Yield and Compositional Profile of Eggplant Fruits as Affected by Phosphorus Supply, Genotype and Grafting
Abstract
1. Introduction
2. Materials and Methods
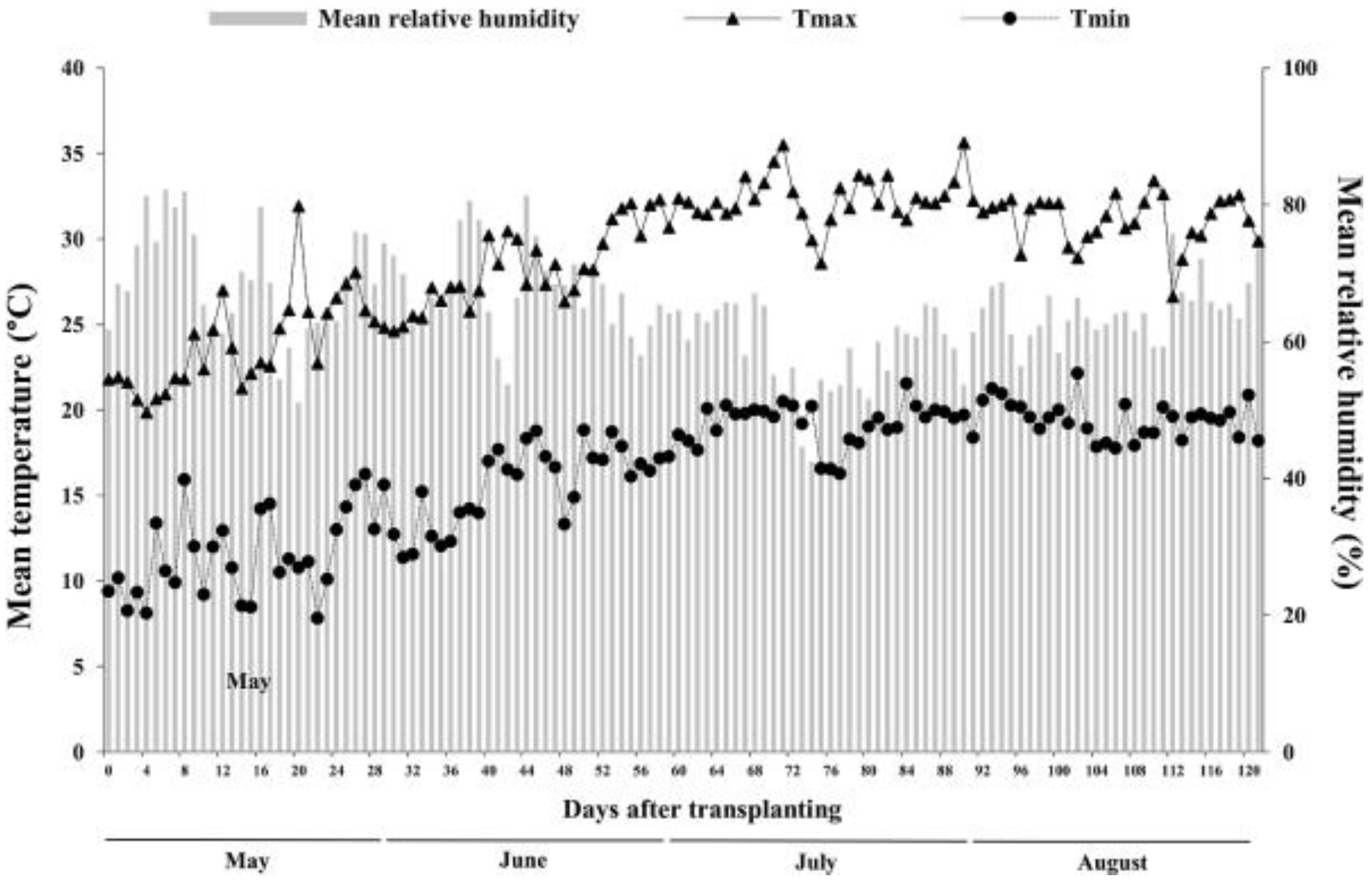
2.1. Experimental Site
2.2. Experimental Design and Crop Management
2.3. Fruit Collection, Carpometric Determinations and Sample Preparation
2.4. Determination of Total Phenols Content
2.5. Determination of Total N and Mineral Profile
2.6. Statistical Procedures
3. Results
3.1. Yield and Carpometric Traits
3.2. Fruit N and Mineral Profile
3.2.1. Macronutrients
3.2.2. Mesonutrients
3.2.3. Micronutrients
3.2.4. Correlation among Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Obersteiner, M.; Peñuelas, J.; Ciais, P.; Van Der Velde, M.; Janssens, I.A. The phosphorus trilemma. Nat. Geosci. 2013, 6, 897–898. [Google Scholar] [CrossRef]
- El Bilali, H.; Callenius, C.; Strassner, C.; Probst, L. Food and nutrition security and sustainability transitions in food systems. Food Energy Secur. 2019, 8, e00154. [Google Scholar] [CrossRef]
- Cordell, D.; Drangert, J.O.; White, S. The story of phosphorus: Global food security and food for thought. Glob. Environ. Chang. 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Bindraban, P.S.; Dimkpa, C.O.; Pandey, R. Exploring phosphorus fertilizers and fertilization strategies for improved human and environmental health. Biol. Fertil. Soils 2020, 56, 299–317. [Google Scholar] [CrossRef]
- Wang, R.; Min, J.; Kronzucker, H.J.; Li, Y.; Shi, W. N and P runoff losses in China’s vegetable production systems: Loss characteristics, impact, and management practices. Sci. Total Environ. 2019, 663, 971–979. [Google Scholar] [CrossRef]
- Wang, R.; Shi, W.; Li, Y. Phosphorus supply and management in vegetable production systems in China. Front. Agric. Sci. Eng. 2019, 6, 348–356. [Google Scholar] [CrossRef]
- Kalkhajeh, Y.K.; Huang, B.; Sørensen, H.; Holm, P.E.; Hansen, H.C.B. Phosphorus accumulation and leaching risk of greenhouse vegetable soils in Southeast China. Pedosphere 2021, 31, 683–693. [Google Scholar] [CrossRef]
- Buturi, C.V.; Mauro, R.P.; Fogliano, V.; Leonardi, C.; Giuffrida, F. Mineral biofortification of vegetables as a tool to improve human diet. Foods 2021, 10, 223. [Google Scholar] [CrossRef]
- Ierna, A.; Mauro, R.P.; Leonardi, C.; Giuffrida, F. Shelf-life of bunched carrots as affected by nitrogen fertilization and leaf presence. Agronomy 2020, 10, 1982. [Google Scholar] [CrossRef]
