The True Identity of the “Second Pollen Morphology” of Camellia oleifera—Stomium Cells
Abstract
:1. Introduction
2. Material and Methods
2.1. Material Acquisition
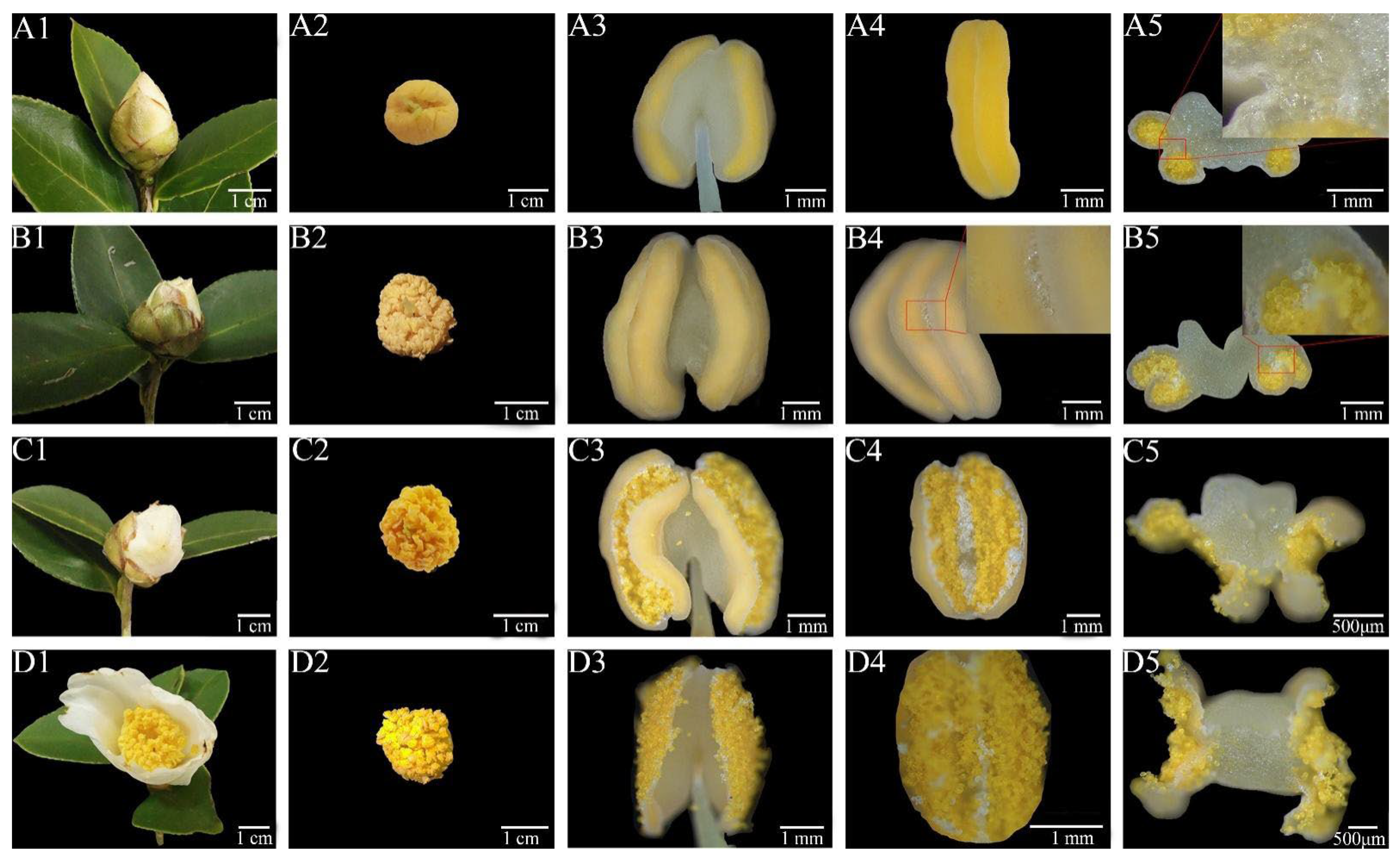
2.2. Morphology of Anther Dehiscence
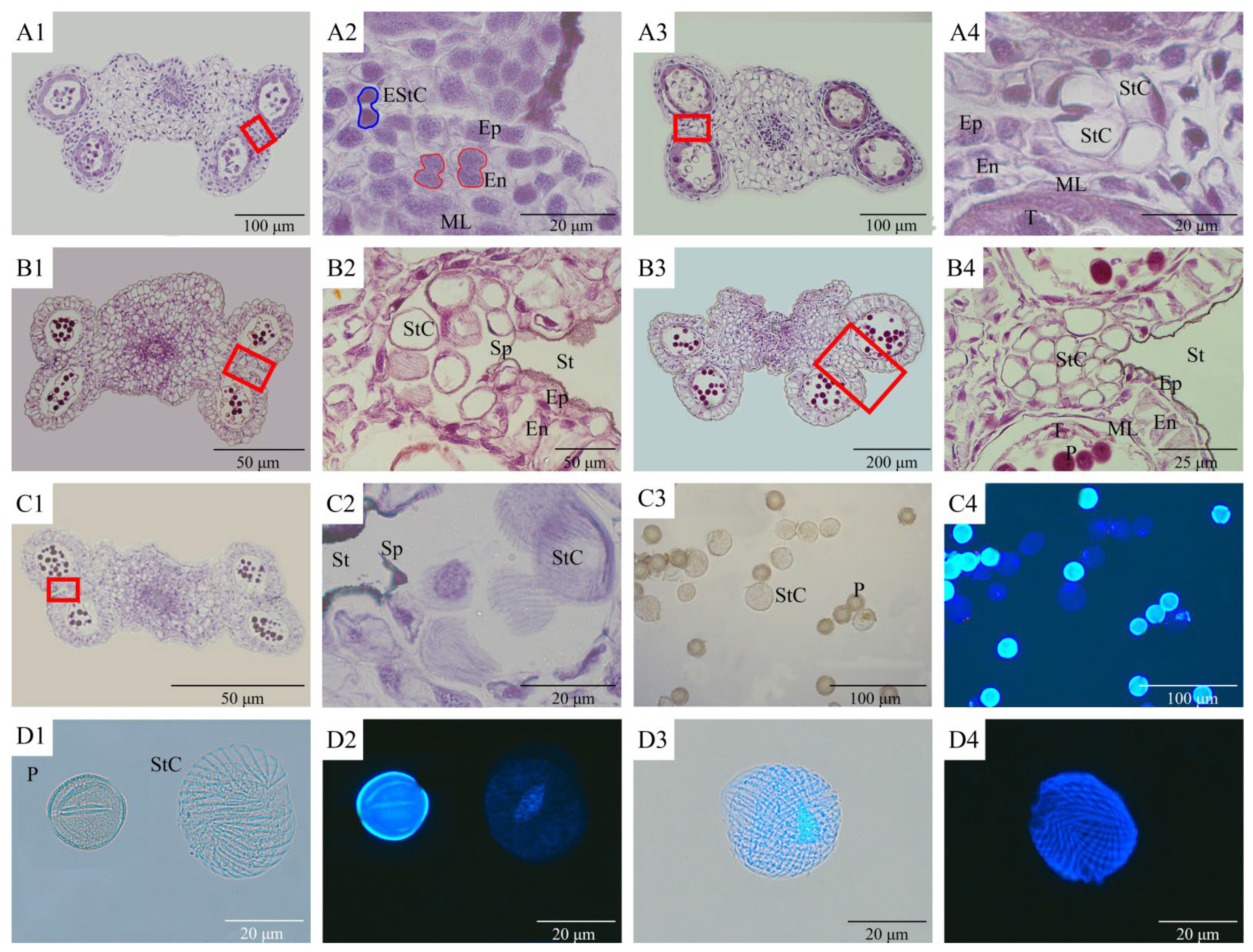
2.3. Internal Structure of Anther Dehiscence
2.4. SEM Observation of Anther Dehiscence
2.5. Fluorescence Observation of Pollen and Second Pollen
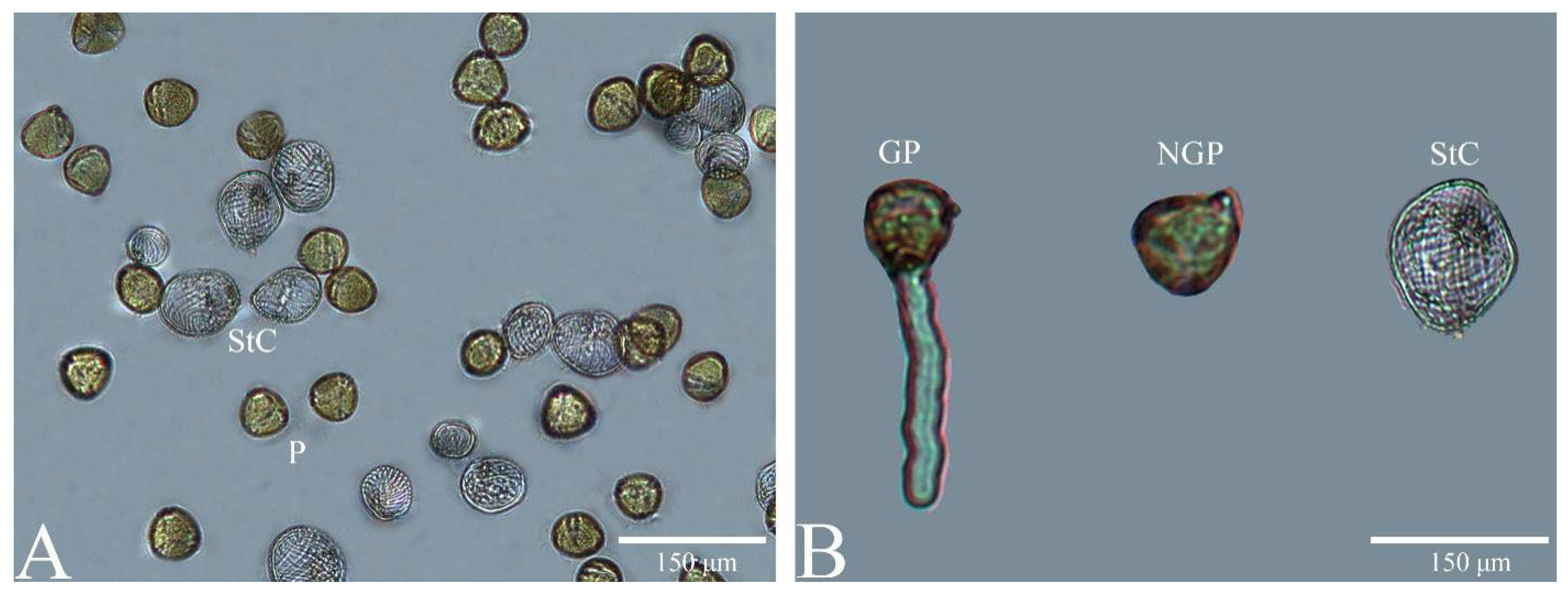
2.6. In Vitro Germination of Pollen and Second Pollen
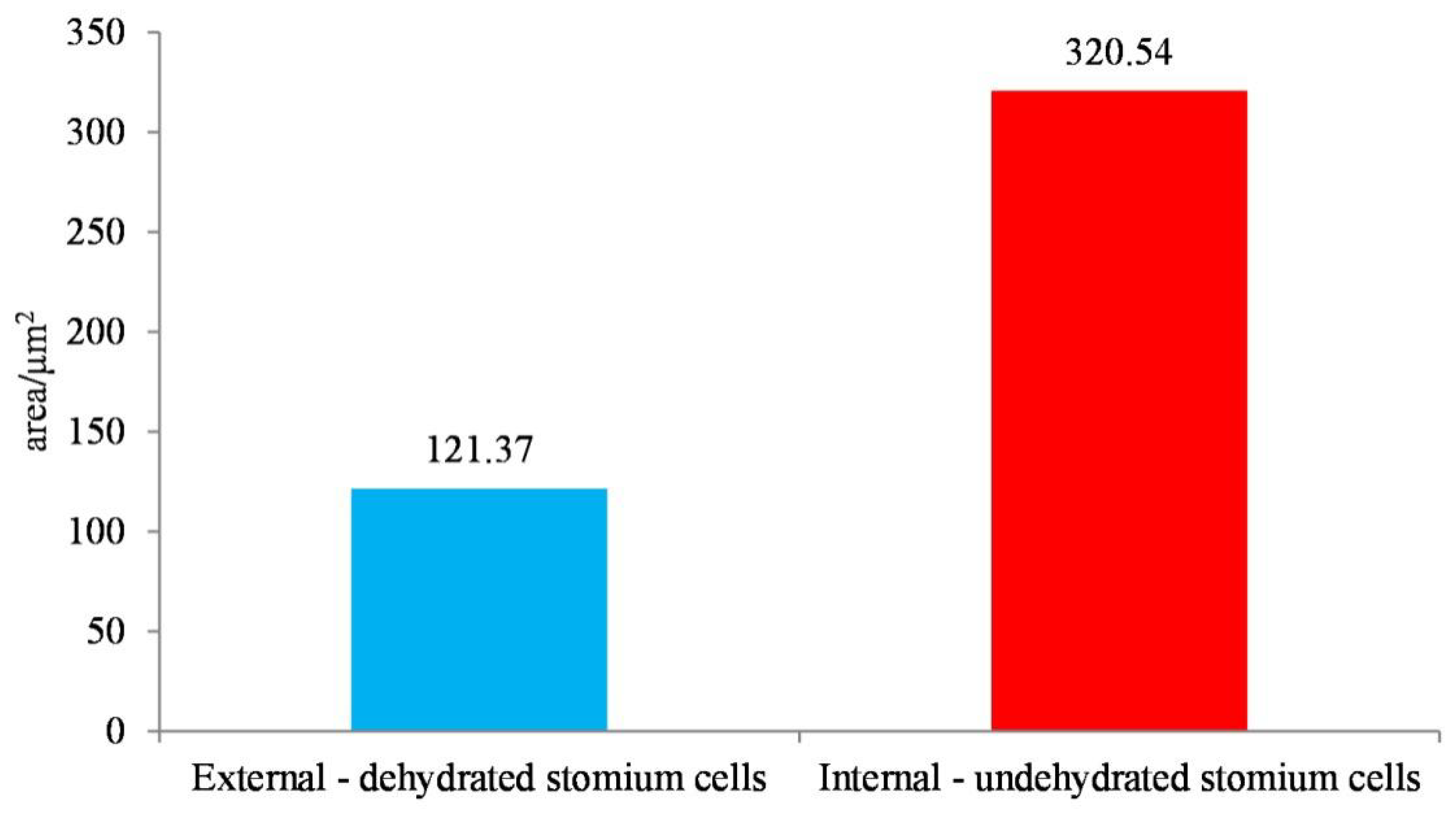
2.7. Area Characteristics of the Cross-Section of the Stomium Cells
3. Results and Analysis
3.1. Morphology Changes in the Anther Dehiscence Process
3.2. SEM Observations of Anther Dehiscence Process
3.3. Microscopic Observation and Fluorescence Observation of Stomium Cell Cytogenesis during Anther Dehiscence
3.4. In Vitro Germination Ability of Pollen and Stomium Cells
4. Discussion
| Species | Labeling or Description | Literature |
|---|---|---|
| C. gauchowensis | Types | [16] |
| C. magniflora | Abnormal pollen | [47] |
| C. sinensis | Pseudopollen | [41] |
| C. tenuifolia | Pseudopollen | [41] |
| C. oleifera | Empty pollen | [48] |
| C. oleifera | false pollen | [17] |
| C. oleifera | Male sterile pollen (with photo) | [37] |
| C. oleifera | Striate pollen | [15] |
| C. oleifera | Pseudopollen | [24] |
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, Y.; Gao, C.; Deng, Q.E.; Qiu, J.; Wei, H.L.; Yang, L.; Xie, J.J.; Liao, D.S. Anatomical Characteristics of Petalized Anther Abortion in Male Sterile Camellia oleifera Plants. J. Am. Soc. Hortic. Sci. 2021, 146, 411–423. [Google Scholar] [CrossRef]
- Sanders, P.M.; Bui, A.Q.; Weterings, K.; McIntire, K.N.; Hsu, Y.C.; Lee, P.Y.; Truong, M.T.; Beals, T.P.; Goldberg, R.B. Anther developmental defects in Arabidopsis thaliana male-sterile mutants. Sex. Plant Reprod. 1999, 11, 297–322. [Google Scholar] [CrossRef]
- Yuan, D.Y.; Zou, F.; Tan, X.F.; He, C.Y.; Yuan, J.; Fan, X.M. Flower bud differentiation and development of male and female gametophytes in Camellia oleifera. J. Cent. South Univ. For. Technol. 2011, 31, 65–70. [Google Scholar] [CrossRef]
