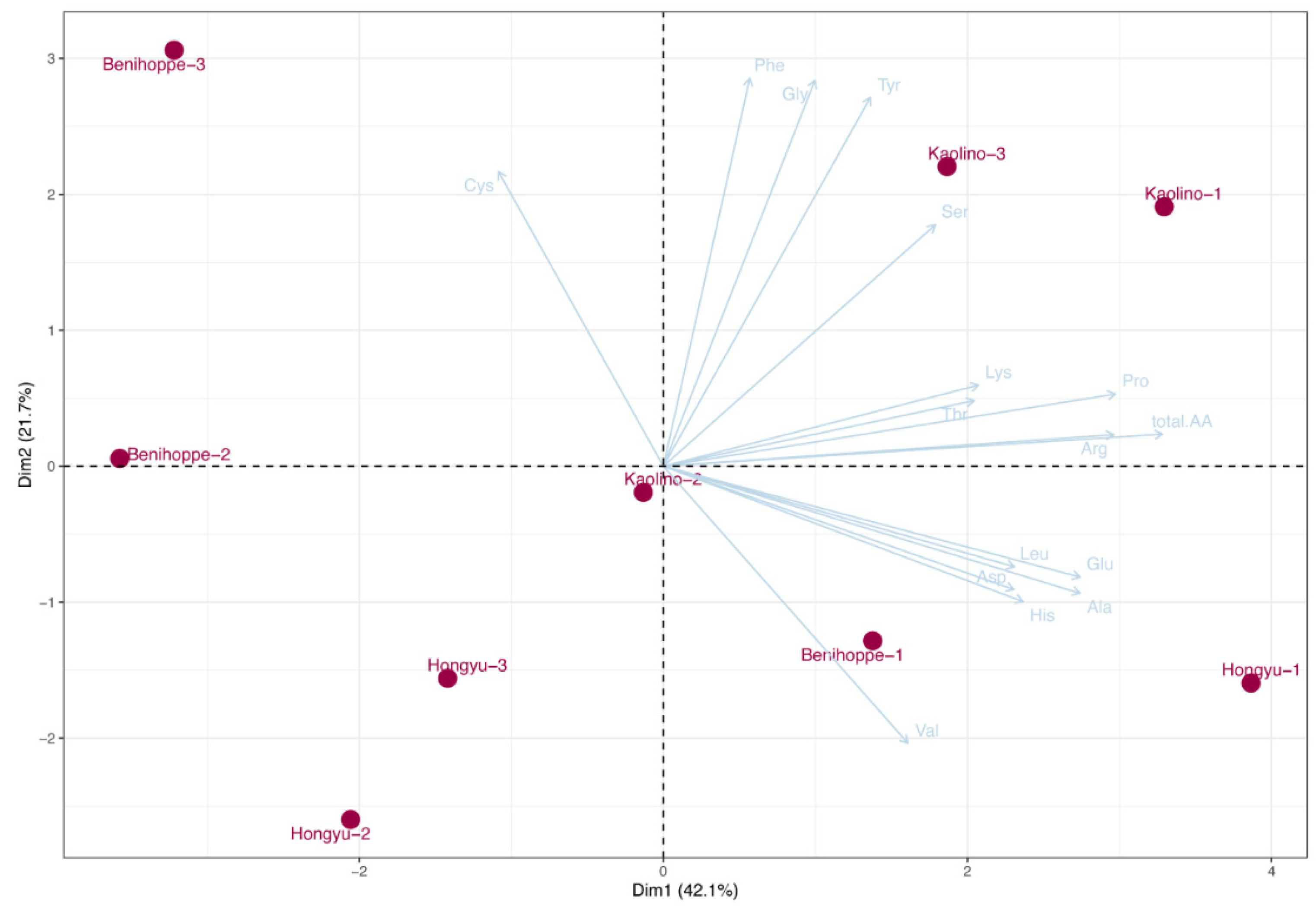
3.5. Amino Acid
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Afrin, S.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Reboredo-Rodriguez, P.; Mezzetti, B.; Varela-López, A.; Giampieri, F.; Battino, M. Promising health benefits of the strawberry: A focus on clinical studies. J. Agric. Food Chem. 2016, 64, 4435–4449. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, F.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Alvarez-Suarez, J.M.; Afrin, S.; Bompadre, S.; Quiles, J.L.; Mezzetti, B.; Battino, M. Strawberry as a health promoter: An evidence based review. Food Funct. 2015, 6, 1386–1398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battino, M.; Giampieri, F.; Cianciosi, D.; Ansary, J.; Chen, X.; Zhang, D.; Gil, E.; Forbes-Hernández, T. The roles of strawberry and honey phytochemicals on human health: A possible clue on the molecular mechanisms involved in the prevention of oxidative stress and inflammation. Phytomedicine 2021, 86, 153170. [Google Scholar] [CrossRef]
- Krüger, E.; Josuttis, M.; Nestby, R.; Toldam-Andersen, T.; Carlen, C.; Mezzetti, B. Influence of growing conditions at different latitudes of europe on strawberry growth performance, yield and quality. J. Berry Res. 2012, 2, 143–157. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Wang, H.; Luo, W.; Xue, S.; Hayat, F.; Gao, Z. Prediction of loquat soluble solids and titratable acid content using fruit mineral elements by artificial neural network and multiple linear regression. Sci. Hortic. 2021, 278, 109873. [Google Scholar] [CrossRef]
- Saridas, M.A.; Simsek, O.; Donmez, D.; Kacar, Y.A.; Kargi, S.P. Genetic diversity and fruit characteristics of new superior hybrid strawberry (Fragaria× ananassa Duchesne ex Rozier) genotypes. Genet. Resour. Crop. Evol. 2021, 68, 741–758. [Google Scholar] [CrossRef]
- Ito, H.; Ueno, H.; Kikuzaki, H. Free amino acid compositions for fruits. J. Nutr. Diet. Pract. 2017, 1, 001–005. [Google Scholar]
- Zhang, H.; Wang, Z.-Y.; Yang, X.; Zhao, H.-T.; Zhang, Y.-C.; Dong, A.-J.; Jing, J.; Wang, J. Determination of free amino acids and 18 elements in freeze-dried strawberry and blueberry fruit using an amino acid analyzer and ICP-MS with micro-wave digestion. Food Chem. 2014, 147, 189–194. [Google Scholar] [CrossRef]
- Dzhanfezova, T.; Barba-Espín, G.; Müller, R.; Joernsgaard, B.; Hegelund, J.N.; Madsen, B.; Larsen, D.H.; Vega, M.M.; Toldam-Andersen, T.B. Anthocyanin profile, antioxidant activity and total phenolic content of a strawberry (Fragaria× ananassa Duch) genetic resource collection. Food Biosci. 2020, 36, 100620. [Google Scholar] [CrossRef]
