Insight into a Successful Development of Biocontrol Agents: Production, Formulation, Packaging, and Shelf Life as Key Aspects
Abstract
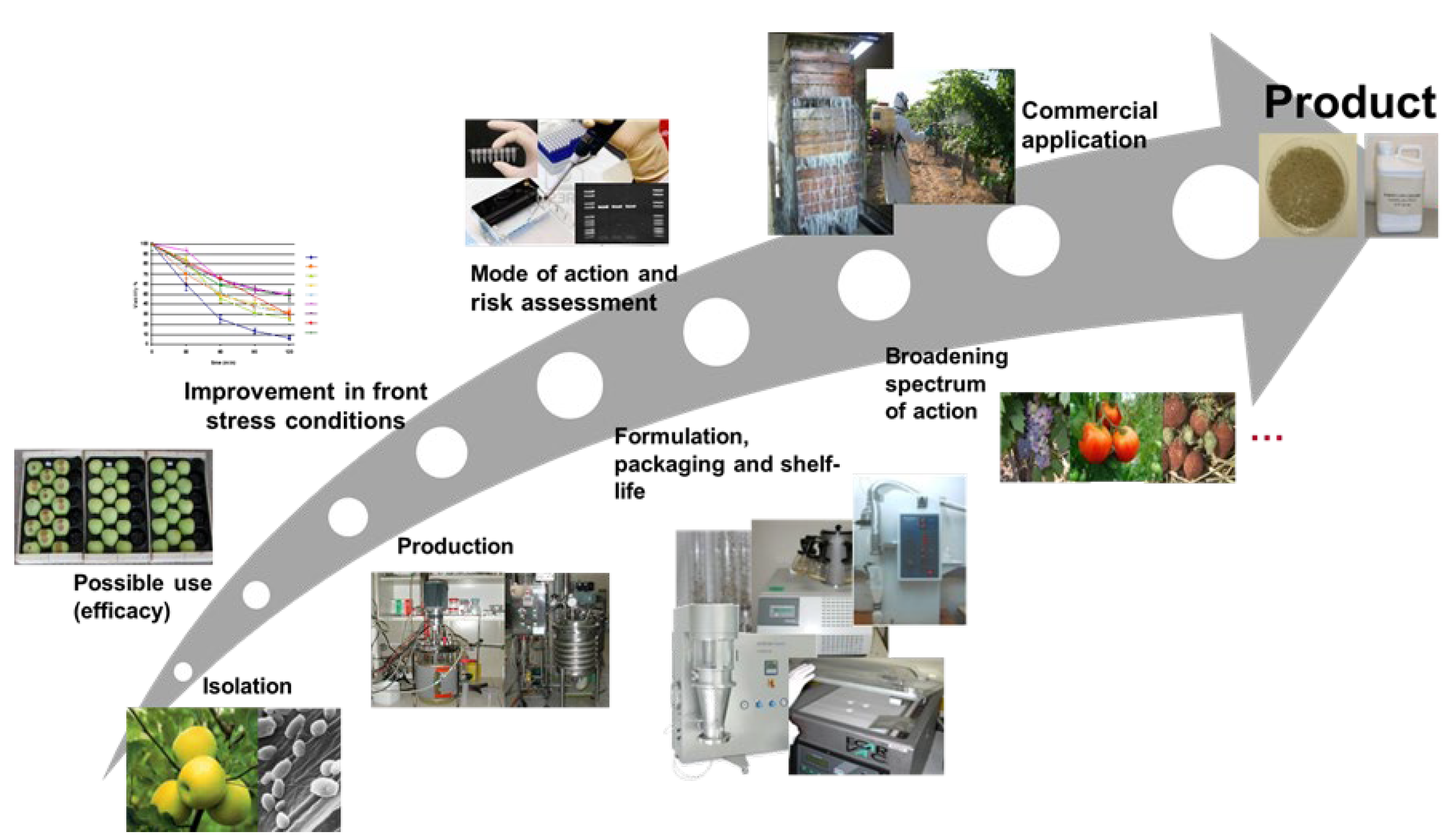
1. Introduction
2. Mass Production of BCAs
3. BCA Formulation Development
3.1. Liquid Formulation
3.2. Solid Formulation
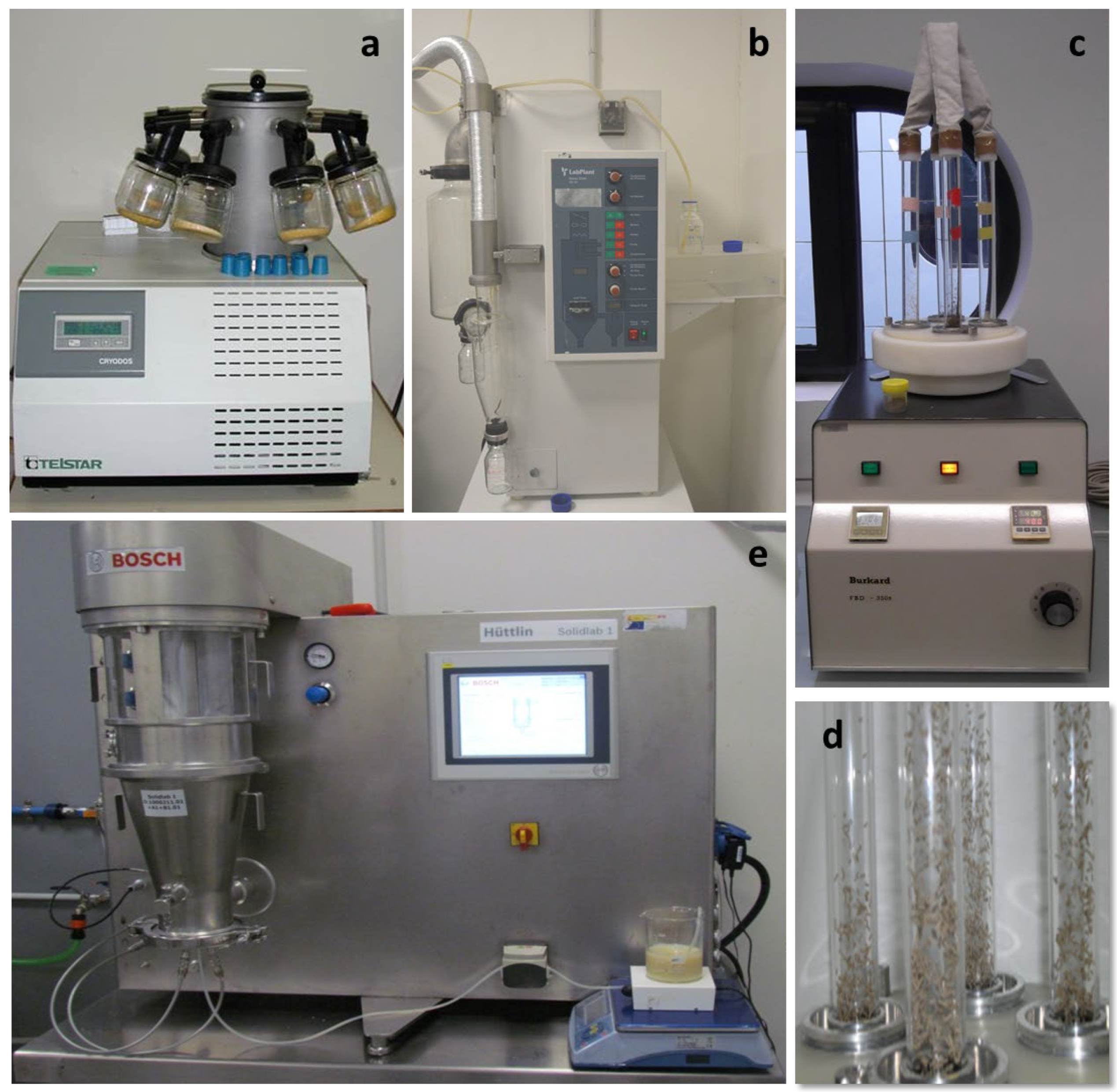
3.2.1. Freeze-Drying
3.2.2. Spray-Drying
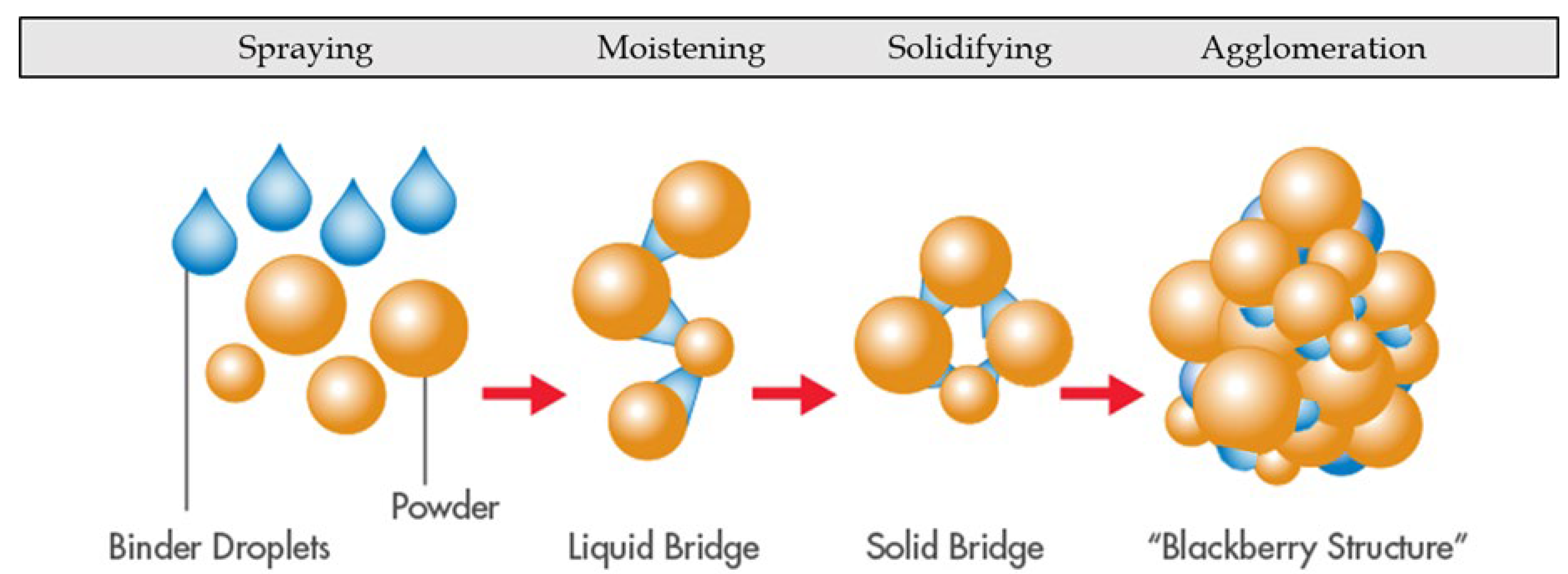
3.2.3. Fluid-Bed Drying
3.2.4. Fluid-Bed Spray-Drying
3.2.5. Encapsulation
3.3. Cell Damages and Possible Improvements
4. Packaging and Shelf Life
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Droby, S.; Wisniewski, M.; Macarisin, D.; Wilson, C. Twenty years of postharvest biocontrol research: Is it time for a new paradigm? Postharvest Biol. Technol. 2009, 52, 137–145. [Google Scholar] [CrossRef]
- Droby, S.; Wisniewski, M.; Teixidó, N.; Spadaro, D.; Jijakli, M.H. The science, development, and commercialization of postharvest biocontrol products. Postharvest Biol. Technol. 2016, 122, 22–29. [Google Scholar] [CrossRef]
- Usall, J.; Torres, R.; Teixidó, N. Biological control of postharvest diseases on fruit: A suitable alternative? Curr. Opin. Food Sci. 2016, 11, 51–55. [Google Scholar] [CrossRef]
- Köhl, J.; Postma, J.; Nicot, P.; Ruocco, M.; Blum, B. Stepwise screening of microorganisms for commercial use in biological control of plant-pathogenic fungi and bacteria. Biol. Control 2011, 57, 1–12. [Google Scholar] [CrossRef]
- Teixidó, N.; Torres, R.; Abadias, M.; Usall, J. Biological control of postharvest diseases in fruit and vegetables. In Protective Cultures, Antimicrobial Metabolites and Bacteriophages for Food and Beverage; Lacroix, C., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2011; pp. 364–402. [Google Scholar]
- Jones, K.A.; Burges, H.D. Technology of formulation and application. In Formulation of Microbial Biopesticides; Burges, H.D., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1998; pp. 7–30. [Google Scholar]
- Nunes, C. Biological control of postharvest diseases of fruit. Eur. J. Plant Pathol. 2012, 133, 181–196. [Google Scholar] [CrossRef]
- Stambury, P.F.; Whitaker, A.; Hall, S.J. Media for industrial fermentations. In Principles of Fermentation Technology; Stambury, P.F., Whitaker, A., Hall, S.J., Eds.; Pergamon Press: Oxford, UK, 1995; pp. 93–121. [Google Scholar]
- Ubando, A.T.; Felix, C.B.; Chen, W.H. Biorefineries in circular bioeconomy: A comprehensive review. Bioresour. Technol. 2020, 299, 122585. [Google Scholar] [CrossRef]
- Costa, E.; Teixidó, N.; Usall, J.; Atares, E.; Viñas, I. Production of the biocontrol agent Pantoea agglomerans strain CPA-2 using commercial products and by-products. Appl. Microbiol. Biotechnol. 2001, 56, 367–371. [Google Scholar] [CrossRef]
- Manso, T.; Nunes, C.; Raposo, S.; Lima-Costa, M.E. Carob pulp as raw material for production of the biocontrol agent P. agglomerans PBC-1. J. Ind. Microbiol. Biotechnol. 2010, 37, 1145–1155. [Google Scholar] [CrossRef]
- Manso, T.; Nunes, C.; Raposo, S.; Lima-Costa, M.E. Production of the biocontrol agent Pantoea agglomerans PBC-1 in a stirred tank reactor by batch and fed-batch cultures. World J. Microbiol. Biotechnol. 2010, 26, 725–735. [Google Scholar] [CrossRef]