- Liao, G.L.; Xu, X.B.; Huang, C.H.; Zhong, M.; Jia, D.F. Resource evaluation and novel germplasm mining of Actinidia eriantha. Sci. Hortic. 2021, 282, 9. [Google Scholar] [CrossRef]
- Wei, Z.X.; Min, T.L.; Zavada, M.S. Pollen morphology of Camellia (Theaceae) and its taxonomic significance. Plant Divers. 1992, 14, 275–282, 347–354. [Google Scholar]
- Penny, R.H.; Steven, J.C. Sexual dimorphism in pollen grain size in cryptically dioecious Thalictrum macrostylum. Plant Syst. Evol. 2009, 279, 11–19. [Google Scholar] [CrossRef]
- Wang, L.L.; Zhang, C.; Yang, M.L.; Zhang, G.P.; Zhang, Z.Q.; Yang, Y.P.; Duan, Y.W. Intensified wind pollination mediated by pollen dimorphism after range expansion in an ambophilous biennial Aconitum gymnandrum. Ecol. Evol. 2017, 7, 541–549. [Google Scholar] [CrossRef]
- Gao, C.; Yuan, D.Y.; Yang, Y.; Wang, B.F.; Liu, D.M.; Zou, F.; Tan, X.F. Anatomical Characteristics of Self-Incompatibility in Camellia oleifera. Sci. Silvae Sin. 2015, 51, 60–68. [Google Scholar] [CrossRef]
- Gao, C.; Yuan, D.Y.; Yang, Y.; Wang, B.F.; Liu, D.M.; Zou, F. Pollen Tube Growth and Double Fertilization in Camellia oleifera. J. Am. Soc. Hortic. Sci. 2015, 140, 12–18. [Google Scholar] [CrossRef] [Green Version]
- He, C.Y.; Tan, X.F.; Yuan, D.Y.; Hu, Q.S.; Zou, F. Determination of the Pollen Number and Pollen Germination Rate of Seven Camellia Species. J. Cent. South Univ. For. Technol. 2009, 29, 74–78. [Google Scholar] [CrossRef]
- Wang, X.N.; Chen, Y.Z.; Wang, R.; Zhu, C.Y.; Peng, S.F.; Chen, L.S.; Ma, L. Pollen viability and chapiter receptivity of Camellia oleifera. J. Cent. South Univ. For. Technol. 2012, 32, 17–22. [Google Scholar] [CrossRef]
- Deng, Y.Y.; Yu, X.L.; Luo, Y.B. The role of native bees on the reproductive success of Camellia oleifera in Hunan Province, Central South China. Acta Ecol. Sin. 2010, 30, 4427–4436. [Google Scholar]
- Liao, T.; Yuan, D.Y.; Peng, S.F.; Zou, F. A fluorescence microscope observation on self and cross-pollination of pollen tubes in Camellia oleifera. J. Cent. South Univ. For. Technol. 2012, 32, 34–37. [Google Scholar] [CrossRef]