- Villamil-Galindo, E.; Van de Velde, F.; Piagentini, A.M. Extracts from strawberry by-products rich in phenolic compounds reduce the activity of apple polyphenol oxidase. LWT-Food Sci. Technol. 2020, 133, 110097. [Google Scholar] [CrossRef]
- Winardiantika, V.; Lee, Y.H.; Park, N.I.; Yeoung, Y.-R. Effects of cultivar and harvest time on the contents of antioxidant phytochemicals in strawberry fruits. Hortic. Environ. Biotechnol. 2015, 56, 732–739. [Google Scholar] [CrossRef]
- Samykanno, K.; Pang, E.; Marriott, P.J. Genotypic and environmental effects on flavor attributes of ‘Albion’ and ‘Juliette’ strawberry fruits. Sci. Hortic. 2013, 164, 633–642. [Google Scholar] [CrossRef]
- Šamec, D.; Maretić, M.; Lugarić, I.; Mešić, A.; Salopek-Sondi, B.; Duralija, B. Assessment of the differences in the physical, chemical and phytochemical properties of four strawberry cultivars using principal component analysis. Food Chem. 2016, 194, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Kim, D.S.; Kim, D.Y.; Chun, C. Variation of bioactive compounds content of 14 oriental strawberry cultivars. Food Chem. 2015, 184, 196–202. [Google Scholar] [CrossRef]
- Pelayo-Zaldívar, C.; Ebeler, S.E.; Kader, A.A. Cultivar and harvest date effects on flavor and other quality attributes of california strawberries. J. Food Qual. 2005, 28, 78–97. [Google Scholar] [CrossRef]
- Chandler, C.K.; Herrington, M.; Slade, A. Effect of harvest date on soluble solids and titratable acidity in fruit of strawberry grown in a winter, annual hill production system. Acta Hortic. 2003, 626, 345–346. [Google Scholar] [CrossRef]
- Dotto, M.C.; Martínez, G.A.; Civello, P.M. Expression of expansin genes in strawberry varieties with contrasting fruit firmness. Plant Physiol. Biochem. 2006, 44, 301–307. [Google Scholar] [CrossRef]
- Liu, C.; Zheng, H.; Sheng, K.; Liu, W.; Zheng, L. Effects of melatonin treatment on the postharvest quality of strawberry fruit. Postharvest Biol. Technol. 2018, 139, 47–55. [Google Scholar] [CrossRef]
- Zhang, J.; Kirkham, M. Antioxidant responses to drought in sunflower and sorghum seedlings. New Phytol. 1996, 132, 361–373. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, W.; Peng, X.; Sun, B.; Wang, X.; Tang, H. Characterization of anthocyanin and proanthocyanidin biosynthesis in two strawberry genotypes during fruit development in response to different light qualities. J. Photochem. Photobiol. B Biol. 2018, 186, 225–231. [Google Scholar] [CrossRef]
- Molan, A.L.; Flanagan, J.; Wei, W.; Moughan, P. Selenium-containing green tea has higher antioxidant and prebiotic activities than regular green tea. Food Chem. 2009, 114, 829–835. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Dong, H.; Wu, D.; Chen, H.; Qin, W.; Liu, W.; Yang, W.; Zhang, Q. Nutritional evaluation of whole soybean curd made from different soybean materials based on amino acid profiles. Food Qual. Saf. 2020, 4, 41–50. [Google Scholar] [CrossRef]
