A Study of Shoot Growth, Leaf Photosynthesis, and Nutrients in ‘Lingfengjing’ Litchi Grafted onto Seedlings of Different Cultivars
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Grafting Success and Tree Growth
2.3. Leaf Greenness and Photosynthetic Rate
2.4. Leaf Mineral Analysis
2.5. Statistics
3. Results and Analysis
3.1. Survival Rate of Grafted Trees on Different Rootstocks
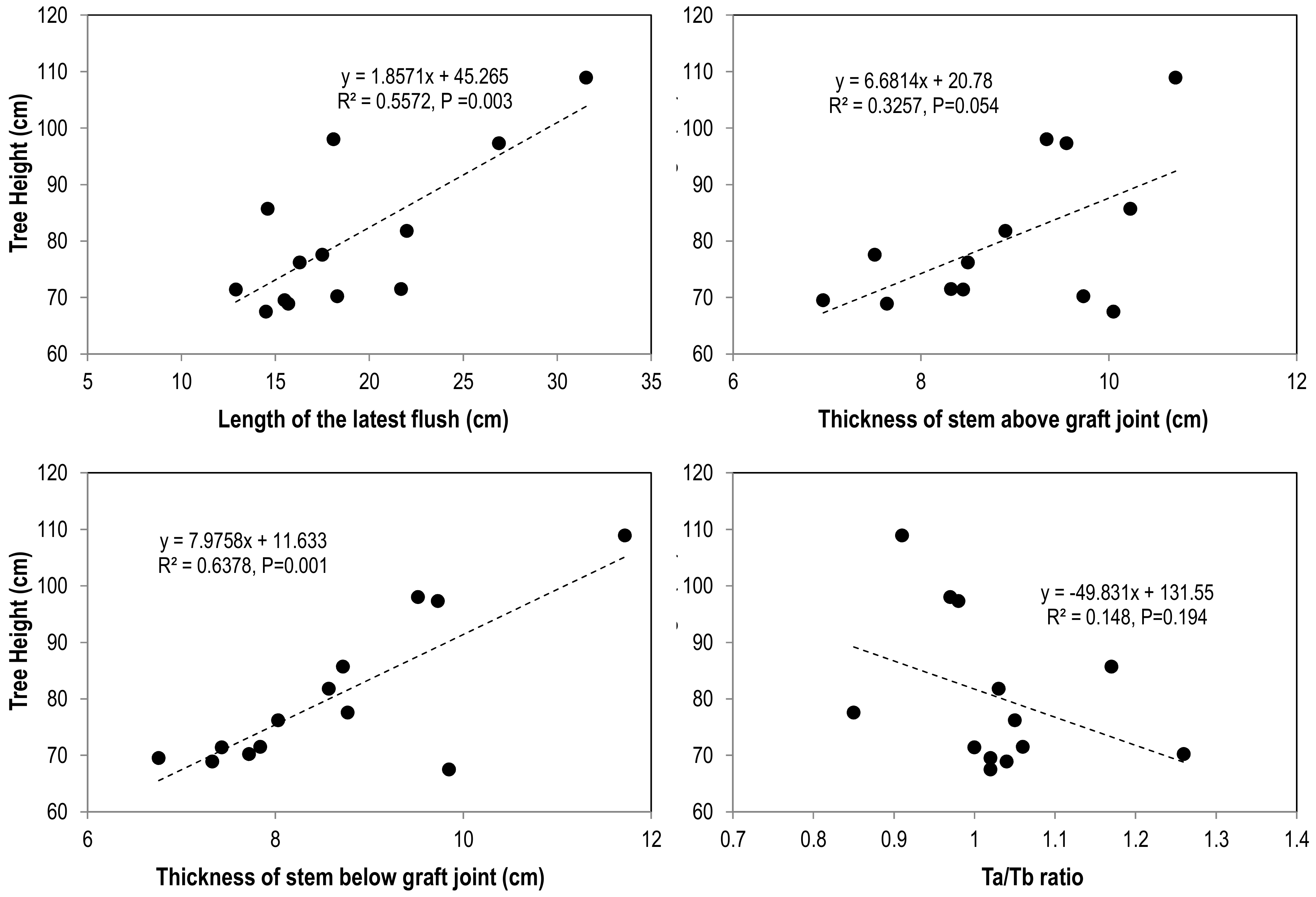
3.2. Tree Growth
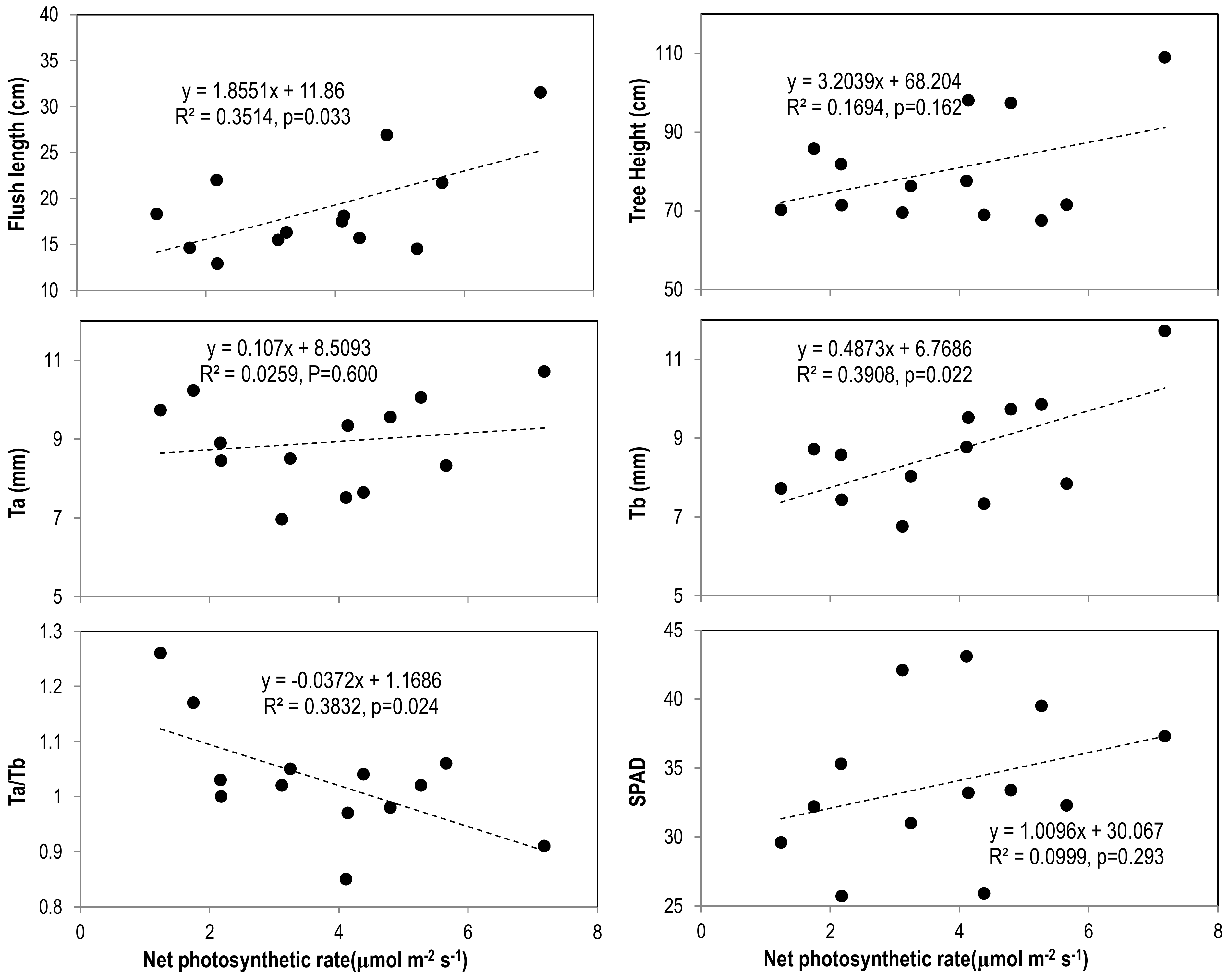
3.3. Leaf Greenness and Photosynthetic Capacity
3.4. Leaf Mineral Nutrients and Their Correlations with Tree Vigor and Photosynthesis
4. Discussion
4.1. ‘Lingfengnuo’ Shows Differential Graft Compatibility with Seedlings of Different Cultivars
4.2. Rootstocks Exert Influence on Mineral Nutrients
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, X.; Subhadrabandhu, S.; Mitra, S.K.; Ben-Arie, R.; Stern, R.A. Origin, history, production and processing. In Litchi and Longan: Botany, Production and Uses; Menzel, C.M., Waite, G.K., Eds.; CAB International: Wallingford, UK, 2005; pp. 1–23. [Google Scholar]
- Wu, S.X. Encyclopaedia of China Fruits: Litchi; China Forestry Press: Beijing, China, 1998. [Google Scholar]
- Menzel, C.; Huang, X.; Liu, C. Cultivars and plant improvement. In Litchi and Longan: Botany, Production and Uses; Menzel, C.M., Waite, G.K., Eds.; CAB International: Wallingford, UK, 2005; pp. 59–86. [Google Scholar]
- Huang, X. Achieving sustainable cultivation of litchi. In Achieving Sustainable Cultivation of Tropical Fruits; Yahia, E.M., Ed.; Burleigh Dodds Science Publishing: Cambridge, UK, 2020; pp. 457–471. [Google Scholar]
- Fan, Y.; Yin, J.H.; Liu, C.M.; Luo, S.; Zhou, J.; Huang, X.M. A new high quality litchi cultivar, Lingfengnuo. J. Fruit Sci. 2010, 27, 852–853. [Google Scholar]
- Fan, Y.; Yin, J.H.; Huang, X.M.; Chen, Y.H. Research on photosynthesis indicators of new litchi cultivar Lingfengnuo. South China Fruits 2011, 40, 13–15. [Google Scholar]