- Liao, T.; Yuan, D.Y.; Gao, C.; Zou, F.; Tang, J.; Tan, X. Pollination, Fertilization and Early Embryonic Development of Camellia oleifera. Sci. Silvae Sin. 2014, 50, 50–55. [Google Scholar] [CrossRef]
- Xiao, D.X.; Dong, J.S. Microsporogenesis and pollen development in Camellia oleifera abel. Acta. Agric. Univ. Jiangxiensis 1986, 8, 43–48. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, Y.; Xi, R.C.; Huang, R.R. Pollen Characteristics of Camellia gauchowensis Chang. For. Res. 2019, 32, 90–96. [Google Scholar] [CrossRef]
- Deng, Q.E.; Li, J.A.; Gao, C.; Cheng, J.Y.; Deng, X.Z.; Jiang, D.Z.; Li, L.; Yan, P. New perspective for evaluating the main Camellia oleifera cultivars in China. Sci. Rep. 2020, 10, 20676. [Google Scholar] [CrossRef]
- Zhuang, R.L.; Wang, J.F. Study on the biology of Camellia oleifera and the effect of artificial pollination. J. Zhejiang Agric. Sci. 1965, 8, 406–409. [Google Scholar] [CrossRef]
- Zhuang, R.L. Preliminary Report on the Storage and Artificial Germination of Camellia oleifera Pollen. For. Sci. Technol. 1981, 2, 15–17. [Google Scholar] [CrossRef]
- Tan, X.F.; Yuan, D.Y.; Yuan, J.; Liao, T. Pollen germination in Camellia oleifera with ascorbic acid and plant growth regulators. J. Zhejiang A F Univ. 2010, 27, 941–944. [Google Scholar] [CrossRef]
- Yuan, D.Y.; Wang, R.; Yuan, J.; Liao, T.; Cui, X.; Cai, L. The influence of nutrient elements on pollen germination percentage in Camellia oleifera. J. Fujian Agric. Fore. Univ. 2010, 39, 471–474. [Google Scholar] [CrossRef]
- Liu, L.X.; Zeng, H.T.; Xu, H.; Yao, X.H. Effects of phytohormones on pollen germination and pollen tube growth of 4 Camellia plants. Chin. J. Oil Crop Sci. 2021, 43, 700–707. [Google Scholar] [CrossRef]
- Gao, C.; Yuan, D.Y.; Yuan, J.; Liao, T.; Zou, F.; Duan, W.H. The Effect of Spraying Nutrient Elements and Growth Regulators at Bloom on Fruit Setting Rate of Camellia oleifera. Acta. Agric. Univ. Jiangxiensis 2012, 34, 505–510. [Google Scholar] [CrossRef]