- Yánez-Mendizábal, V.; Viñas, I.; Usall, J.; Torres, R.; Solsona, C.; Teixidó, N. Production of the postharvest biocontrol agent Bacillus subtilis CPA-8 using low cost commercial products and by-products. Biol. Control 2012, 60, 280–289. [Google Scholar] [CrossRef]
- Gotor-Vila, A.; Usall, J.; Torres, R.; Solsona, C.; Teixido, N. Biocontrol products based on Bacillus amyloliquefaciens CPA-8 using fluid-bed spray-drying process to control postharvest brown rot in stone fruit. LWT-Food Sci. Technol. 2017, 82, 274–282. [Google Scholar] [CrossRef][Green Version]
- Cooper, D.G.; Macdonald, C.R.; Duff, S.J.B.; Kosaric, N. Enhanced production of surfactin from Bacillus subtilis by continuous product removal and metal cation additions. Appl. Environ. Microbiol. 1981, 42, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mawgoud, A.M.; Aboulwafa, M.M.; Hassouna, N.A.H. Optimization of surfactin production by Bacillus subtilis isolate BS5. Appl. Biochem. Biotechnol. 2008, 150, 305–325. [Google Scholar] [CrossRef] [PubMed]
- Cantabella, D.; Dolcet-Sanjuan, R.; Solsona, C.; Vilanova, L.; Torres, R.; Teixidó, N. Optimization of a food industry-waste-based medium for the production of the plant growth promoting microorganism Pseudomonas oryzihabitans PGP01 based on agro-food industries by-products. Biotechnol. Rep. 2021, 32, e00675. [Google Scholar] [CrossRef]
- Abadias, M.; Teixidó, N.; Usall, J.; Viñas, I. Optimization of growth conditions of the postharvest biocontrol agent Candida sake CPA in a lab-scale fermenter. J. Appl. Microbiol. 2003, 95, 301–309. [Google Scholar] [CrossRef]
- Patiño-Vera, M.; Jiménez, B.; Balderas, K.; Ortiz, M.; Allende, R.; Carrillo, A.; Galindo, E. Pilot-scale production and liquid formulation of Rhodotorula minuta, a potential biocontrol agent of mango anthracnose. J. Appl. Microbiol. 2005, 99, 540–550. [Google Scholar] [CrossRef]
- Spadaro, D.; Ciavorella, A.; Dianpeng, Z.; Garibaldi, A.; Gullino, M.L. Effect of culture media and pH on the biomass production and biocontrol efficacy of a Metschnikowia pulcherrima strain to be used as a biofungicide for postharvest disease control. Can. J. Microbiol. 2010, 56, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, G.; Sui, Y. Optimization of culture medium enhances viable biomass production and biocontrol efficacy of the antagonistic yeast, Candida diversa. Front. Microbiol. 2017, 8, 2021. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A. Solid-state fermentation. Biochem. Eng. J. 2003, 13, 81–84. [Google Scholar] [CrossRef]
- Larena, I.; De Cal, A.; Melgarejo, P. Solid substrate production of Epicoccum nigrum conidia for biological control of brown rot on stone fruits. Int. J. Food Microbiol. 2004, 94, 161–167. [Google Scholar] [CrossRef]
- Prasad, R.D.; Rangeshwaran, R.; Anuroop, C.P.; Phanikumar, P.R. Bioefficacy and shelf life of conidial and chlamydospore formulations of Trichoderma harzianum Rifai. J. Biol. Control 2002, 16, 145–148. [Google Scholar]
