The Bioactive Compounds and Fatty Acid Profile of Bitter Apple Seed Oil Obtained in Hot, Arid Environments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site and Plant Material
2.2. Lipid Extraction
2.3. Fatty Acid Profiling of Tumba Seed Oil Using GC-MS/MS
2.4. Nutraceutical Composition and Antioxidant Activity Determination of Tumba Seed Oil
2.4.1. Methanolic Extractives of Tumba Seed Oil
2.4.2. Determination of Total Phenolic Content (TPC) and Total Flavonoid Content (TFC)
2.4.3. Estimation of Lignan, Oryzanol, and Carotenoid Content
2.4.4. Total Antioxidant Activity
2.5. Statistical Analysis
3. Results and Discussion
3.1. The Fatty Acid Composition of Tumba Seed Oil
3.2. Nutraceutical Composition and Antioxidant Activity of Tumba Seed Oil
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Berwal, M.K.; Haldhar, S.M.; Ram, C.; Shil, S.; Kumar, R.; Gora, J.S.; Singh, D.; Samadia, D.K.; Kumar, M.; Mekhemar, M. Calligonum polygonoides L. as Novel Source of Bioactive Compounds in Hot Arid Regions: Evaluation of Phytochemical Composition and Antioxidant Activity. Plants 2021, 10, 1156. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Khapte, P.S.; Meghwal, P.R. Genetic Diversity of Vegetables in Arid Region. In Horticulture Based Integrated Farming System; Shukla, A.K., Gupta, D.K., Jangid, B.L., Keerthika, A., Noor Mohammad, M.B., Mehta, R.S., Eds.; New India Publishing Agency: New Delhi, India, 2021; pp. 35–41. [Google Scholar]
- Javadzadeh, H.R.; Davoudi, A.; Davoudi, F.; Valizadegan, G.; Goodarzi, H.; Mahmoodi, S.; Ghane, M.R.; Faraji, M. Citrullus colocynthis as the Cause of Acute Rectorrhagia. Case Rep. Emerg. Med. 2013, 2013, 652192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bikdeloo, M.; Colla, G.; Rouphael, Y.; Hassandokht, M.R.; Soltani, F.; Salehi, R.; Kumar, P.; Cardarelli, M. Morphological and Physio-Biochemical Responses of Watermelon Grafted onto Rootstocks of Wild Watermelon [Citrullus colocynthis (L.) Schrad] and Commercial Interspecific Cucurbita Hybrid to Drought Stress. Horticulturae 2021, 7, 359. [Google Scholar] [CrossRef]
- Dane, F.; Liu, J.; Zhang, C. Phylogeography of the bitter apple, Citrullus colocynthis. Genet. Resour. Crop Evol. 2006, 54, 327–336. [Google Scholar] [CrossRef]
- Hassanane, M.S.; EL-Fiky, S.; Abd EL-Bbaset, S.A. A genotoxic study of the Citrullus colocynthis extract. Bull. Natl. Res. Cent. 2001, 26, 223–235. [Google Scholar]
- Rahimi, R.; Amin, G.; Ardekani, M.R. A review on Citrullus colocynthis Schrad.: From traditional Iranian medicine to modern phytotherapy. J. Altern. Complement. Med. 2012, 18, 551–554. [Google Scholar] [CrossRef]
- Berwal, M.K.; Haldhar, S.M.; Ram, C.; Saroj, P.L. Determination of total phenolic & flavonoids and antioxidant activity in Calligonum polygonoides L. from Thar Desert. J. Environ. Biol. 2021, 42, 1347–1354. [Google Scholar] [CrossRef]