- Chen, Y.; Yuan, D.Y.; Li, Y.M.; Zhang, J.; Hu, G.X.; Zou, F. Floral morphological and breeding characteristics of the F1 generation of Camellia. J. Fujian Agric. For. Univ. 2020, 49, 440–446. [Google Scholar] [CrossRef]
- Chen, Y.; Yuan, D.Y.; Li, K.; Yang, Y.H.; Jin, Y.J.; Lin, M.H.; Zou, F. Viability Determination and Preservation of Pollen of Camellia oleifera ‘Huashuo’, ‘Huaxin’ and ‘Huajin’. Acta. Agric. Univ. Jiangxiensis 2020, 42, 118–126. [Google Scholar] [CrossRef]
- Xie, Y.Q. Flower characteristics and stigma receptivity of Camellia oleifera and Camellia meiocarpa. J. For. Environ. 2015, 35, 249–254. [Google Scholar] [CrossRef]
- Wang, X.N.; Chen, Y.Z.; Jiang, L.J.; Liu, Z.L.; Peng, S.F.; Wang, R.; Ma, L.; Yang, X.H. Electron microscopic scanning on pollen morphology characters of Camellia oleifera superior clones. J. Cent. South Univ. For. Technol. 2010, 30, 67–71, 90. [Google Scholar] [CrossRef]
- Li, H.P. Plant Microscopy Technique, 2nd ed.; Science Press: Beijing, China, 2009; pp. 9–39. ISBN 978-7-03-023688-3. [Google Scholar]
- Southworth, D. Solubility of Pollen Exines. Am. J. Bot. 1974, 61, 36–44. [Google Scholar] [CrossRef]
- Voragen, A.G.J.; Coenen, G.J.; Verhoef, R.P.; Schols, H.A. Pectin, a versatile polysaccharide present in plant cell walls. Struct. Chem. 2009, 20, 263–275. [Google Scholar] [CrossRef] [Green Version]
- Bahadur, S.; Ahmad, M.; Mir, S.; Zafar, M.; Sultana, S.; Ashfaq, S.; Arfan, M. Identification of monocot flora using pollen features through scanning electron microscopy. Microsc. Res. Tech. 2018, 81, 599–613. [Google Scholar] [CrossRef]
- Gallardo, A.; Ocete, R.; Lopez, M.A.; Lara, M.; Rivera, D. Assessment of pollen dimorphism in populations of Vitis vinifera L. subsp sylvestris (Gmelin) Hegi in Spain. Vitis 2009, 48, 59–62. [Google Scholar] [CrossRef]
- Koski, M.H.; Galloway, L.F. Geographic variation in pollen color is associated with temperature stress. New Phytol. 2018, 218, 370–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Wang, L.L.; Lan, D.; Yang, Y.P.; Duan, Y.W. Pollination ecology of Arnebia szechenyi (Boraginaceae), a Chinese endemic perennial characterized by distyly and heteromorphic self-incompatibility. Ann. Bot. Fenn. 2014, 51, 297–304. [Google Scholar] [CrossRef]