- Sabatino, L.; Iapichino, G.; Consentino, B.B.; D’Anna, F.; Rouphael, Y. Rootstock and arbuscular mycorrhiza combinatorial effects on eggplant crop performance and fruit quality under greenhouse conditions. Agronomy 2020, 10, 693. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/faostat/en/#home (accessed on 26 January 2022).
- Ierna, A.; Mauro, R.P.; Mauromicale, G. Improved yield and nutrient efficiency in two globe artichoke genotypes by balancing nitrogen and phosphorus supply. Agron. Sustain. Dev. 2012, 32, 773–780. [Google Scholar] [CrossRef]
- Zhang, B.; Fu, Z.; Huang, J.; Wang, J.; Xu, S.; Zhang, L. Consumers’ perceptions, purchase intention, and willingness to pay a premium price for safe vegetables: A case study of Beijing, China. J. Clean. Prod. 2018, 197, 1498–1507. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Leonardi, C.; Bie, Z. Role of grafting in vegetable crops grown under saline conditions. Sci. Hortic. 2010, 127, 147–155. [Google Scholar] [CrossRef]
- Mauro, R.P.; Agnello, M.; Distefano, M.; Sabatino, L.; San Bautista Primo, A.; Leonardi, C.; Giuffrida, F. Chlorophyll fluorescence, photosynthesis and growth of tomato plants as affected by long-term oxygen root zone deprivation and grafting. Agronomy 2020, 10, 137. [Google Scholar] [CrossRef]
- Semiz, G.D.; Suarez, D.L. Impact of grafting, salinity and irrigation water composition on eggplant fruit yield and ion relations. Sci. Rep. 2019, 9, 19373. [Google Scholar] [CrossRef]
- Maršić, N.K.; Mikulić-Petkovšek, M.; Štampar, F. Grafting influences phenolic profile and carpometric traits of fruits of greenhouse-grown eggplant (Solanum melongena L.). J. Agric. Food Chem. 2014, 62, 10504–10514. [Google Scholar] [CrossRef]
- Hopkins, B.G. Phosphorus. In Handbook of Plant Nutrition; Barker, A.V., Pilbeam, D.J., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 65–126. [Google Scholar]
- Leonardi, C.; Giuffrida, F. Growth rate and carpometric characteristics during eggplant fruit growth. Acta Hortic. 2009, 807, 175–180. [Google Scholar] [CrossRef]
- Sabatino, L.; Iapichino, G.; Maggio, A.; D’Anna, E.; Bruno, M.; D’Anna, F. Grafting affects yield and phenolic profile of Solanum melongena L. landraces. J. Integr. Agric. 2016, 15, 1017–1024. [Google Scholar] [CrossRef]