- Rani, R.; Dushyant, S.; Monika, C.; Jaya, P.Y. Antibacterial activity of twenty different endophytic fungi isolated from Calotropis procera and time kill assay. Clin. Microbiol. 2017, 6, 3. [Google Scholar] [CrossRef]
- Abdel-Hassan, I.A.; Mohammeda, S.T. The hypoglycaemic and antihyperglycaemic effect of Citrullus colocynthis fruit aqueous extract in normal and alloxan diabetic rabbits. J. Ethnopharmacol. 2000, 71, 325–330. [Google Scholar] [CrossRef]
- Ardekani, M.R.S.; Rahimi, R.; Javadi, B.; Abdi, L.; Khanavi, M. Relationship between temperaments of medicinal plants and their major chemical compounds. J. Tradit. Chin. Med. 2011, 31, 27–31. [Google Scholar] [CrossRef] [Green Version]
- Huseini, H.F.; Darvishzadeh, F.; Heshmat, R.; Jafariazar, Z.; Raza, M.; Larijani, B. The clinical investigation of Citrullus colocynthis (L.) schrad fruit in treatment of type II diabetic patients: A randomized, double blind, placebo-controlled clinical trial. Phytother. Res. 2009, 23, 1186–1189. [Google Scholar] [CrossRef]
- Rahbar, A.R.; Nabipour, I. The hypolipidemic effect of Citrullus colocynthis on patients with hyperlipidemia. Pak. J. Biol. Sci. 2010, 13, 1202–1207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marzouk, B.; Marzouk, Z.; Haloui, E.; Fenina, N.; Bouraoui, A.; Aouni, M. Screening of analgesic and anti-inflammatory activities of Citrullus colocynthis from southern Tunisia. J. Ethnopharmacol. 2010, 128, 15–19. [Google Scholar] [CrossRef]
- Tannin-Spitz, T.; Bergman, M.; Grossman, S. Cucurbitacin glucosides: Antioxidant and free-radical scavenging activities. Biochem. Biophys. Res. Commun. 2007, 364, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Nmila, R.; Gross, R.; Rchid, H.; Roye, M.; Manteghetti, M.; Petit, P.; Tijane, M.; Ribes, G.; Sauvaire, Y. Insulinotropic effect of Citrullus colocynthis fruit extracts. Planta Med. 2000, 66, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, F.; Panjehshahin, M.R. The toxic effect of alcoholic extract of Citrullus colocynthis on rat liver. Iran. J. Pharm. Ther. 2006, 5, 117–119. [Google Scholar]
- Jouad, H.; Haloui, M.; Rhiouani, H.; El Hilaly, J.; Eddouks, M. Ethnobotanical survey of medicinal plants used for the treatment of diabetes, cardiac and renal diseases in the North centre region of Morocco (Fez-Boulemane). J. Ethnopharmacol. 2001, 77, 175–182. [Google Scholar] [CrossRef]
- Kamalakar, K.; Sai-Manoj, G.N.V.T.; Prasad, R.B.N.; Karuna, M.S.L. Thumba (Citrullus colocynthis L.) seed oil: A potential bio-lubricant base-stock. Grasas Aceites 2015, 66, e055. [Google Scholar] [CrossRef] [Green Version]
- Mariod, A.A.; Mirghani, M.E.S.; Hussein, I. Citrullus colocynthis Colocynth, Bitter Apple, Bitter Gourd. In Unconventional Oilseeds and Oil Sources; Mariod, A.A., Mirghani, M.E.S., Hussein, I., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 99–105. [Google Scholar] [CrossRef]
- Ashish, K.; Naveen, K.; Hasan, M.M.; Rajeev, C.; Arshad, N.S.; Zahid, A.K. Production of biodiesel from thumba oil: Optimization of process parameters. Iran. J. Energy Environ. 2010, 1, 352–358. [Google Scholar]