- Ickert-Bond, S.M.; Skvarla, J.J.; Chissoe, W.F. Pollen dimorphism in Ephedra L. (Ephedraceae). Rev. Palaeobot. Palynol. 2003, 124, 325–334. [Google Scholar] [CrossRef]
- Bolinder, K.; Ivarsson, L.N.; Humphreys, A.M.; Ickert-Bond, S.M.; Han, F.; Hoorn, C.; Rydin, C. Pollen morphology of Ephedra (Gnetales) and its evolutionary implications. Grana 2016, 55, 24–51. [Google Scholar] [CrossRef]
- Chen, Y.Z.; Wang, D.B.; Liu, Y.X.; Peng, S.F.; Su, L.G.; Chen, L.S. Research on selection of superior male-sterile clone of Camellia oleifer. J. Cent. South Univ. For. Technol. 2011, 31, 1–6. [Google Scholar] [CrossRef]
- Luo, L.; Yu, C.; Guo, X.L.; Pan, H.T.; Zhang, Q.X. Morphological Variation and Palynomorphology of Rosa laxa in Xinjiang, China. J. Am. Soc. Hortic. Sci. 2018, 143, 409–417. [Google Scholar] [CrossRef] [Green Version]
- Zhao, R.; Hu, X.; Yuan, D.Y.; Masabni, J.; Xiong, H.; Zou, F. Orthogonal test design for optimizing culture medium for in vitro pollen germination of interspecific oil tea hybrids. An. Acad. Bras. Cienc. 2021, 93, 10. [Google Scholar] [CrossRef]
- Li, C.L.; Yang, S.P.; Yao, X.H.; Reng, H.D.; Wang, K.L.; Lin, P. Characteristics of Pollen Germination in Vitro of Camellia oleifera. For. Res. 2011, 24, 212–217. [Google Scholar] [CrossRef]
- Tsou, C.H. Embryology of the Theaceae-Anther and ovule development of Camellia, Franklinia, and Schima. Am. J. Bot. 1997, 84, 369–381. [Google Scholar] [CrossRef]
- Davies, K.L.; Turner, M.P. Pseudopollen in Dendrobium unicum Seidenf. (Orchidaceae): Reward or deception? Ann. Bot. 2004, 94, 129–132. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.Q.; Liang, X.W.; Zhao, L.; Zhang, Z.Y.; Xue, X.F.; Wang, K.; We, L.M. UPLC-Q-Exactive Orbitrap/MS-Based Lipidomics Approach To Characterize Lipid Extracts from Bee Pollen and Their in Vitro Anti-Inflammatory Properties. J. Agric. Food Chem. 2017, 65, 6848–6860. [Google Scholar] [CrossRef]
- Iqbal, M.C.M.; Wijesekara, K.B. Cells of the connective tissue differentiate and migrate into pollen sacs. Naturwissenschaften 2002, 89, 39–42. [Google Scholar] [CrossRef]