- Jeyarajan, R. Prospects of indigenous mass production and formulation of Trichoderma. In Proceedings of the Group Meeting on Antagonistic Organisms in Plant Disease Management Held at Project Directorate of Biological Control, Bangalore, India, 10–11 July 2006. [Google Scholar]
- Angeli, D.; Saharan, K.; Segarra, G.; Sicher, C.; Pertot, I. Production of Ampelomyces quisqualis conidia in submerged fermentation and improvements in the formualtion for increases shelf-lefe. Crop Prot. 2017, 97, 135–144. [Google Scholar] [CrossRef]
- Carbó, A.; Torres, R.; Usall, J.; Ballesta, J.; Teixidó, N. Biocontrol potential of Ampelomyces quisqualis strain CPA-9 against powdery mildew: Conidia production in liquid medium and efficacy on zucchini leaves. Sci. Hortic. 2020, 267, 109337. [Google Scholar] [CrossRef]
- Hynes, R.K.; Boyetchko, S.M. Research initiatives in the art and science of biopesticide formulations. Soil Biol. Biochem. 2006, 38, 845–849. [Google Scholar] [CrossRef]
- Gotor-Vila, A.; Usall, J.; Torres, R.; Abadias, M.; Teixidó, N. Formulation of the biocontrol agent Bacillus amyloliquefaciens CPA-8 using different approaches: Liquid, freeze-drying and fluid-bed spray-drying. Biocontrol 2017, 62, 545–555. [Google Scholar] [CrossRef]
- Fravel, D.R.; Connick, W.J.; Lewis, J.A. Formulation of microorganisms to control plant diseases. In Formulation of Microbial Biopesticides; Burges, H.D., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1998; pp. 187–202. [Google Scholar]
- Brar, S.K.; Verma, M.; Tyagi, R.G.; Valéro, J.R. Recent advances in dowstream processing and formulations of Bacillus thuringiensis based biopestices. Process Biochem. 2006, 41, 323–342. [Google Scholar] [CrossRef]
- Navarta, L.G.; Calvo, J.; Calvente, V.; Benuzzi, D.; Sanz, M.I. Freezing and freeze-drying of the bacterium Rahnella aquatilis BNM 0523: Study of protecting agents, rehydration media and freezing temperatures. Lett. Appl. Microbiol. 2011, 53, 565–571. [Google Scholar] [CrossRef]
- Batta, Y.A. Postharvest biological control of apple gray mold by Trichoderma harzianum Rifai formulated in an invert emulsion. Crop Prot. 2004, 23, 19–26. [Google Scholar] [CrossRef]
- Rhodes, D.J. Formulation of biological control agents. In Exploitation of Microorganims; Jones, D.G., Ed.; Chapman & Hall: London, UK, 1993; pp. 411–439. [Google Scholar]
- Torres, R.; Usall, J.; Teixidó, N.; Abadias, M.; Viñas, I. Liquid formulation of the biocontrol agent Candida sake by modifying water activity or adding protectants. J. Appl. Microbiol. 2003, 94, 330–339. [Google Scholar] [CrossRef]
- Abadias, M.; Usall, J.; Teixidó, N.; Viñas, I. Liquid formulation of the postharvest biocontrol agent Candida sake CPA-1 in isotonic solutions. Phytopathology 2003, 93, 436–442. [Google Scholar] [CrossRef]
- Melin, P.; Håkansson, S.; Schnürer, J. Optimisation and comparison of liquid and dry formulations of the biocontrol yeast Pichia anomala J121. Appl. Microbiol. Biotechnol. 2007, 73, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Nandhini, M.; Harish, S.; Aiyanathan, K.E.A.; Durgadevi, D.; Beaulah, A. Glycerol-based liquid formulation of the epiphytic yeast Hanseniaspora guilliermondii isolate YBB3 with multiple modes of action controls postharvest Aspergillus rot in grapes. J. Plant Pathol. 2021, 103, 1253–1264. [Google Scholar] [CrossRef]