- Padula, M.C.; Lepore, L.; Milella, L.; Ovesna, J.; Malafronte, N.; Martelli, G.; de Tommasi, N. Cultivar based selection and genetic analysis of strawberry fruits with high levels of health promoting compounds. Food Chem. 2013, 140, 639–646. [Google Scholar] [CrossRef]
- Szajdek, A.; Borowska, E. Bioactive compounds and health-promoting properties of berry fruits: A review. Plant Foods Hum. Nutr. 2008, 63, 147–156. [Google Scholar] [CrossRef]
- Fecka, I.; Nowicka, A.; Kucharska, A.Z.; Sokół-Łętowska, A. The effect of strawberry ripeness on the content of polyphenols, cinnamates, L-ascorbic and carboxylic acids. J. Food Compos. Anal. 2021, 95, 103669. [Google Scholar] [CrossRef]
- Jouquand, C.; Chandler, C.; Plotto, A.; Goodner, K. A sensory and chemical analysis of fresh strawberries over harvest dates and seasons reveals factors that affect eating quality. J. Am. Soc. Hortic. Sci. 2008, 133, 859–867. [Google Scholar] [CrossRef] [Green Version]
- Hadjipieri, M.; Christofi, M.; Goulas, V.; Manganaris, G.A. The impact of genotype and harvesting day on qualitative attributes, postharvest performance and bioactive content of loquat fruit. Sci. Hortic. 2020, 263, 108891. [Google Scholar] [CrossRef]
- Redpath, L.E.; Gumpertz, M.; Ballington, J.R.; Bassil, N.; Ashrafi, H. Genotype, environment, year, and harvest effects on fruit quality traits of five blueberry (Vaccinium corymbosum L.) cultivars. Agronomy 2021, 11, 1788. [Google Scholar] [CrossRef]
- Hirsch, M.; Langer, S.E.; Marina, M.; Rosli, H.G.; Civello, P.M.; Martínez, G.A.; Villarreal, N.M. Expression profiling of endo-xylanases during ripening of strawberry cultivars with contrasting softening rates. Influence of postharvest and hormonal treatments. J. Sci. Food Agric. 2021, 101, 3676–3684. [Google Scholar] [CrossRef] [PubMed]
- Villarreal, N.M.; Rosli, H.G.; Martínez, G.A.; Civello, P.M. Polygalacturonase activity and expression of related genes during ripening of strawberry cultivars with contrasting fruit firmness. Postharvest Biol. Technol. 2008, 47, 141–150. [Google Scholar] [CrossRef]
- Neocleous, D. Effects of cultivars and coco-substrates on soilless strawberry production in cyprus. J. Berry Res. 2012, 2, 207–213. [Google Scholar] [CrossRef] [Green Version]
- Shaw, D. Genotypic variation and genotypic correlations for sugars and organic acids of strawberries. J. Am. Soc. Hortic. Sci. 1988, 113, 770–774. [Google Scholar]