- Davies, K.L.; Winters, C.; Turner, M.P. Pseudopollen: Its structure and development in Maxillaria (Orchidaceae). Ann. Bot. 2000, 85, 887–895. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Hao, Q.; Guo, X.; Liu, Q.C.; Sun, Y.K.; Liu, Q.H.; Wang, K.L. Anther and ovule development in Camellia japonica (Naidong) in relation to winter dormancy: Climatic evolution considerations. Flora 2017, 233, 127–139. [Google Scholar] [CrossRef]
- Jia, W.Q.; Wang, S.P.; Li, J.Y. Pollen Morphology, Storage Condition and Physiologically Dynamic Change during Storage of Camellia magniflora. Acta. Bot. Boreali-Occident. Sin. 2015, 35, 754–760. [Google Scholar] [CrossRef]
- Li, Y.M.; Ye, T.W.; Han, C.X.; Ye, Z.H.; Zhang, J.; Xiao, S.X.; Yuan, D.Y. Cytogenetic analysis of interspecific hybridization in oil-tea (Camellia oleifera). Euphytica 2021, 217, 12. [Google Scholar] [CrossRef]
- Scott, R.J.; Spielman, M.; Dickinson, H.G. Stamen structure and function. Plant Cell 2004, 16, S46–S60. [Google Scholar] [CrossRef]
- Sanders, P.M.; Bui, A.Q.; Le, B.H.; Goldberg, R.B. Differentiation and degeneration of cells that play a major role in tobacco anther dehiscence. Sex. Plant Reprod. 2005, 17, 219–241. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Nishimura, M.; Hara-Nishimura, I.; Noguchi, T. Behavior of vacuoles during microspore and pollen development in Arabidopsis thaliana. Plant Cell Physiol. 2003, 44, 1192–1201. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Y.R.; Ni, Z.J. Effects of the climate during flowering period on post-flowering fruit setting in Camellia oleifera. J. Zhejiang For. Univ. 2010, 27, 323–328. [Google Scholar] [CrossRef]
- Yang, C.Y.; Xu, Z.Y.; Song, J.; Conner, K.; Barrena, G.V.; Wilson, Z.A. Arabidopsis MYB26/MALE STERILE35 regulates secondary thickening in the endothecium and is essential for anther dehiscence. Plant Cell 2007, 19, 534–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, M.R.; Band, L.R.; Dyson, R.J.; Lessinnes, T.; Wells, D.M.; Yang, C.; Everitt, N.M.; Jensen, O.E.; Wilson, Z.A. A biomechanical model of anther opening reveals the roles of dehydration and secondary thickening. New Phytol. 