- Cassaniti, C.; Giuffrida, F.; Scuderi, D.; Leonardi, C. The effect of rootstock and nutrient solution concentration on eggplant grown in a soilless system. J. Food Agric. Environ. 2011, 9, 252–256. [Google Scholar]
- Mauro, R.P.; Agnello, M.; Onofri, A.; Leonardi, C.; Giuffrida, F. Scion and rootstock differently influence growth, yield and quality characteristics of cherry tomato. Plants 2020, 9, 1725. [Google Scholar] [CrossRef]
- Zubillaga, M.M.; Aristi, J.P.; Lavado, R.S. Effect of phosphorus and nitrogen fertilization on sunflower (Helianthus annus L.) nitrogen uptake and yield. J. Agron. Crop Sci. 2002, 188, 267–274. [Google Scholar] [CrossRef]
- Giuffrida, F.; Agnello, M.; Mauro, R.P.; Ferrante, A.; Leonardi, C. Cultivation under salt stress conditions influences postharvest quality and glucosinolates content of fresh-cut cauliflower. Sci. Hortic. 2018, 236, 166–174. [Google Scholar] [CrossRef]
- Niño-Medina, G.; Urías-Orona, V.; Muy-Rangel, M.D.; Heredia, J.B. Structure and content of phenolics in eggplant (Solanum melongena)—A review. S. Afr. J. Bot. 2017, 111, 161–169. [Google Scholar] [CrossRef]
- Komatsu, W.; Itoh, K.; Akutsu, S.; Kishi, H.; Ohhira, S. Nasunin inhibits the lipopolysaccharide-induced pro-inflammatory mediator production in RAW264 mouse macrophages by suppressing ROS-mediated activation of PI3 K/Akt/NF-κB and p38 signaling pathways. Biosci. Biotechnol. Biochem. 2017, 81, 1956–1966. [Google Scholar] [CrossRef]
- Mennella, G.; Lo Scalzo, R.; Fibiani, M.; DAlessandro, A.; Francese, G.; Toppino, L.; Acciarri, N.; De Almeida, A.E.; Rotino, G.L. Chemical and bioactive quality traits during fruit ripening in eggplant (S. melongena L.) and allied species. J. Agric. Food Chem. 2012, 60, 11821–11831. [Google Scholar] [CrossRef]
- Mauro, R.P.; Agnello, M.; Rizzo, V.; Graziani, G.; Fogliano, V.; Leonardi, C.; Giuffrida, F. Recovery of eggplant field waste as a source of phytochemicals. Sci. Hortic. 2020, 261, 109023. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Panayiotou, C.; Tzortzakis, N. Nitrogen and phosphorus levels affected plant growth, essential oil composition and antioxidant status of lavender plant (Lavandula angustifolia Mill.). Ind. Crops Prod. 2016, 83, 577–586. [Google Scholar] [CrossRef]
- Valentinuzzi, F.; Mason, M.; Scampicchio, M.; Andreotti, C.; Cesco, S.; Mimmo, T. Enhancement of the bioactive compound content in strawberry fruits grown under iron and phosphorus deficiency. J. Sci. Food Agric. 2015, 95, 2088–2094. [Google Scholar] [CrossRef]