- Shaw, D.V. Response to selection and associated changes in genetic variance for soluble solids and titratable acids contents in strawberries. J. Am. Soc. Hortic. Sci. 1990, 115, 839–843. [Google Scholar] [CrossRef]
- Agulheiro-Santos, A.; Ricardo-Rodrigues, S.; Laranjo, M.; Melgão, C.; Velázquez, R. Non-destructive prediction of total soluble solids in strawberry using near infrared spectroscopy. J. Sci. Food Agric. 2022. [Google Scholar] [CrossRef]
- Josuttis, M.; Carlen, C.; Crespo, P.; Nestby, R.; Toldam-Andersen, T.; Dietrich, H.; Krüger, E. A comparison of bioactive compounds of strawberry fruit from europe affected by genotype and latitude. J. Berry Res. 2012, 2, 73–95. [Google Scholar] [CrossRef] [Green Version]
- Viljevac, V.M.; Krunoslav, D.; Ines, M.; Vesna, T.; Dominik, V.; Zvonimir, Z.; Boris, P.; Zorica, J. Season, location and cultivar influence on bioactive compounds of sour cherry fruits. Plant Soil Environ. 2017, 63, 389–395. [Google Scholar] [CrossRef] [Green Version]
- Cocco, C.; Magnani, S.; Maltoni, M.L.; Quacquarelli, I.; Cacchi, M.; Antunes, L.E.C.; D’Antuono, L.F.; Faedi, W.; Baruzzi, G. Effects of site and genotype on strawberry fruits quality traits and bioactive compounds. J. Berry Res. 2015, 5, 145–155. [Google Scholar] [CrossRef] [Green Version]
- Pradas, I.; Medina, J.J.; Ortiz, V.; Moreno-Rojas, J.M. ‘Fuentepina’and ‘amiga’, two new strawberry cultivars: Evaluation of genotype, ripening and seasonal effects on quality characteristics and health-promoting compounds. J. Berry Res. 2015, 5, 157–171. [Google Scholar] [CrossRef] [Green Version]
- Anttonen, M.J.; Hoppula, K.I.; Nestby, R.; Verheul, M.J.; Karjalainen, R.O. Influence of fertilization, mulch color, early forcing, fruit order, planting date, shading, growing environment, and genotype on the contents of selected phenolics in strawberry (Fragaria× ananassa Duch.) fruits. J. Agric. Food Chem. 2006, 54, 2614–2620. [Google Scholar] [CrossRef] [PubMed]
- Sorrequieta, A.; Ferraro, G.; Boggio, S.B.; Valle, E.M. Free amino acid production during tomato fruit ripening: A focus on l-glutamate. Amino Acids 2010, 38, 1523–1532. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.; Suh, J.H.; Chambers, A.H.; Crane, J.; Wang, Y. Relationship between sensory attributes and chemical composition of different mango cultivars. J. Agric. Food Chem. 2019, 67, 5177–5188. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, B.; Hildebrandt, T.M. The role of amino acid metabolism in signaling and metabolic adaptation to stress-induced energy deficiency in plants. J. Exp. Bot. 2021, 72, 4634–4645. [Google Scholar] [CrossRef]
- Keutgen, A.J.; Pawelzik, E. Contribution of amino acids to strawberry fruit quality and their relevance as stress indicators under nacl salinity. Food Chem. 2008, 111, 642–647. [Google Scholar] [CrossRef]