2012, 196, 1030–1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.B.; Luo, X.; Zhu, L. Cytological analysis and genetic control of rice anther development. J. Genet. Genomics 2011, 38, 379–390. [Google Scholar] [CrossRef]
- Wei, D.H.; Liu, M.J.; Chen, H.; Zheng, Y.; Liu, Y.X.; Wang, X.; Yang, S.H.; Zhou, M.Q.; Lin, J. Inducer of CBF expression 1 is a male fertility regulator impacting anther dehydration in Arabidopsis. PLoS Genet. 2018, 14, 32. [Google Scholar] [CrossRef]
- Mitsumoto, K.; Yabusaki, K.; Aoyagi, H. Classification of pollen species using autofluorescence image analysis. J. Biosci. Bioeng. 2009, 107, 90–94. [Google Scholar] [CrossRef]
- Xue, J.S.; Zhang, B.C.; Zhan, H.D.; Lv, Y.L.; Jia, X.L.; Wang, T.H.; Yang, N.Y.; Lou, Y.X.; Zhang, Z.B.; Hu, W.J.; et al. Phenylpropanoid Derivatives Are Essential Components of Sporopollenin in Vascular Plants. Mol. Plant. 2020, 13, 1644–1653. [Google Scholar] [CrossRef]
- Bubert, H.; Lambert, J.; Steuernagel, S.; Ahlers, F.; Wiermann, R. Continuous decomposition of sporopollenin from pollen of Typha angustifolia L. by acidic methanolysis. Z. Naturforsch. (C) 2002, 57, 1035–1041. [Google Scholar] [CrossRef]
- Kapuscinski, J. DAPI-A DNA-Specific Fluorescent-Probe. Biotech. Histochem. 1995, 70, 220–233. [Google Scholar] [CrossRef]
- Bonne, D.; Heusele, C.; Simon, C.; Pantaloni, D. 4′,6-Diamidino-2-Phenylindole, A Fluorescent-Probe for Tubulin and Microtubules. J. Biol. Chem. 1985, 260, 2819–2825. [Google Scholar] [CrossRef]
- Garcia, C.C. An approach to the diversity of endothecial thickenings in Solanaceae. Flora 2002, 197, 214–223. [Google Scholar] [CrossRef]

| Phase | Main Characteristics | Phase | Main Characteristics |
|---|---|---|---|
| 1 | The stamen primordium has only three layers of cells (i.e., inner, medium, and outer) which develop into the connective tissue, sporogenous cells, and epidermis, respectively | 8 | Pollen mother cell completes meiosis I |