- Gurudeeban, S.; Satyavani, K.; Ramanathan, T. Bitter apple (Citrullus colocynthis): An overview of chemical composition and biomedical potentials. Asian J. Plant Sci. 2010, 9, 394–401. [Google Scholar] [CrossRef]
- Kulkarni, A.S.; Khotpal, R.R.; Karadbhajane, V.Y.; More, V.I. Physico-chemical Composition and lipid classes of Aegle marmelos (Bael) and Citrullus colocynthis (Tumba) Seed Oils. J. Chem. Pharm. Res. 2012, 4, 1486–1488. [Google Scholar]
- Solomon, G.; Luqman, C.A.; Nor, M.A. Investigating “Egusi” (Citrullus colocynthis L.) Seed Oil as Potential Biodiesel Feedstock. Energies 2010, 3, 607–618. [Google Scholar] [CrossRef]
- Yaniv, Z.; Shabelsky, E.; Schafferman, D. Perspectives on new crops and new uses. In Colocynth: Potential Arid Land Oilseed from an Ancient Cucurbit; Janick, J., Ed.; ASHS Press: Alexandria, VA, USA, 1999; pp. 257–261. [Google Scholar]
- Al-Hwaiti, M.S.; Alsbou, E.M.; Abu Sheikha, G.; Bakchiche, B.; Pham, T.H.; Thomas, R.H.; Bardaweel, S.K. Evaluation of the anticancer activity and fatty acids composition of “Handal” (Citrullus colocynthis L.) seed oil, a desert plant from south Jordan. Food Sci. Nutr. 2021, 9, 282–289. [Google Scholar] [CrossRef]
- Bireche, M.; Gherib, A.; Bakchiche, B.; Berrabah, M.; Maatallah, M. Positional distribution of fatty acids in the triglycerides of Citrullus colocynthis seed oil growing in Algeria. J. Mater. Environ. Sci. 2017, 8, 622–627. [Google Scholar]
- AOCS. Official Methods and Recommended Practices of the American Oil Chemist’s Society; AOCS: Champaign, IL, USA, 2003. [Google Scholar]
- Berwal, M.K.; Haldhar, S.M.; Ram, C.; Shil, S.; Gora, J.S. Effect of extraction solvent on total phenolics, flavonoids and antioxidant capacity of flower bud and foliage of Calligonum polygonoides L. Indian J. Agric. Biochem. 2021, 34, 61–67. [Google Scholar] [CrossRef]
- Medini, F.; Fellah, H.; Ksouri, R.; Abdelly, C. Total phenolic, flavonoid and tannin contents and antioxidant and antimicrobial activities of organic extracts of shoots of the plant Limonium delicatulum. J. Taibah Univ. Sci. 2014, 8, 216–224. [Google Scholar] [CrossRef] [Green Version]
- Bhatnagar, A.S.; Hemavathy, J.; Gopala Krishna, A.G. Development of a rapid method for determination of lignans content in sesame oil. Food Sci. Technol. 2015, 52, 521–527. [Google Scholar] [CrossRef]
- Gopala Krishna, A.G.; Hemakumar, K.H.; Khatoon, S. Study on the composition of rice bran oil and its higher free fatty acids value. J. Am. Oil Chem. Soc. 2006, 83, 117–120. [Google Scholar] [CrossRef]
- Kumar, S.S.; Manasa, V.; Tumaney, A.W.; Bettadaiah, B.K.; Chaudari, S.R.; Giridhar, P. Chemical composition, nutraceuticals characterization, NMR confirmation of squalene and antioxidant activities of Basella rubra L. seed oil. RSC Adv. 2020, 10, 31863–31873. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin, E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Kostik, V.; Memeti, S.; Bauer, B. Fatty Acid Composition of Edible Oils and Fats. J. Hyg. Eng. Design 2013, 4, 112–116. [Google Scholar]