- Chen, R.; Song, S.; Li, X.; Liu, H.; Huang, D. Phosphorus deficiency restricts plant growth but induces pigment formation in the flower stalk of Chinese kale. Hortic. Environ. Biotechnol. 2013, 54, 243–248. [Google Scholar] [CrossRef]
- Postma, J.A.; Dathe, A.; Lynch, J.P. The optimal lateral root branching density for maize depends on nitrogen and phosphorus availability. Plant Physiol. 2014, 166, 590–602. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Imtiaz, M.; Kong, Q.; Cheng, F.; Ahmed, W.; Huang, Y.; Bie, Z. Grafting: A technique to modify ion accumulation in horticultural crops. Front. Plant Sci. 2016, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kyriacou, M.C.; Rouphael, Y.; Colla, G.; Zrenner, R.; Schwarz, D. Vegetable grafting: The implications of a growing agronomic imperative for vegetable fruit quality and nutritive value. Front. Plant Sci. 2017, 8, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, O.M.; Luzuriaga-McPherson, A.; Lin, Y.; Gilbert, L.C.; Ha, S.W.; Beck, G.R. Impact of phosphorus-based food additives on bone and mineral metabolism. J. Clin. Endocrinol. Metab. 2015, 100, 4264–4271. [Google Scholar] [CrossRef] [PubMed]
- Gumz, M.L.; Rabinowitz, L.; Wingo, C.S. Disorders of fluids and electrolytes: An integrated view of potassium homeostasis. N. Engl. J. Med. 2015, 373, 60–72. [Google Scholar] [CrossRef]
- De Iorio, A.F.; Gorgoschide, L.; Rendina, A.; Barros, M.J. Effect of phosphorus, copper, and zinc addition on the phosphorus/copper and phosphorus/zinc interaction in lettuce. J. Plant Nutr. 1996, 19, 481–491. [Google Scholar] [CrossRef]
- Irfan, M.; Abbas, M.; Shah, J.A.; Depar, N.; Memon, M.Y.; Sial, N.A. Interactive effect of phosphorus and boron on plant growth, nutrient accumulation and grain yield of wheat grown on calcareous soil. Eurasian J. Soil Sci. 2019, 8, 17–26. [Google Scholar] [CrossRef]
- Awan, Z.I.; Abbasi, M.K. Interactive effect of phosphorus and copper on maize growth. Pak. J. Agric. Res. 2000, 16, 105–108. [Google Scholar]
- Barker, A.V.; Eaton, T.E. Zinc. In Handbook of Plant Nutrition; Barker, A.V., Pilbeam, D.J., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 537–566. [Google Scholar]
- Shaaban, M.M. Role of boron in plant nutrition and human health. Am. J. Plant Physiol. 2010, 5, 224–240. [Google Scholar] [CrossRef]