- Abadias, M.; Teixidó, N.; Usall, J.; Solsona, C.; Viñas, I. Survival of the postharvest biocontrol yeast Candida sake CPA-1 after dehydration by spray-drying. Biocontrol Sci. Technol. 2005, 15, 835–846. [Google Scholar] [CrossRef]
- Adams, G. The principles of Freeze-Drying. In Cryopreservation and Freeze-Drying Protocols, 2nd ed.; Day, J.G., Stacey, G.N., Eds.; Humana Press Inc.: Totowa, NJ, USA, 2007; pp. 15–38. [Google Scholar]
- Prakash, O.; Nimonkar, Y.; Shouche, Y.S. Practice and prospects of microbial preservation. FEMS Microbiol. Lett. 2013, 339, 1–9. [Google Scholar] [CrossRef]
- Costa, E.; Usall, J.; Teixidó, N.; García, N.; Viñas, I. Effect of protective agents, rehydration media and initial cell concentration on viability of Pantoea agglomerans strain CPA-2 subjected to freeze-drying. J. Appl. Microbiol. 2000, 89, 793–800. [Google Scholar] [CrossRef]
- Abadias, M.; Benabarre, A.; Teixidó, N.; Usall, J.; Viñas, I. Effect of freeze drying and protectants on viability of the biocontrol yeast Candida sake. Int. J. Food Microbiol. 2001, 65, 173–182. [Google Scholar] [CrossRef]
- Abadias, M.; Teixidó, N.; Usall, J.; Benabarre, A.; Viñas, I. Viability, efficacy, and storage stability of freeze-dried biocontrol agent Candida sake using different protective and rehydration media. J. Food Prot. 2001, 64, 856–861. [Google Scholar] [CrossRef]
- Melin, P.; Schnürer, J.; Håkansson, S. Formulation and stabilisation of the biocontrol yeast Pichia anomala. Antonie Van Leeuwenhoek 2011, 99, 107–112. [Google Scholar] [CrossRef]
- Li, B.Q.; Tian, S. Effect of intracellular trehalose in Cryptococcus laurentii and exogenous lyoprotectants on its viability and biocontrol efficacy on Penicillium expansum in apple fruit. Lett. Appl. Microbiol. 2007, 44, 437–442. [Google Scholar] [CrossRef]
- Navarta, L.G.; Calvo, J.; Posetto, P.; Cerutti, S.; Raba, J.; Benuzzi, D.; Sanz, M.I. Postharvest control of gray mold in apples with lyophilized formulations of Cryptococcus laurentii: The effect of cold stress in the survival and effectiveness of the yeast. Food Bioprocess Technol. 2014, 7, 2962–2968. [Google Scholar] [CrossRef]
- Cabrefiga, J.; Francés, J.; Montesinos, E.; Bonaterra, A. Improvement of a dry formulation of Pseudomonas fluorescens EPS62e for fire blight disease biocontrol by combination of culture osmoadaptation with a freeze-drying lyoprotectant. J. Appl. Microbiol. 2014, 117, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Stephan, D.; Da Silva, A.P.M.; Bisutti, I.L. Optimization of a freeze-drying process for the biocontrol agent Pseudomonas spp. and its influence on viability, storability and efficacy. Biol. Control 2016, 94, 74–81. [Google Scholar]
- Strasser, S.; Neureiter, M.; Geppl, M.; Braun, R.; Danner, H. Influence of lyophilization, fluidized bed drying, addition of protectants, and storage on the viability of lactic acid bacteria. J. Appl. Microbiol. 2009, 107, 167–177. [Google Scholar] [CrossRef]