- Zambiazi, R.C.; Przybylski, R.; Zambiazi, M.W.; Mendonca, C.B. Fatty Acid Composition of Vegetable Oils and Fats. Bol. Cent. Pesqui. Processamento Aliment. 2007, 25, 111–120. [Google Scholar]
- Orsavova, J.; Misurcova, L.; Ambrozova, J.V.; Vicha, R.; Mlcek, J. Fatty Acids Composition of Vegetable Oils and Its Contribution to Dietary Energy Intake and Dependence of the Cardiovascular Mortality on Dietary Intake of Fatty Acids. Int. J. Mol. Sci. 2015, 16, 12871–12890. [Google Scholar] [CrossRef] [PubMed]
- Venn-Watson, S.; Lumpkin, R.; Dennis, E.A. Efficacy of dietary odd-chain saturated fatty acid pentadecanoic acid parallels broad associated health benefits in humans: Could it be essential? Sci. Rep. 2020, 10, 8161. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Cepeda, A.; Davila-Said, G.; Orea-Tejeda, A.; González-Islas, D.; Elizondo-Montes, M.; Pérez-Cortes, G.; Keirns-Davies, C.; Castillo-Aguilar, L.F.; Verdeja-Vendrell, L.; Peláez-Hernández, V.; et al. Dietary intake of fatty acids and its relationship with FEV1/FVC in patients with chronic obstructive pulmonary disease. Clin. Nutr. ESPEN 2019, 29, 92–96. [Google Scholar] [CrossRef]
- Huang, L.; Lin, J.S.; Aris, I.M.; Yang, G.; Chen, W.Q.; Li, L.J. Circulating Saturated Fatty Acids and Incident Type 2 Diabetes: A Systematic Review and Meta-Analysis. Nutrients 2019, 11, 998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aglago, E.K.; Biessy, C.; Torres-Mejia, G.; Angeles-Llerenas, A.; Gunter, M.J.; Romieu, I.; Chajes, V. Association between serum phospholipid fatty acid levels and adiposity in Mexican women. J. Lipid Res. 2017, 58, 1462–1470. [Google Scholar] [CrossRef] [Green Version]
- Kurotani, K.; Sato, M.; Yasuda, K.; Kashima, K.; Tanaka, S.; Hayashi, T.; Shirouchi, B.; Akter, S.; Kashino, I.; Hayabuchi, H.; et al. Even- and odd-chain saturated fatty acids in serum phospholipids are differentially associated with adipokines. PLoS ONE 2017, 12, e0178192. [Google Scholar] [CrossRef] [Green Version]
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef]
- Thusoo, S.; Gupta, S.; Sudan, R.; Kour, J.; Bhagat, S.; Hussain, R.; Bhagat, M. Antioxidant Activity of Essential Oil and Extracts of Valeriana jatamansi Roots. BioMed Res. Int. 2014, 2014, 614187. [Google Scholar] [CrossRef]
- Chang, S.T.; Wu, J.H.; Wang, S.Y.; Kang, P.L.; Yang, N.S.; Shyur, L.F. Antioxidant activity of extracts from Acacia confusa Bark and Heartwood. J. Agric. Food Chem. 2001, 49, 3420–3424. [Google Scholar] [CrossRef] [PubMed]
- Janu, C.; Kumar, D.S.; Reshma, M.V.; Jayamurthy, P.; Sundaresan, A.; Nisha, P. Comparative Study on the Total Phenolic Content and Radical Scavenging Activity of Common Edible Vegetable Oils. J. Food Biochem. 2014, 38, 38–49. [Google Scholar] [CrossRef]