- Alejandro, S.; Höller, S.; Meier, B.; Peiter, E. Manganese in plants: From acquisition to subcellular allocation. Front. Plant Sci. 2020, 11, 1–23. [Google Scholar] [CrossRef]
- Natasha, N.; Shahid, M.; Bibi, I.; Iqbal, J.; Khalid, S.; Murtaza, B.; Bakhat, H.F.; Farooq, A.B.U.; Amjad, M.; Hammad, H.M.; et al. Zinc in soil-plant-human system: A data-analysis review. Sci. Total Environ. 2022, 808, 152024. [Google Scholar] [CrossRef]
- Savvas, D.; Colla, G.; Rouphael, Y.; Schwarz, D. Amelioration of heavy metal and nutrient stress in fruit vegetables by grafting. Sci. Hortic. 2010, 127, 156–161. [Google Scholar] [CrossRef]
- Rouphael, Y.; Cardarelli, M.; Rea, E.; Colla, G. Grafting of cucumber as a means to minimize copper toxicity. Environ. Exp. Bot. 2008, 63, 49–58. [Google Scholar] [CrossRef]
- Olivares, M.; Walter, T.; Hertrampf, E.; Pizarro, F. Anaemia and iron deficiency disease in children. Br. Med. Bull. 1999, 55, 534–543. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Beleggia, R.; Fragasso, M.; Miglietta, F.; Cattivelli, L.; Menga, V.; Nigro, F.; Pecchioni, N.; Fares, C. Mineral composition of durum wheat grain and pasta under increasing atmospheric CO2 concentrations. Food Chem. 2018, 242, 53–61. [Google Scholar] [CrossRef]
- Arao, T.; Takeda, H.; Nishihara, E. Reduction of cadmium translocation from roots to shoots in eggplant (Solanum melongena) by grafting onto Solanum torvum rootstock. Soil Sci. Plant Nutr. 2008, 54, 555–559. [Google Scholar] [CrossRef]
- Saenchai, C.; Bouain, N.; Kisko, M.; Prom-u-thai, C.; Doumas, P.; Rouached, H. The involvement of OsPHO1;1 in the regulation of iron transport through integration of phosphate and Zinc deficiency signaling. Front. Plant Sci. 2016, 7, 1–9. [Google Scholar] [CrossRef]
- Rai, S.; Singh, P.K.; Mankotia, S.; Swain, J.; Satbhai, S.B. Iron homeostasis in plants and its crosstalk with copper, zinc, and manganese. Plant Stress 2021, 1, 100008. [Google Scholar] [CrossRef]
| Soil Characteristic | Unit | Value |
|---|---|---|
| Clay | % | 30 |
| Silt | % | 13 |
| Sand | % | 57 |
| Organic matter | % | 2.8 |
| pH | log[H+] | 7.5 |
| Cation exchange capacity | cmolc kg−1 | 19.1 |
| Total N | mg kg−1 | 1.4 |
| Available P | mg kg−1 | 1.5 |
| Exchangeable K | mg kg−1 | 278 |
| Mg | mg kg−1 | 433 |
| Ca | mg kg−1 | 5245 |
| Na | mg kg−1 | 125 |
| Fe | mg kg−1 | 107 |
| Cu | mg kg−1 | 12 |
| Zn | mg kg−1 | 6 |