| 2 | Sporogenous cell formation, with one layer of anther wall (epidermal cells) | 9 | Pollen mother cells complete meiosis II to form tetrads |
| 3 | Sporogenous cells divide to produce primordial cytoplasmic cells and primordial wall cells with two layers of anther walls | 10 | The callus enclosing the tetrad descends to liberate a single microspore, and the nucleus of the microspore remains in the center of the cell, often called the mononuclear phase |
| 4 | Primary cytoplasmic cells divide to form secondary cytoplasmic cells, and primary wall cells divide to form two layers of primary walls | 11 | The microspores form large vesicles while the nucleus moves to the edge of the cell, often called the mononuclear leaning phase |
| 5 | The development of the anther wall is basic, producing a total of five layers of anther wall, and secondary spore-forming cells begin to divide and proliferate | 12 | The nucleus of the microspore divides asymmetrically, producing a large and a small nucleus for nutrition and reproduction |
| 6 | Production of pollen mother cells surrounded by a common callus | 13 | Microspore development is mature, inner wall cells are radially thickened, and stomium cells are formed |
| 7 | Degradation of callus releases individual pollen mother cells | 14 | Anthers dehiscent from the stomium cells for dispersal of pollen |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Gao, C. The True Identity of the “Second Pollen Morphology” of Camellia oleifera—Stomium Cells. Horticulturae 2022, 8, 347. https://doi.org/10.3390/horticulturae8040347
Hu Y, Gao C. The True Identity of the “Second Pollen Morphology” of Camellia oleifera—Stomium Cells. Horticulturae. 2022; 8(4):347. https://doi.org/10.3390/horticulturae8040347
Chicago/Turabian StyleHu, Yang, and Chao Gao. 2022. "The True Identity of the “Second Pollen Morphology” of Camellia oleifera—Stomium Cells" Horticulturae 8, no. 4: 347. https://doi.org/10.3390/horticulturae8040347
APA StyleHu, Y., & Gao, C. (2022). The True Identity of the “Second Pollen Morphology” of Camellia oleifera—Stomium Cells. Horticulturae, 8(4), 347. https://doi.org/10.3390/horticulturae8040347