- Fan, Y.; Yin, J.H.; Huang, X.M. The cracking-resistance, hanging life on tree and storability of new litchi varieties Lingfengnuo. Chin. J. Trop. Crops 2014, 35, 2469–2473. [Google Scholar]
- Fan, Y.; Li, J.; Li, Z.Y.; Xie, B.X.; Liang, X.W.; Fan, Y.J.; Zhang, H.Y.; Huang, X.M. Research on the fruit setting ability and fruit quality performance on different litchi rootstock varieties. J. Anhui Agric. Sci. 2018, 46, 50–53. [Google Scholar]
- Menzel, C.M. Propagation of lychee: A review. Sci. Hortic. 1985, 25, 31–48. [Google Scholar] [CrossRef]
- Saúco, V.G.; Jahiel, M.; Modesto, P.H.D.; Huang, X.M.; Mitra, S.; Yamanishi, O.Y.; de Andrade, R.A. Litchi (Litchi chinensis Sonn) propagation. New Technologies and innovations. Rev. Bras. Frutic. Jaboticabal 2018, 40, e-575. [Google Scholar] [CrossRef]
- Hu, G.B.; Huang, X.M.; Bu, J.H. Shoot growth from scions top-grafted on different litchi cultivars. Acta Hortic. 2010, 863, 449–452. [Google Scholar] [CrossRef]
- Cai, J.X.; Lin, B.H. A preliminary report of the performance of different scion-rootstock combinations involved in top grafting on ‘Wuye’. Fujian Fruits 2011, 2, 14–16. [Google Scholar]
- Huang, F.Z.; Peng, H.X.; Zhu, J.H.; Li, H.L.; Li, D.P.; Xu, N.; Qin, X.Q.; Ding, F.; Lu, G.F. Studies on grafting affinity of new varieties of litchi ‘Guifeihong’ and ‘Caomeili.Subtropical’. Plant Sci. 2011, 40, 8–11. [Google Scholar]
- Sun, H.X.; Lu, C.Y.; Lin, W.M.; Huang, X.F. A survey of survival rate of grafted ‘Jinggagnhonnuo’. Fujian Fruits 2011, 4, 15–16. [Google Scholar]
- Li, W.C.; Wei, Y.Z.; Shi, S.Y.; Wang, Y.C.; Liu, L.Q.; Hu, G.B. Preliminary study on different rootstockscion combinations in ‘Shuangjianyuhebao’ litchi. Acta Hortic. 2014, 1029, 73–80. [Google Scholar] [CrossRef]
- Pires, M.D.; Yamanish, O.K.; Peixoto, J.R. Top working of ‘Bengal’ lychee trees in the state o Sao Paulo, Brazil. Rev. Bras. Frutic. Jaboticabal 2014, 36, 680–685. [Google Scholar] [CrossRef][Green Version]
- Chen, Z.; Zhao, J.; Qin, Y.; Hu, G. Study on the graft compatibility between ‘Jingganghongnuo’ and other litchi cultivars. Sci. Hortic. 2016, 199, 56–62. [Google Scholar] [CrossRef]