| Variable | Phosphorus Level | Cultivar | Rootstock | Mean | |||
|---|---|---|---|---|---|---|---|
| P30 | P90 | ‘Birgah’ | ‘Dalia’ | Control | ‘Espina’ | ||
| Marketable yield (kg plant−1) | 2.80 ± 0.74 a | 2.80 ± 0.50 a | 2.97 ± 0.68 a | 2.64 ± 0.53 b | 2.36 ± 0.49 b | 3.25 ± 0.57 a | 2.80 ± 0.62 |
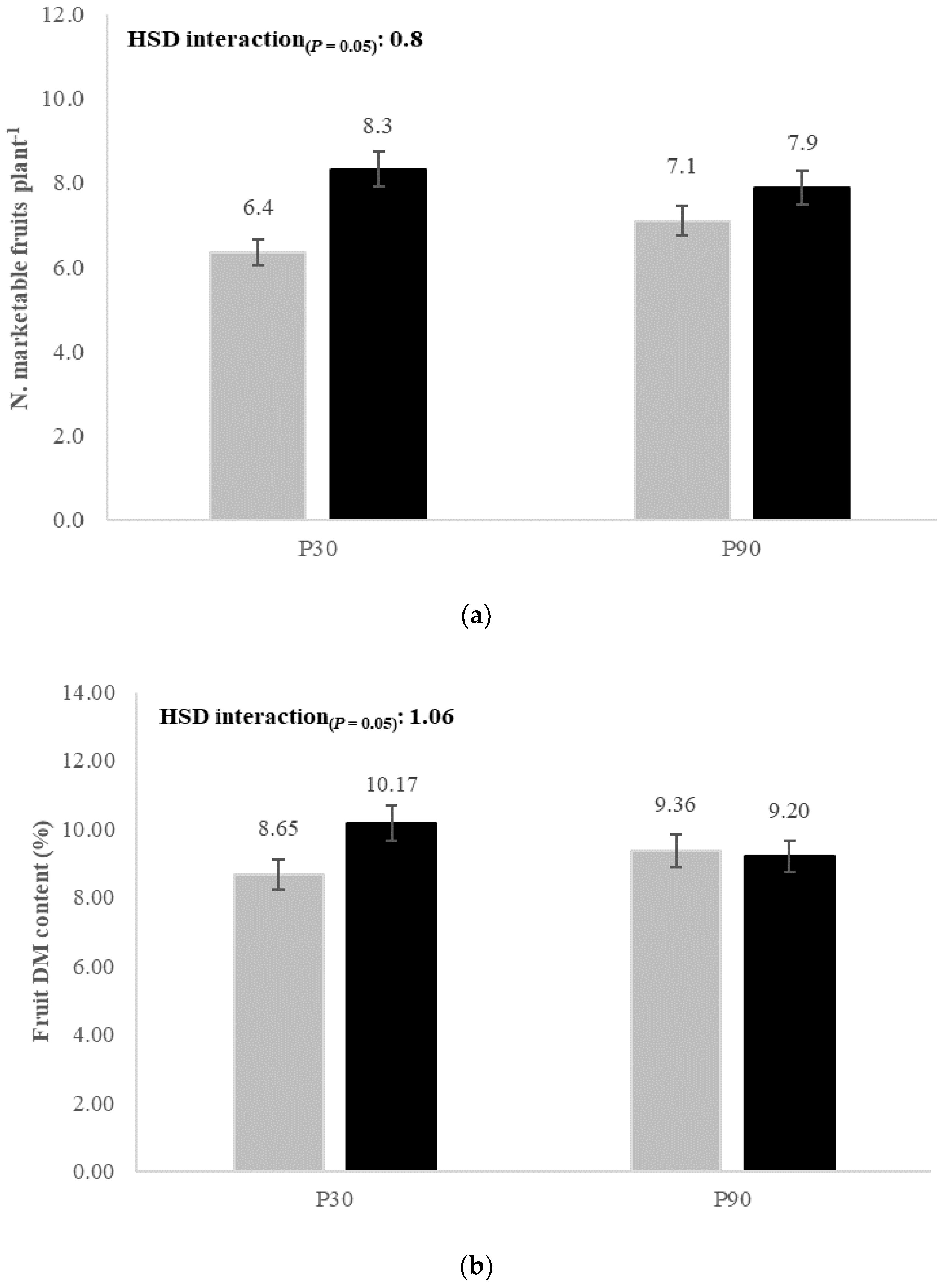
| Marketable fruits (n. plant−1) | 7.34 ± 2.10 a | 7.49 ± 1.97 a | 5.71 ± 1.86 b | 9.12 ± 1.70 a | 6.73 ± 1.81 b | 8.11 ± 2.00 a | 7.42 ± 1.99 |
| Fruit FW (g) | 402 ± 125 a | 399 ± 128 a | 514 ± 105 a | 287 ± 127 b | 371 ± 109 b | 430 ± 142 a | 401 ± 124 |
| Fruit DM content (%) | 9.70 ± 1.06 a | 9.00 ± 0.90 b | 9.43 ± 0.98 a | 9.27 ± 1.13 a | 9.01 ± 0.90 b | 9.69 ± 1.08 a | 9.35 ± 1.03 |
| Fruit firmness (N) | 14.6 ± 2.7 a | 13.5 ± 2.2 b | 16.0 ± 1.6 a | 12.0 ± 1.2 b | 13.5 ± 2.4 b | 14.5 ± 2.6 a | 14.0 ± 2.5 |
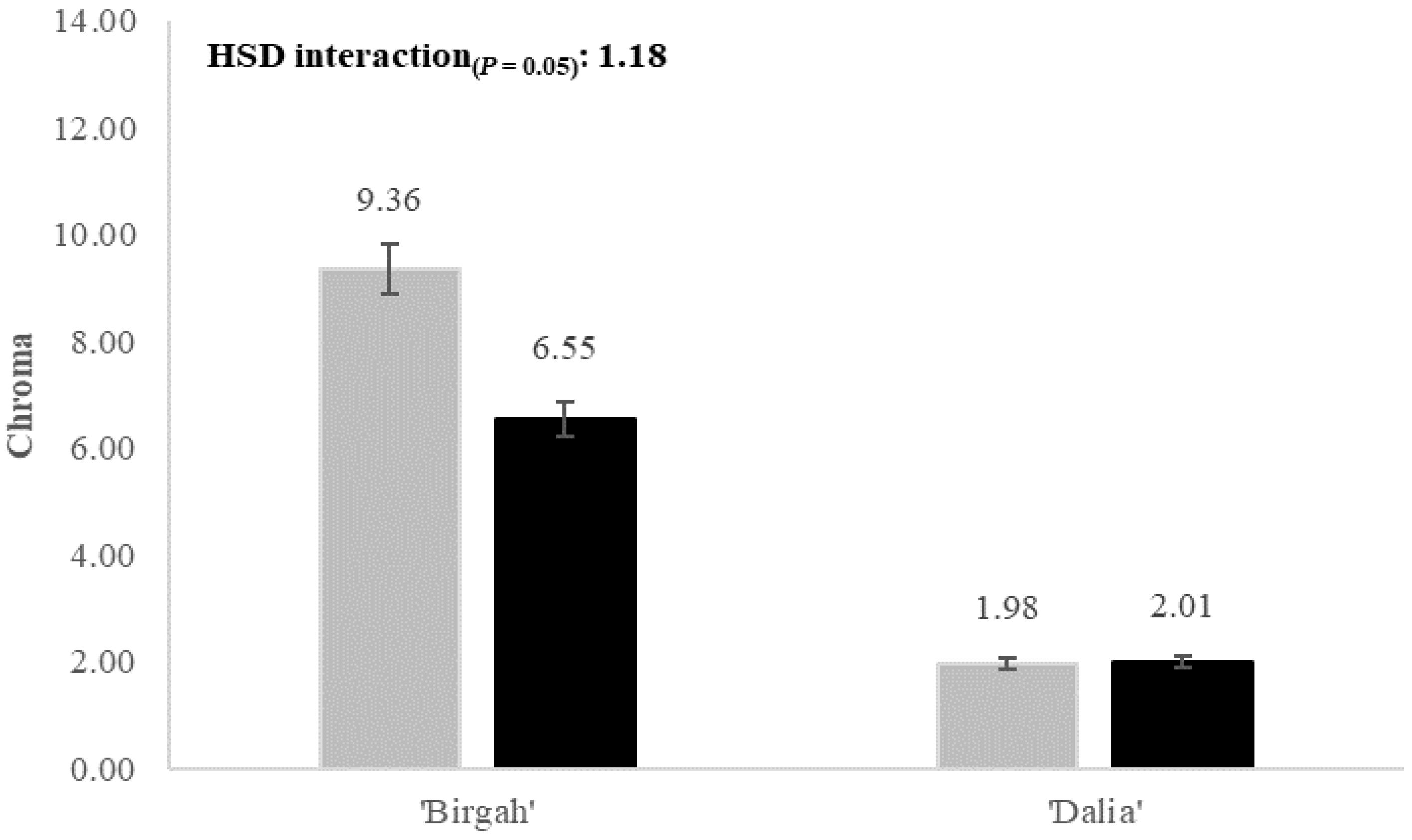
| Chroma (adimensional) | 5.67 ± 1.06 a | 4.28 ± 1.40 b | 7.95 ± 2.07 a | 2.00 ± 1.09 b | 4.90 ± 1.70 a | 5.05 ± 1.81 a | 4.97 ± 2.05 |
| TPC (mg CAE kg−1 FW) | 2012 ± 380 a | 1721 ± 373 b | 1538 ± 268 b | 2196 ± 377 a | 1878 ± 415 a | 1795 ± 398 a | 1837 ± 456 |
| Variable | Phosphorus Level | Cultivar | Rootstock | Mean | |||
|---|---|---|---|---|---|---|---|
| P30 | P90 | ‘Birgah’ | ‘Dalia’ | Control | ‘Espina’ | ||
| Macronutrients (mg kg−1 FW) | |||||||
| N | 140 ± 14 a | 119 ± 19 b | 122 ± 22 b | 137 ± 13 a | 139 ± 19 a | 118 ± 18 b | 130 ± 19 |
| P | 165 ± 16 a | 148 ± 12 b | 150 ±21 a | 162 ± 8 a | 157 ± 16 a | 155 ± 18 a | 156 ± 17 |
| K | 625 ± 45 a | 575 ± 75 b | 561 ± 54 b | 640 ± 53 a | 580 ± 79 b | 620 ± 53 a | 600 ± 66 |
| Mesonutrients (mg kg−1 FW) | |||||||
| Mg | 102 ± 8 a | 98 ± 9 a | 97 ± 9 a | 103 ± 8 a | 100 ± 11 a | 100 ± 6 a | 100 ± 9 |
| Ca | 69 ± 20 a | 78 ± 36 a | 56 ± 10 b | 91 ±31 a | 62 ± 10 b | 85 ± 37 a | 73 ± 29 |
| S | 9.5 ± 1.5 a | 8.4 ± 1.8 b | 9.1 ± 1.9 a | 8.8 ± 1.6 a | 10.3 ± 0.9 a | 7.5 ± 1.0 b | 8.9 ± 1.7 |
| Na | 15.8 ± 8.8 a | 11.5 ± 6.7 b | 9.4 ± 3.1 b | 17.9 ± 9.1 a | 18.0 ± 9.2 a | 9.2 ± 2.2 b | 13.6 ± 7.9 |
| Micronutrients (µg kg−1 FW) | |||||||
| Fe | 2900 ± 857 b | 3339 ± 871 a | 3702 ± 757 a | 2538 ± 535 b | 3139 ± 930 a | 3101 ± 856 a | 3120 ± 874 |
| Mn | 521 ± 63 a | 512 ± 76 a | 512 ± 62 a | 512 ± 78 a | 466 ± 64 b | 558 ±35 a | 512 ± 69 |
| Cu | 495 ± 42 a | 376 ± 105 b | 423 ± 125 a | 448 ± 67 a | 463 ± 63 a | 408 ±121 b | 436 ± 99 |