- Bhandari, B.R.; Patel, K.C.; Chen, X.D. Spray-drying of food materials-process and product characteristics. In Drying Technologies in Food Processing; Chen, X.D., Majumdar, A.S., Eds.; Blackwell Publishing Ltd.: Oxford, UK, 2008; pp. 113–159. [Google Scholar]
- Costa, S.S.; Machado, B.A.S.; Martin, A.R.; Bagnara, F.; Ragadalli, S.A.; Alves, A.R.C. Drying by spray drying in the food industry: Micro-encapsulation, process parameters and main carriers used. Afr. J. Food Sci. 2015, 9, 462–470. [Google Scholar]
- Yánez-Mendizábal, V.; Viñas, I.; Usall, J.; Cañamás, T.; Teixidó, N. Endospore production allows using spray-drying as a possible formulation system of the biocontrol agent Bacillus subtilis CPA-8. Biotechnol. Lett. 2012, 34, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Yánez-Mendizábal, V.; Viñas, I.; Usall, J.; Torres, R.; Solsona, C.; Abadias, M.; Teixidó, N. Formulation development of the biocontrol agent Bacillus subtilis strain CPA-8 by spray-drying. J. Appl. Microbiol. 2012, 112, 954–965. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.H.; Wei, H.G.; Li, Y.G.; Li, S.L.; Luo, Y.C.; Zhang, D.J.; Ni, L. Optimization of the spray drying of a Paenibacillus polymyxa-based biopesticide on pilot plant and production scales. Biocontrol Sci. Technol. 2014, 24, 426–435. [Google Scholar] [CrossRef]
- Prabakaran, G.; Hoti, S.L. Optimization of spray-drying conditions for the large-scale preparation of Bacillus thuringiensis var. israelensis after downstream processing. Biotechnol. Bioeng. 2008, 100, 103–107. [Google Scholar] [CrossRef]
- Costa, E.; Teixidó, N.; Usall, J.; Fons, E.; Gimeno, V.; Delgado, J.; Viñas, I. Survival of Pantoea agglomerans strain CPA-2 in a spray-drying process. J. Food Prot. 2002, 65, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Strasser, S. Innovative Product Formulations Applying the Fluidised Bed Technology. Ph.D. Thesis, University of Natural Resources and Life Sciences, Vienna, Austria, 2008. [Google Scholar]
- Larena, I.; De Cal, A.; Linan, M.; Melgarejo, P. Drying of Epicoccum nigrum conidia for obtaining a shelf-stable biological product against brown rot disease. J. Appl. Microbiol. 2003, 94, 508–514. [Google Scholar] [CrossRef]
- Larena, I.; Melgarejo, P.; De Cal, A. Drying of conidia of Penicillium oxalicum, a biological control agent against Fusarium wilt of tomato. J. Phytopathol. 2003, 151, 600–606. [Google Scholar] [CrossRef]
- Guijarro, B.; Larena, I.; Melgarejo, P.; De Cal, A. Effect of drying on conidial viability of Penicillium frequentans, a biological control agent against peach brown rot disease caused by Monilinia spp. Biocontrol Sci. Technol. 2006, 16, 257–269. [Google Scholar] [CrossRef]
- Mounir, R.; Durieux, A.; Bodo, E.; Allard, C.; Simon, J.P.; Achbani, E.H.; El-Jaafari, S.; Douira, A.; Jijakli, M.H. Production, formulation and antagonistic activity of the biocontrol like-yeast Aureobasidium pullulans against Penicillium expansum. Biotechnol. Lett. 2007, 29, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Mokiou, S.; Magan, N. Physiological manipulation and formulation of the biocontrol yeast Pichia anomala for control of Penicillium verrucosum and ochratoxin A contamination of moist grain. Biocontrol Sci. Technol. 2008, 18, 1063–1073. [Google Scholar] [CrossRef]