- Chen, Z.; Hu, F.; Fa, H.; Zhao, J.; Wang, X.; Hu, G. The correlative analysis between genetic relationship and graft compatibilities in litchi cultivars. Mol. Plant Breed. 2018, 16, 8111–8120. [Google Scholar]
- Ning, Z. Handbook of Food Component Analysis; China Light Industry Publisher: Beijing, China, 1998. [Google Scholar]
- Koepke, T.; Dhingra, A. Rootstock scion somatogenetic interactions in perennial composite plants. Plant Cell Rep. 2013, 32, 1321–1337. [Google Scholar] [CrossRef]
- Hu, G.B.; Huang, X.M. (Eds.) Pictural Description of New Litchi Cultivars and Top Grafting Techniques; Guangdong Science and Technology Press: Guangzhou, China, 2018. [Google Scholar]
- Zhong, Q.C.; Feng, J.Z.; Huang, B.Z.; Zhong, Y.W.; Qiu, Y.P. A preliminary report of the nutrient exhange between scion and rootstock in litchi. Nucl. Agric. Bull. 1989, 5, 211–212. [Google Scholar]
- Wang, Z.J.; Jin, K.Y. Study on determine the adaptability and compatibility of Pinussibirica (loud) Mayr scion and rootstock by using 32P tracer isotope technology. North Hortiuclt. 2015, 1, 76–78. [Google Scholar]
- Ermel, F.F.; Poessel, J.L.; Faurobert, M.; Catesson, A.M. Early scion/stock junction in compatible and imcompatible pear/pear and pear/quince grafts: A histo-cytological study. Ann. Bot. 1997, 79, 505–515. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, J.; Hu, F.; Qin, Y.H.; Wang, X.; Hu, G. Transcriptome changes between compatible and incompatible graft combination of Litchi chinensis by digtal gene expression profile. Sci. Rep. 2017, 7, 3954. [Google Scholar] [CrossRef]
- Zhou, J. Construction of a High Density Genetic Linkage Map and SRAP Analysis of Several Important Germplasm in Litchi. Master’s Thesis, South China Agricultural University, Guangzhou, China, 2009. [Google Scholar]
- Smith, P.F.; Reuther, W.; Specht, A.W. The influence of rootstock on the mineral composition of Valencia orange leaves. Plant Physiol. 1949, 24, 455–461. [Google Scholar] [CrossRef]