| B | 1809 ± 972 a | 1374 ± 661 b | 1246 ± 416 b | 1937 ± 1025 a | 1053 ± 294 b | 2131 ±874 a | 1592 ± 843 |
| Zn | 3106 ±1832 a | 2172 ± 1224 b | 2198 ± 564 b | 3080 ±2124 a | 1657 ± 604 b | 3621 ±1692 a | 2639 ± 1597 |
| Variable | P30 | P90 | HSDinteraction (p = 0.05) | ||
|---|---|---|---|---|---|
| ‘Birgah’ | ‘Dalia’ | ‘Birgah’ | ‘Dalia’ | ||
| K (mg kg−1 FW) | 596 ± 14 | 636 ± 59 | 527 ± 38 | 643 ± 51 | 54 |
| Mg (mg kg−1 FW) | 106 ± 7 | 106 ± 9 | 88 ± 7 | 100 ± 6 | 14 |
| Mn (µg kg−1 FW) | 543 ± 31 | 499 ± 80 | 480 ± 69 | 525 ± 81 | 57 |
| Cu (µg kg−1 FW) | 521 ± 34 | 469 ± 32 | 325 ± 101 | 427 ± 88 | 67 |
| Variable | P30 | P90 | HSDinteraction (p = 0.05) | ||
|---|---|---|---|---|---|
| Control | ‘Espina’ | Control | ‘Espina’ | ||
| Fe (µg kg−1 FW) | 3176 ± 1161 | 2625 ± 290 | 3102 ± 741 | 3577 ± 993 | 550 |
| B (µg kg−1 FW) | 1113 ± 304 | 2505 ± 907 | 993 ± 298 | 1756 ± 722 | 319 |
| Zn (µg kg−1 FW) | 1813 ± 600 | 4399 ± 1736 | 1501 ± 621 | 2843 ± 1353 | 441 |
| Variable | ‘Birgah’ | ‘Dalia’ | HSDinteraction (p = 0.05) | ||
|---|---|---|---|---|---|
| Control | ‘Espina’ | Control | ‘Espina’ | ||
| K (mg kg−1 FW) | 546 ±59 | 577 ± 49 | 612 ± 42 | 667 ± 53 | 52 |
| Ca (mg kg−1 FW) | 53 ± 8 | 59 ± 12 | 69 ± 3 | 112 ± 32 | 20 |
| Na (mg kg−1 FW) | 10.5 ± 3.3 | 8.3 ± 2.6 | 25.5 ± 6.4 | 10.2 ± 1.4 | 3.7 |
| Cu (µg kg−1 FW) | 477 ± 76 | 369 ± 147 | 450 ± 52 | 447 ± 85 | 67 |
| B (µg kg−1 FW) | 1055 ± 365 | 1437 ± 400 | 1051 ± 237 | 2824 ± 607 | 319 |
| Zn (µg kg−1 FW) | 2130 ± 344 | 2266 ± 755 | 1184 ± 384 | 4976 ± 1150 | 441 |
| Marketable Yield | Marketable Fruits | Fruit FW | Fruit DM Content | Fruit Firmness | Chroma | TPC | N | P | K | Mg | Ca | S | Na | Fe | Mn | Cu | B | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Marketable fruits | NS | - | ||||||||||||||||
| Fruit FW | 0.543 ** | NS | - | |||||||||||||||
| Fruit DM content | 0.539 ** | NS | NS | - | ||||||||||||||
| Fruit firmness | 0.464 * | −0.535 ** | 0.868 *** | 0.602 ** | - | |||||||||||||
| Chroma | NS | NS | 0.879 *** | NS | 0.864 *** | - | ||||||||||||
| TPC | NS | 0.574 ** | NS | NS | −0.583 ** | −0.550 ** | - | |||||||||||
| N | NS | NS | −0.434 * | NS | NS | NS | 0.474 * | - | ||||||||||
| P | NS | NS | NS | NS | NS | NS | 0.502 * | 0.658 *** | - | |||||||||
| K | NS | 0.455 * | −0.546 ** | NS | NS | NS | 0.713 *** | 0.450 * | 0.516 ** | - | ||||||||