- Carbó, A.; Torres, R.; Usall, J.; Fons, E.; Teixidó, N. Dry formulations of the biocontrol agent Candida sake CPA-1 using fluidised bed drying to control the main postharvest diseases on fruits. J. Sci. Food Agric. 2017, 97, 3691–3698. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Mishra, G. Fluid bed technology: Overview and parameters for process selection. Int. J. Pharm. Sci. Res. 2010, 2, 236–246. [Google Scholar]
- Santivarangkna, C.; Kulozik, U.; Foerst, P. Alternative drying processes for the industrial preservation of lactic acid starter cultures. Biotechnol. Prog. 2007, 23, 302–315. [Google Scholar] [CrossRef]
- Hemati, A.; Cherif, R.; Saleh, K.; Pont, V. Fluidized bed coating and granulation: Influence of process-related variables and physicochemical properties on the growth kinetics. Powder Technol. 2003, 130, 18–34. [Google Scholar] [CrossRef]
- Planinsek, O.; Pisek, R.; Trojak, A.; Srcic, S. The utilization of surface free-energy parameters for the selection of a suitable binder in fluidized bed granulation. Int. J. Pharm. 2000, 207, 77–88. [Google Scholar] [CrossRef]
- Nag, A.; Das, S. Improving ambient temperature stability of probiotics with stress adaptation and fluidized bed drying. J. Funct. Foods 2013, 5, 170–177. [Google Scholar] [CrossRef]
- Carbó, A.; Torres, R.; Usall, J.; Solsona, C.; Teixidó, N. Fluidized-bed spray-drying formulations of Candida sake CPA-1 by adding biodegradable coatings to enhance their survival under stress conditions. Appl. Microbiol. Biotechnol. 2017, 101, 7865–7876. [Google Scholar] [CrossRef] [PubMed]
- Carbó, A.; Torres, R.; Usall, J.; Marín, A.; Contreras, C.; Chiralt, A.; Teixidó, N. Dehydration of Ampelomyces quisqualis CPA-9 conidia by adding biodegradable coatings: Biocontrol activity against powdery mildew and physical characterization of the formulated product. Biol. Control 2021, 161, 104701. [Google Scholar] [CrossRef]
- Vemmer, M.; Patel, A.V. Review of encapsulation methods suitable for microbial biological control agents. Biol. Control 2013, 67, 380–389. [Google Scholar] [CrossRef]
- González, L.E.; Bashan, Y. Increased growth of the microalga Chlorella vulgaris when coimmobilized and cocultured in alginate beads with the plant-growth-promoting bacterium Azospirillum brasilense. Appl. Environ. Microbiol. 2000, 66, 1527–1531. [Google Scholar] [CrossRef]
- Lumsden, R.D.; Walter, J.F. Development of the biocontrol fungus Gliocladium virens: Risk assessment and approval for horticultural use. In Biological Control: Benefits and Risks; Hokkanen, H.M.T., Lynch, J.M., Eds.; Cambridge University Press: Cambridge, UK, 1995; pp. 263–269. [Google Scholar]
- Sui, Y.; Wisniewski, M.; Droby, S.; Liu, J. Responses of yeast biocontrol agents to environmental stress. Appl. Environ. Microbiol. 2015, 81, 2968–2975. [Google Scholar] [CrossRef]
- Santivarangkna, C.; Kulozik, U.; Foerst, P. Inactivation mechanisms of lactic acid starter cultures preserved by drying processes. J. Appl. Microbiol. 2008, 105, 1–13. [Google Scholar] [CrossRef]