- Jiménez, S.; Pinochet, J.; Gogorcena, Y.; Betrán, J.A.; Moreno, M.A. Influence of different vigour cherry rootstocks on leaves and shoots mineral composition. Sci. Hortic. 2007, 112, 73–79. [Google Scholar] [CrossRef]
- Wu, W.M.; Wang, X.C.; Wang, Q.L.; Cai, W.J.; Zhao, M.Z. Effect of rootstock on vegetative growth, fruit quality and minerals in ‘Summer Black’ grape. Sino-Overseas Grapevine Wine 2014, 5, 14–17. [Google Scholar]
- Lazare, S.; Haberman, A.; Yermiyahu, U.; Erel, R.; Simenski, E.; Dag, A. Avocado rootstock influences scion leaf mineral content. Arch. Agron. Soil Sci. 2020, 66, 1399–1409. [Google Scholar] [CrossRef]

| Seedling Cultivar | Number of Grafted Trees | Number of Survived Trees | Grafting Success Rate (%) |
|---|---|---|---|
| HY | 12 | 5 | 41.7 |
| BL | 32 | 22 | 68.8 |
| SD | 12 | 5 | 41.7 |
| BTY | 11 | 6 | 54.5 |
| HZ | 21 | 18 | 85.7 |
| JGHN | 11 | 7 | 63.6 |
| SJYHB | 5 | 2 | 40 |
| CZ | 5 | 2 | 40 |
| MGL | 9 | 4 | 44.4 |
| SSH | 11 | 5 | 45.5 |
| XJZ | 8 | 8 | 100 |
| HHDHL | 15 | 4 | 26.7 |
| SK | 6 | 3 | 50 |
| Rootstock | Latest Shoot Length (cm) | Tree Height (cm) | Thickness above Graft Joint (Ta) (cm) | Thickness below Graft Joint (Tb) (cm) | Ta/Tb | Flowering Rate (%) |
|---|---|---|---|---|---|---|
| HY | 18.3 ± 4.4ab | 70.2 ± 7.9cd | 9.73 ± 1.26ab | 7.72 ± 0.99bc | 1.26 * | 34.4 |
| BL | 22.0 ± 1.9ab | 81.8 ± 5.4bc | 8.90 ± 0.79b | 8.57 ± 0.53bc | 1.03 | 15.4 |
| SD | 14.6 ± 3.4bc | 85.7 ± 5.6bc | 10.23 ± 1.18a | 8.72 ± 1.06bc | 1.17 * | 0 |
| BTY | 18.1 ± 1.9ab | 98.0 ± 6.0ab | 9.34 ± 1.07ab | 9.52 ± 0.84ab | 0.97 | 7.0 |
| HZ | 31.4 ± 4.9a | 108.9 ± 8.5a | 10.71 ± 1.24a | 11.74 ± 1.15a | 0.91 * | 0 |
| JGHN | 26.9 ± 5.4ab | 97.3 ± 9.7ab | 9.55 ± 1.00ab | 9.73 ± 0.93ab | 0.98 | 0 |
| SJYHB | 16.3 ± 3.2ab | 76.2 ± 5.2cd | 8.50 ± 0.54b | 8.03 ± 0.30bc | 1.05 | 0 |
| MGL | 21.7 ± 8.9a | 71.5 ± 10.9cd | 8.32 ± 1.41b | 7.84 ± 0.09bc | 1.06 * | 0 |
| SSH | 15.7 ± 3.2bc | 68.9 ± 5.0d | 7.64 ± 0.68bc | 7.33 ± 0.61bc | 1.04 | 0 |
| XJZ | 12.9 ± 2.9c | 71.4 ± 2.8cd | 8.45 ± 0.58b | 8.43 ± 0.73bc | 1.00 | 25 |
| CZ | 17.5 ± 7.2ab | 77.5 ± 7.2cd | 7.51 ± 1.88bc | 8.77 ± 0.70bc | 0.85 | 0 |