- Qamar, A.; Mysore, K.; Senthil-Kumar, M. Role of proline and pyrroline-5-carboxylate metabolism in plant defense against invading pathogens. Front. Plant Sci. 2015, 6, 503. [Google Scholar] [CrossRef] [Green Version]
- Batista-Silva, W.; Heinemann, B.; Rugen, N.; Nunes-Nesi, A.; Araújo, W.L.; Braun, H.P.; Hildebrandt, T.M. The role of amino acid metabolism during abiotic stress release. Plant Cell Environ. 2019, 42, 1630–1644. [Google Scholar] [CrossRef] [Green Version]

| Quality Trait | F-Value (Significance) | ||
|---|---|---|---|
| Harvest Date | Cultivar | Harvest Date × Cultivar | |
| F | 21.183 ** | 40.416 ** | 17.339 ** |
| L* | 156.776 ** | 125.703 ** | 4.576 ** |
| b* | 16.537 ** | 89.085 ** | 12.414 ** |
| a* | 80.503 ** | 32.401 ** | 19.431 ** |
| TSS | 110.55 ** | 20.098 ** | 5.062 ** |
| TA | 692.841 ** | 183.217 ** | 170.539 ** |
| TSS/TA | 692.841 ** | 183.217 ** | 170.539 ** |
| AsA | 39.143 ** | 44.581 ** | 141.297 ** |
| TP | 6.942 ** | 5.719 * | 4.457 * |
| ANT | 19.979 ** | 274.147 ** | 12.206 ** |
| DPPH | 6.635 ** | 11.879 ** | 1.212 |
| FRAP | 2.634 | 2.947 | 0.396 |
| TAA | 12.073 ** | 12.328 ** | 0.502 |
| Harvest Date | Cultivar | Asp | Thr | Ser | Glu | Ala | Gly | Cys | Val |
|---|---|---|---|---|---|---|---|---|---|
| Kaolino | 1.06 ± 0.09 a | 0.15 ± 0.00 b | 0.18 ± 0.02 b | 1.48 ± 0.21 a | 0.18 ± 0.01 a | 0.20 ± 0.02 abcd | 0.01 ± 0.00 a | 0.11 ± 0.01 b | |
| January | Benihoppe | 0.91 ± 0.04 ab | 0.11 ± 0.01 c | 0.12 ± 0.01 b | 1.19 ± 0.09 ab | 0.11 ± 0.00 cd | 0.20 ± 0.00 abc | 0.00 ± 0.00 c | 0.16 ± 0.01 a |
| Hongyu | 1.05 ± 0.10 a | 0.11 ± 0.00 c | 0.17 ± 0.02 b | 1.37 ± 0.09 ab | 0.11 ± 0.00 bc | 0.21 ± 0.00 a | 0.00 ± 0.00 c | 0.15 ± 0.02 a | |
| Kaolino | 1.00 ± 0.02 ab | 0.10 ± 0.00 c | 0.12 ± 0.01 b | 1.33 ± 0.05 ab | 0.13 ± 0.01 b | 0.18 ± 0.00 cd | 0.00 ± 0.00 c | 0.11 ± 0.01 b | |
| February | Benihoppe | 0.86 ± 0.06 b | 0.15 ± 0.01 b | 0.13 ± 0.01 b | 1.06 ± 0.10 b | 0.07 ± 0.01 e | 0.18 ± 0.01 d | 0.01 ± 0.00 ab | 0.10 ± 0.01 b |
| Hongyu | 0.95 ± 0.03 ab | 0.10 ± 0.00 c | 0.13 ± 0.01 b | 1.14 ± 0.08 b | 0.10 ± 0.00 cd | 0.19 ± 0.00 abcd | 0.00 ± 0.00 c | 0.13 ± 0.00 ab | |
| Kaolino | 0.85 ± 0.05 b | 0.30 ± 0.01 a | 0.26 ± 0.06 a | 1.18 ± 0.02 ab | 0.17 ± 0.01 a | 0.20 ± 0.00 ab | 0.00 ± 0.00 bc | 0.12 ± 0.01 ab | |
| March | Benihoppe | 0.84 ± 0.03 b | 0.11 ± 0.01 c | 0.14 ± 0.02 b | 0.74 ± 0.07 c | 0.19 ± 0.00 a | 0.18 ± 0.00 bcd | 0.01 ± 0.00 a | 0.11 ± 0.00 b |
| Hongyu | 0.88 ± 0.04 ab | 0.12 ± 0.00 c | 0.12 ± 0.00 b | 1.05 ± 0.07 b | 0.09 ± 0.00 de | 0.20 ± 0.01 ab | 0.00 ± 0.00 c | 0.13 ± 0.01 ab |