- Biegerdose, A.; Dose, K.; Meffert, R.; Mehler, M.; Risi, S. Extreme dryness and DNA protein cross-links. In Life Sciences and Space Research; Oro, J., Horneck, G., Greenberg, J.M., Raulin, F., Schwartz, A.W., Dose, K., Friedman, E.I., Eds.; Pergamon Press Ltd.: Oxford, UK, 1992; pp. 411–439. [Google Scholar]
- Fu, N.; Chen, X.D. Towards a maximal cell survival in convective thermal drying processes. Food Res. Int. 2011, 44, 1127–1149. [Google Scholar] [CrossRef]
- Collins, D.P.; Jacobsen, B.J. Optimizing a Bacillus subtilis isolate for biological control of sugar beet cercospora leaf spot. Biol. Control 2003, 26, 153–161. [Google Scholar] [CrossRef]
- Minh, H.N.T.; Durand, A.; Loison, P.; Perrier-Cornet, J.M.; Gervais, P. Effect of sporulation conditions on the resistance of Bacillus subtilis spores to heat and high pressure. Appl. Microbiol. Biotechnol. 2011, 90, 1409–1417. [Google Scholar] [CrossRef]
- Teixidó, N.; Cañamás, T.P.; Usall, U.; Torres, R.; Magan, N.; Viñas, I. Accumulation of the compatible solutes, glycine–betaine andectoine, in osmotic stress adaptation and heat shock cross-protection in the biocontrol agent Pantoea agglomerans CPA-2. Lett. Appl. Microbiol. 2005, 41, 248–252. [Google Scholar] [CrossRef]
- Teixidó, N.; Cañamás, T.P.; Abadias, M.; Usall, J.; Solsona, C.; Casals, C.; Viñas, I. Improving low water activity and desiccation tolerance of the biocontrol agent Pantoea agglomerans CPA-2 by osmotic treatments. J. Appl. Microbiol. 2006, 101, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Teixido, N.; Segarra, G.; Casals, C.; Usall, J.; Torres, R. Formulations to improve biocontrol products shelf-life and/or ecosystem adaptation. In How Research Can Stimulate the Development of Commercial Biological Control Against Plant Diseases; Progress in Biological Control; De Cal, A., Melgarejo, P., Magan, N., Eds.; Springer: Cham, Switzerland, 2020; Volume 2, pp. 257–273. [Google Scholar]
- Sabuquillo, P.; De Cal, A.; Melgarejo, P. Development of a dried Penicillium oxalicum conidial formulation for use as a biological agent against Fusarium wilt of tomato: Selection of optimal additives and storage conditions for maintaining conidial viability. Biol. Control 2010, 54, 221–229. [Google Scholar] [CrossRef]
- Leslie, S.B.; Israeli, E.; Lighthart, B.; Crowe, J.H.; Crowe, L.M. Trehalose and sucrose protect both membranes and proteins in intact bacteria during drying. Appl. Environ. Microbiol. 1995, 61, 3592–3597. [Google Scholar] [CrossRef] [PubMed]
- Morgan, C.A.; Herman, N.; White, P.A.; Vesey, G. Preservation of micro-organisms by drying; A review. J. Microbiol. Methods 2006, 66, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Alamprese, C.; Cappa, C.; Ratti, S.; Limbo, S.; Signorelli, M.; Fessas, D.; Lucisano, M. Shelf-life extension of whole-wheat bread sticks: Formulation and packaging strategies. Food Chem. 2017, 230, 532–539. [Google Scholar] [CrossRef]
- Costa, E.; Usall, J.; Teixido, N.; Torres, R.; Vinas, I. Effect of package and storage conditions on viability and efficacy of the freeze-dried biocontrol agent Pantoea agglomerans strain CPA-2. J. Appl. Microbiol. 2002, 92, 873–878. [Google Scholar] [CrossRef]