| HHDHL | 15.5 ± 5.2bc | 69.5 ± 0.5d | 6.96 ± 1.31c | 6.76 ± 1.08c | 1.02 | 0 |
| SK | 14.5 ± 3.1bc | 67.5 ± 7.5d | 10.05 ± 2.61a | 9.85 ± 0.21ab | 1.02 | 0 |
| Rooststock Cultivar | Leaf Greenness (SPAD) | Net Photosynthetic Rate (µmol m−2 s−1) |
|---|---|---|
| HY | 29.6 ± 1.76cd | 1.24 ± 0.26c |
| BL | 35.3 ± 2.57bc | 2.17 ± 0.22bc |
| SD | 32.2 ± 2.26bc | 1.75 ± 0.41c |
| BTY | 33.2 ± 1.74bc | 4.14 ± 1.01b |
| HZ | 37.3 ± 1.32ab | 7.18 ± 0.86a |
| JGHN | 33.4 ± 1.44bc | 4.80 ± 1.01ab |
| SJYHB | 31.0 ± 1.34bc | 3.24 ± 0.60bc |
| MGL | 32.3 ± 3.05bc | 5.66 ± 1.35ab |
| SSH | 25.9 ± 2.05d | 4.38 ± 0.65b |
| XJZ | 25.7 ± 1.27d | 2.18 ± 0.60bc |
| CZ | 43.1 ± 1.16a | 4.11 ± 1.07b |
| HHDHL | 42.1 ± 3.18a | 3.12 ± 0.70bc |
| SK | 39.5 ± 2.02ab | 5.37 ± 0.63ab |
| Rootstock Cultivar | N | P | K | Ca | Mg |
|---|---|---|---|---|---|
| (g/kg) | (g/kg) | (g/kg) | (g/kg) | (g/kg) | |
| HY | 14.53 ± 0.76a | 1.12 ± 0.06a | 13.16 ± 0.43a | 2.86 ± 0.14ab | 1.72 ± 0.14ab |
| BL | 14.20 ± 0.85a | 1.14 ± 0.03a | 12.62 ± 2.27a | 3.14 ± 0.34ab | 1.54 ± 0.15b |
| SD | 13.75 ± 2.27a | 0.97 ± 0.06a | 11.43 ± 0.71ab | 3.36 ± 0.43ab | 1.77 ± 0.31ab |
| BTY | 15.03 ± 1.25a | 1.22 ± 0.12a | 10.48 ± 1.13ab | 3.31 ± 0.74ab | 1.75 ± 0.48ab |
| HZ | 15.12 ± 0.51a | 1.38 ± 0.15a | 8.98 ± 0.60ab | 2.07 ± 0.15b | 0.91 ± 0.13b |
| JGHN | 14.87 ± 0.86a | 1.45 ± 0.16a | 11.6 ± 0.79a | 2.96 ± 0.37ab | 1.71 ± 0.08ab |
| SJYHB | 16.20 ± 4.92a | 1.54 ± 0.61a | 12.56 ± 0.51a | 2.23 ± 0.26b | 1.38 ± 0.12b |
| MGL | 13.44 ± 2.09a | 1.49 ± 0.16a | 9.89 ± 0.66ab | 3.77 ± 0.12ab | 1.19 ± 0.26b |
| SSH | 11.86 ± 0.68a | 1.17 ± 0.04a | 9.34 ± 0.53ab | 3.74 ± 0.46ab | 1.77 ± 0.10ab |
| XJZ | 12.74 ± 2.00a | 1.13 ± 0.33a | 9.85 ± 2.17ab | 4.12 ± 0.79ab | 2.58 ± 0.53a |
| CZ | 15.45 ± 2.04a | 1.25 ± 0.12a | 7.77 ± 3.51ab | 3.55 ± 1.49ab | 0.99 ± 0.04b |
| HHDHL | 16.19 ± 0.34a | 1.44 ± 0.27a | 8.18 ± 2.10ab | 3.85 ± 0.51ab | 1.01 ± 0.01b |
| SK | 14.74 ± 2.76a | 1.41 ± 0.16a | 6.07 ± 1.21b | 4.72 ± 0.10a | 1.36 ± 0.18b |