| Mg | NS | NS | NS | NS | NS | NS | 0.497 * | 0.613 ** | 0.503 * | 0.497 * | - | |||||||
| Ca | NS | 0.692 *** | −0.571 ** | NS | −0.499 * | −0.597 ** | NS | NS | NS | NS | NS | - | ||||||
| S | −0.553 ** | NS | NS | NS | NS | NS | NS | 0.644 *** | NS | NS | NS | −0.462 * | - | |||||
| Na | −0.566 ** | NS | −0.595 ** | NS | −0.423 * | NS | 0.588 ** | 0.551 ** | NS | 0.594 ** | NS | NS | 0.439 * | - | ||||
| Fe | NS | −0.598 ** | 0.669 *** | NS | 0.537 ** | 0.535 ** | −0.531 ** | NS | NS | NS | NS | NS | NS | −0.497 * | - | |||
| Mn | 0.534 ** | NS | NS | 0.459 * | NS | NS | NS | NS | NS | NS | NS | 0.437 * | −0.516 ** | −0.466 * | NS | - | ||
| Cu | NS | NS | NS | NS | NS | NS | NS | 0.479 * | 0.615 ** | 0.442 * | NS | NS | 0.424 * | NS | NS | - | ||
| B | NS | 0.665 *** | NS | 0.452 * | NS | NS | NS | NS | NS | NS | 0.452 * | 0.670 *** | −0.411 * | NS | NS | 0.637 *** | NS | - |
| Zn | NS | 0.528 ** | NS | NS | NS | NS | NS | NS | NS | NS | NS | 0.546 ** | NS | NS | −0.460 * | 0.579 ** | NS | 0.901 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mauro, R.P.; Stazi, S.R.; Distefano, M.; Giuffrida, F.; Marabottini, R.; Sabatino, L.; Allevato, E.; Cannata, C.; Basile, F.; Leonardi, C. Yield and Compositional Profile of Eggplant Fruits as Affected by Phosphorus Supply, Genotype and Grafting. Horticulturae 2022, 8, 304. https://doi.org/10.3390/horticulturae8040304
Mauro RP, Stazi SR, Distefano M, Giuffrida F, Marabottini R, Sabatino L, Allevato E, Cannata C, Basile F, Leonardi C. Yield and Compositional Profile of Eggplant Fruits as Affected by Phosphorus Supply, Genotype and Grafting. Horticulturae. 2022; 8(4):304. https://doi.org/10.3390/horticulturae8040304
Chicago/Turabian StyleMauro, Rosario Paolo, Silvia Rita Stazi, Miriam Distefano, Francesco Giuffrida, Rosita Marabottini, Leo Sabatino, Enrica Allevato, Claudio Cannata, Federico Basile, and Cherubino Leonardi. 2022. "Yield and Compositional Profile of Eggplant Fruits as Affected by Phosphorus Supply, Genotype and Grafting" Horticulturae 8, no. 4: 304. https://doi.org/10.3390/horticulturae8040304
APA StyleMauro, R. P., Stazi, S. R., Distefano, M., Giuffrida, F., Marabottini, R., Sabatino, L., Allevato, E., Cannata, C., Basile, F., & Leonardi, C. (2022). Yield and Compositional Profile of Eggplant Fruits as Affected by Phosphorus Supply, Genotype and Grafting. Horticulturae, 8(4), 304. https://doi.org/10.3390/horticulturae8040304