- Torres, R.; Solsona, C.; Viñas, I.; Usall, J.; Plaza, P.; Teixidó, N. Optimization of packaging and storage conditions of a freeze-dried Pantoea agglomerans formulation for controlling postharvest diseases in fruit. J. Appl. Microbiol. 2014, 117, 173–184. [Google Scholar] [CrossRef]
- Gallagher, E.; Kunkel, A.; Gormley, T.R.; Arendt, E.K. The effect of dairy and rice powder addition on loaf and crumb characteristics, and on shelf life (intermediate and long-term) of gluten-free breads stored in a modified atmosphere. Eur. Food Res. Technol. 2003, 218, 44–48. [Google Scholar] [CrossRef]
- Elzein, A.; Kroschel, J.; Marley, P.; Cadisch, G. Does vacuum-packaging or co-delivered amendments enhance shelf-life of Striga-mycoherbicidal products containing Fusarium oxysporum f. sp. strigae during storage? Biocontrol Sci. Technol. 2009, 19, 349–367. [Google Scholar] [CrossRef]
- Gotor-Vila, A.; Usall, J.; Torres, R.; Solsona, C.; Teixidó, N. Enhanced shelf-life of the formulated biocontrol agent Bacillus amyloliquefaciens CPA-8 combining diverse packaging strategies and storage conditions. Int. J. Food Microbiol. 2019, 290, 205–213. [Google Scholar] [CrossRef]
- Carbó, A.; Teixidó, N.; Usall, J.; Torres, R. Verifying the biocontrol activity of novel film-forming formulations of Candida sake CPA-1: Resilience in relation to environmental factors, rainfall episodes, and control of Botrytis cinerea on different hosts. J. Sci. Food Agric. 2019, 99, 4969–4976. [Google Scholar] [CrossRef] [PubMed]
- Teshler, M.P.; Ash, G.J.; Zolotarov, Y.; Watson, A.K. Increased shelf life of a bioherbicide through combining modified atmosphere packaging and low temperatures. Biocontrol Sci. Technol. 2007, 17, 387–400. [Google Scholar] [CrossRef]
- Xue, J.J.; Hou, J.G.; Zhang, Y.A.; Wang, C.Y.; Wang, Z.; Yu, J.J.; Wang, Y.B.; Wang, Y.Z.; Wang, Q.H.; Sung, C.K. Optimization of storage condition for maintaining long-term viability of nematophagous fungus Esteya vermicola as biocontrol agent against pinewood nematode. World J. Microbiol. Biotechnol. 2014, 30, 2805–2810. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teixidó, N.; Usall, J.; Torres, R. Insight into a Successful Development of Biocontrol Agents: Production, Formulation, Packaging, and Shelf Life as Key Aspects. Horticulturae 2022, 8, 305. https://doi.org/10.3390/horticulturae8040305
Teixidó N, Usall J, Torres R. Insight into a Successful Development of Biocontrol Agents: Production, Formulation, Packaging, and Shelf Life as Key Aspects. Horticulturae. 2022; 8(4):305. https://doi.org/10.3390/horticulturae8040305
Chicago/Turabian StyleTeixidó, Neus, Josep Usall, and Rosario Torres. 2022. "Insight into a Successful Development of Biocontrol Agents: Production, Formulation, Packaging, and Shelf Life as Key Aspects" Horticulturae 8, no. 4: 305. https://doi.org/10.3390/horticulturae8040305
APA StyleTeixidó, N., Usall, J., & Torres, R. (2022). Insight into a Successful Development of Biocontrol Agents: Production, Formulation, Packaging, and Shelf Life as Key Aspects. Horticulturae, 8(4), 305. https://doi.org/10.3390/horticulturae8040305