| Rootstock Cultivar | Cu | Zn | Fe | Mn | B |
|---|---|---|---|---|---|
| (mg/kg) | (mg/kg) | (mg/kg) | (mg/kg) | (mg/kg) | |
| HY | 9.46 ± 0.80ab | 22.21 ± 0.47cde | 82.17 ± 24.03a | 37.46 ± 3.09de | 25.43 ± 3.18bc |
| BL | 9.92 ± 0.53ab | 26.79 ± 2.84bcde | 69.64 ± 8.73a | 32.03 ± 7.21e | 19.83 ± 3.32bc |
| SD | 8.61 ± 0.24b | 24.95 ± 1.20bcde | 72.29 ± 16.24a | 43.29 ± 2.36cde | 14.70 ± 3.59c |
| BTY | 10.58 ± 1.36ab | 21.15 ± 0.83de | 59.99 ± 9.10a | 72.52 ± 16.78bcde | 15.38 ± 1.50c |
| HZ | 11.28 ± 0.96ab | 20.56 ± 1.20e | 59.22 ± 3.93a | 71.95 ± 9.11bcde | 14.14 ± 3.59c |
| JGHN | 15.22 ± 1.84ab | 27.06 ± 0.44bcde | 57.39 ± 3.79a | 76.39 ± 8.91bcde | 24.32 ± 3.43bc |
| SJYHB | 17.49 ± 7.45a | 29.61 ± 5.10bcd | 52.42 ± 7.41a | 46.02 ± 10.30cde | 16.89 ± 8.91bc |
| MGL | 15.72 ± 1.62ab | 27.71 ± 2.49bcde | 62.81 ± 9.13a | 110.33 ± 8.25b | 28.51 ± 1.32abc |
| SSH | 9.50 ± 0.92ab | 22.66 ± 1.35cde | 86.45 ± 5.01a | 89.81 ± 5.4b | 30.52 ± 2.45ab |
| XJZ | 15.07 ± 3.15ab | 37.65 ± 3.13a | 87.73 ± 16.35a | 86.80 ± 17.38bc | 42.37 ± 6.03a |
| CZ | 13.45 ± 6.46ab | 24.17 ± 6.65cde | 59.15 ± 3.93a | 104.13 ± 35.65b | 31.25 ± 9.06ab |
| HHDHL | 13.93 ± 2.22ab | 30.37 ± 3.83abc | 69.94 ± 2.73a | 120.48 ± 26.97b | 18.02 ± 1.48bc |
| SK | 10.06 ± 3.72ab | 33.31 ± 0.88ab | 91.12 ± 28.52a | 169.49 ± 20.42a | 23.53 ± 1.95bc |
| FL | TH | Ta | Tb | Ta/Tb | SPAD | Pn | N | P | K | Ca | Mg | Cu | Zn | Fe | Mn | B | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FL | 1 | ||||||||||||||||
| TH | 0.746 ** | 1 | |||||||||||||||
| Ta | 0.423 | 0.571 * | 1 | ||||||||||||||
| Tb | 0.679 * | 0.799 ** | 0.751 ** | 1 | |||||||||||||
| Ta/Tb | −0.315 | −0.385 | 0.237 | −0.427 | 1 | ||||||||||||
| SPAD | 0.187 | 0.133 | −0.089 | 0.330 | −0.486 | 1 | |||||||||||
| Pn | 0.593 * | 0.412 | 0.161 | 0.625 * | −0.619 * | 0.316 | 1 | ||||||||||
| N | 0.196 | 0.259 | 0.020 | 0.261 | −0.246 | 0.700 ** | 0.095 | 1 | |||||||||
| P | 0.330 | 0.028 | −0.174 | 0.150 | −0.364 | 0.353 | 0.622 * | 0.525 | 1 | ||||||||
| K | 0.164 | 0.182 | 0.206 | −0.176 | 0.556 * | −0.542 | −0.552 | −0.036 | −0.265 | 1 | |||||||