| Harvest Date | Cultivar | Leu | Tyr | Phe | Lys | His | Arg | pro | Total AA |
|---|---|---|---|---|---|---|---|---|---|
| Kaolino | 0.11 ± 0.00 b | 0.07 ± 0.00 a | 0.18 ± 0.01 a | 0.11 ± 0.00 e | 0.07 ± 0.00 a | 0.12 ± 0.00 a | 0.11 ± 0.00 a | 4.13 ± 0.35 a | |
| January | Benihoppe | 0.16 ± 0.01 a | 0.06 ± 0.01 ab | 0.17 ± 0.02 a | 0.13 ± 0.01 bc | 0.06 ± 0.00 a | 0.11 ± 0.01 abc | 0.08 ± 0.00 bc | 3.57 ± 0.18 bcd |
| Hongyu | 0.11 ± 0.01 b | 0.05 ± 0.00 bc | 0.15 ± 0.01 ab | 0.15 ± 0.00 a | 0.07 ± 0.00 a | 0.12 ± 0.00 ab | 0.10 ± 0.01 a | 3.92 ± 0.15 ab | |
| Kaolino | 0.08 ± 0.00 cd | 0.06 ± 0.00 ab | 0.17 ± 0.02 a | 0.12 ± 0.00 cd | 0.05 ± 0.00 b | 0.08 ± 0.00 de | 0.08 ± 0.00 bc | 3.59 ± 0.07 bc | |
| February | Benihoppe | 0.06 ± 0.00 d | 0.04 ± 0.00 c | 0.17 ± 0.01 a | 0.11 ± 0.00 e | 0.05 ± 0.00 b | 0.06 ± 0.00 e | 0.00 ± 0.00 d | 3.06 ± 0.13 de |
| Hongyu | 0.08 ± 0.00 cd | 0.04 ± 0.00 c | 0.12 ± 0.00 b | 0.07 ± 0.00 f | 0.05 ± 0.00 b | 0.09 ± 0.01 cd | 0.07 ± 0.00 c | 3.26 ± 0.11 cde | |
| Kaolino | 0.09 ± 0.00 bc | 0.07 ± 0.01 a | 0.18 ± 0.01 a | 0.14 ± 0.00 b | 0.06 ± 0.00 a | 0.10 ± 0.01 bcd | 0.08 ± 0.00 b | 3.81 ± 0.04 ab | |
| March | Benihoppe | 0.07 ± 0.01 cd | 0.07 ± 0.00 a | 0.18 ± 0.00 a | 0.11 ± 0.00 de | 0.03 ± 0.00 c | 0.09 ± 0.01 cd | 0.07 ± 0.00 c | 2.94 ± 0.13 e |
| Hongyu | 0.08 ± 0.00 cd | 0.05 ± 0.00 bc | 0.15 ± 0.00 ab | 0.12 ± 0.00 de | 0.06 ± 0.00 a | 0.08 ± 0.00 de | 0.07 ± 0.00 c | 3.21 ± 0.08 cde |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Yang, M.; Hou, G.; Zhang, Y.; Chen, Q.; Lin, Y.; Li, M.; Wang, Y.; He, W.; Wang, X.; et al. Effect of Genotype and Harvest Date on Fruit Quality, Bioactive Compounds, and Antioxidant Capacity of Strawberry. Horticulturae 2022, 8, 348. https://doi.org/10.3390/horticulturae8040348
Zhang Y, Yang M, Hou G, Zhang Y, Chen Q, Lin Y, Li M, Wang Y, He W, Wang X, et al. Effect of Genotype and Harvest Date on Fruit Quality, Bioactive Compounds, and Antioxidant Capacity of Strawberry. Horticulturae. 2022; 8(4):348. https://doi.org/10.3390/horticulturae8040348
Chicago/Turabian StyleZhang, Yunting, Min Yang, Guoyan Hou, Yong Zhang, Qing Chen, Yuanxiu Lin, Mengyao Li, Yan Wang, Wen He, Xiaorong Wang, and et al. 2022. "Effect of Genotype and Harvest Date on Fruit Quality, Bioactive Compounds, and Antioxidant Capacity of Strawberry" Horticulturae 8, no. 4: 348. https://doi.org/10.3390/horticulturae8040348
APA StyleZhang, Y., Yang, M., Hou, G., Zhang, Y., Chen, Q., Lin, Y., Li, M., Wang, Y., He, W., Wang, X., Tang, H., & Luo, Y. (2022). Effect of Genotype and Harvest Date on Fruit Quality, Bioactive Compounds, and Antioxidant Capacity of Strawberry. Horticulturae, 8(4), 348. https://doi.org/10.3390/horticulturae8040348