| Ca | −0.625 * | −0.623 * | −0.328 | −0.365 | −0.021 | 0.092 | −0.069 | −0.384 | −0.127 | −0.621 * | 1 | ||||||
| Mg | −0.401 | −0.073 | 0.031 | −0.238 | 0.124 | −0.704 ** | −0.394 | −0.608 * | −0.465 | 0.228 | 0.303 | 1 | |||||
| Cu | 0.070 | −0.087 | −0.426 | −0.217 | −0.329 | 0.011 | 0.181 | 0.315 | 0.697 ** | 0.027 | −0.128 | 0.002 | 1 | ||||
| Zn | −0.481 | −0.524 | −0.247 | −0.362 | −0.024 | −0.056 | −0.201 | −0.060 | 0.218 | −0.246 | 0.557 * | 0.434 | 0.479 | 1 | |||
| Fe | −0.545 | −0.603 * | 0.017 | −0.330 | 0.400 | −0.322 | −0.309 | −0.604 * | −0.461 | −0.272 | 0.671 * | 0.404 | −0.488 | 0.376 | 1 | ||
| Mn | −0.222 | −0.357 | −0.251 | 0.025 | −0.392 | 0.443 | 0.514 | 0.027 | 0.447 | −0.913 ** | 0.735 ** | −0.172 | 0.140 | 0.423 | 0.315 | 1 | |
| B | −0.333 | −0.528 | −0.424 | −0.438 | −0.102 | −0.366 | −0.129 | −0.574 * | −0.158 | −0.199 | 0.517 | 0.463 | 0.266 | 0.473 | 0.484 | 0.286 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Y.; Li, Z.; Xie, B.; Liang, X.; Huang, X. A Study of Shoot Growth, Leaf Photosynthesis, and Nutrients in ‘Lingfengjing’ Litchi Grafted onto Seedlings of Different Cultivars. Horticulturae 2022, 8, 282. https://doi.org/10.3390/horticulturae8040282
Fan Y, Li Z, Xie B, Liang X, Huang X. A Study of Shoot Growth, Leaf Photosynthesis, and Nutrients in ‘Lingfengjing’ Litchi Grafted onto Seedlings of Different Cultivars. Horticulturae. 2022; 8(4):282. https://doi.org/10.3390/horticulturae8040282
Chicago/Turabian StyleFan, Yan, Zhiyuan Li, Binxia Xie, Xiaowen Liang, and Xuming Huang. 2022. "A Study of Shoot Growth, Leaf Photosynthesis, and Nutrients in ‘Lingfengjing’ Litchi Grafted onto Seedlings of Different Cultivars" Horticulturae 8, no. 4: 282. https://doi.org/10.3390/horticulturae8040282
APA StyleFan, Y., Li, Z., Xie, B., Liang, X., & Huang, X. (2022). A Study of Shoot Growth, Leaf Photosynthesis, and Nutrients in ‘Lingfengjing’ Litchi Grafted onto Seedlings of Different Cultivars. Horticulturae, 8(4), 282. https://doi.org/10.3390/horticulturae8040282