- Kozlowska, M.; Gruczynska, E.; Scibisz, I.; Rudzinska, M. Fatty acids and sterols composition, and antioxidant activity of oils extracted from plant seeds. Food Chem. 2016, 213, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Harborne, J.B.; Baxter, H.; Moss, G.P. Phytochemical Dictionary: Handbook of Bioactive Compounds from Plants, 2nd ed.; Taylor & Francis: London, UK, 1999. [Google Scholar]
- Xuan, T.D.; Gangqiang, G.; Minh, T.N.; Quy, T.N.; Khanh, T.D. An Overview of Chemical Profiles, Antioxidant and Antimicrobial Activities of Commercial Vegetable Edible Oils Marketed in Japan. Foods 2018, 7, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.W.; Kim, J.B.; Cho, S.M.; Cho, I.K.; Li, O.X.; Jang, H.H.; Lee, S.H.; Lee, Y.M.; Hwang, K.A. Characterization and quantification of γ-oryzanol in grains of 16 Korean rice varieties. Int. J. Food. Sci. Nutr. 2015, 66, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Ajala, A.W.; Ghavami, A. Evaluation of the effectiveness of cereal bran extract for sunflower oil stability during frying. Int. J. Food Stud. 2020, 9, SI52–SI61. [Google Scholar] [CrossRef] [Green Version]
- Moazzami, A.A.; Kamal-Eldin, A. Sesame seed is a rich source of dietary lignans. J. Am. Oil Chem. Soc. 2006, 83, 719–723. [Google Scholar] [CrossRef]
- Reshma, M.V.; Balachandran, C.; Arumughan, C.; Sunderasan, A.; Sukumaran, D.; Thomas, S.; Saritha, S.S. Extraction, separation and characterisation of sesame oil lignan for nutraceutical applications. Food Chem. 2010, 120, 1041–1046. [Google Scholar] [CrossRef]
- Ribayamercado, J.D.; Solon, S.F.; Tang, G.; Cabal-Borza, M.; Perfecto, S.C.; Russel, R.M. Bionconversion of plant carotenoids to vit-A in Filipino school-aged children varies inversely with vit-A status. Am. J. Clin. Nutr. 2000, 72, 455–465. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.D. Carotenoids. In Modern Nutrition in Health and Disease, 11th ed.; Ross, C.A., Caballero, B., Cousins, R.J., Tucker, K.L., Ziegler, T.R., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2014; pp. 427–439. [Google Scholar]
- Chandrasekaram, K.; Ng, M.H.; Choo, Y.M.; Chuah, C.H. Effect of storage temperature on the stability of phytonutrients in palm concentrates. Am. J. Appl. Sci. 2009, 6, 529–533. [Google Scholar] [CrossRef] [Green Version]
- Hemalatha, S.; Ghafoorunissa. Sesame lignans enhance the thermal stability of edible vegetable oils. Food Chem. 2007, 105, 1076–1085. [Google Scholar] [CrossRef]
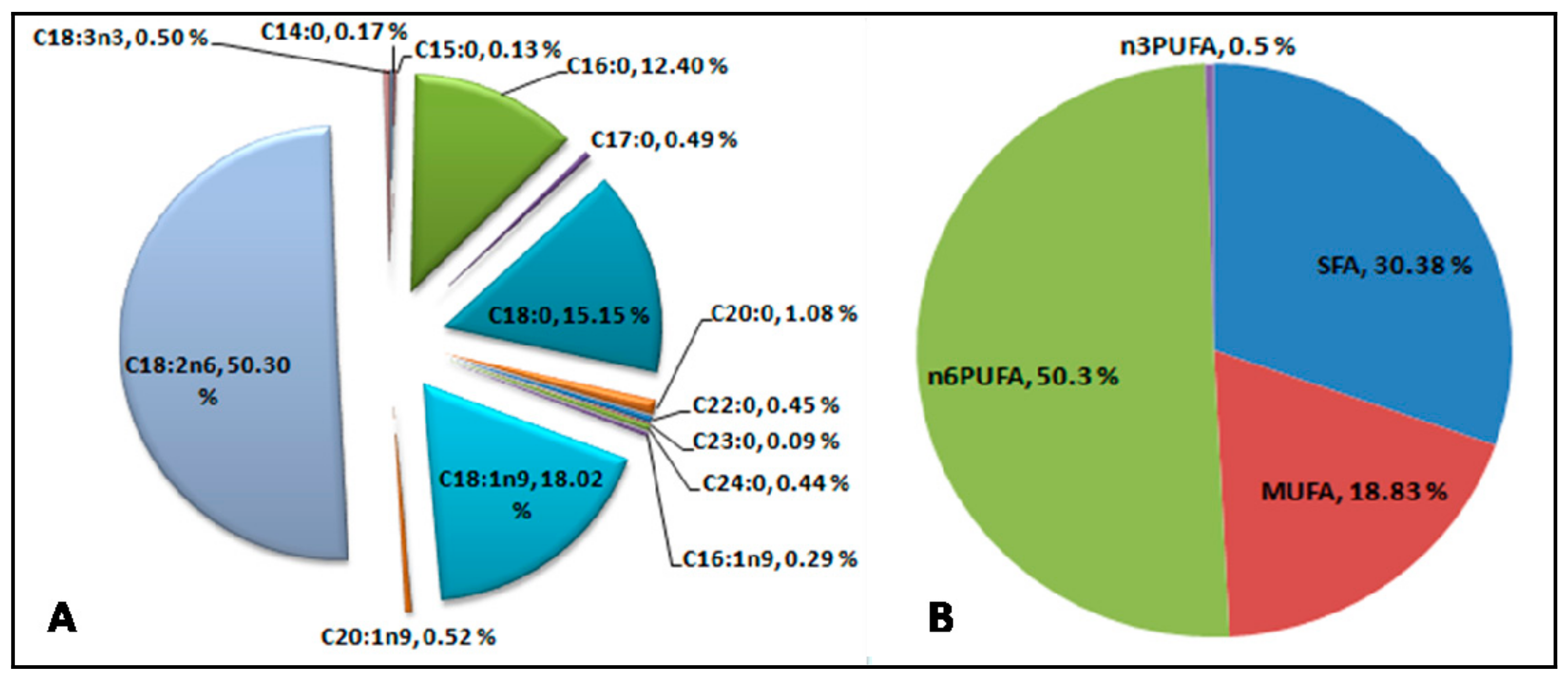
| Name of the Fatty Acid | Common Name | Formula | Peak Area (%) | Type of Fatty Acid | |
|---|---|---|---|---|---|
| 1 | Tetradecanoate | Myristic acid | C14:0 | 0.17 ± 0.01 | Saturated |
| 2 | Pentadecanoic acid | Pentadecylic acid | C15:0 | 0.13 ± 0.01 | Saturated |
| 3 | Hexadecanoic acid | Palmitic acid | C16:0 | 12.41 ± 0.02 | Saturated |
| 4 | 9-Hexadecenoic acid, (Z)- | Palmitelaidic acid | C16:1n9 | 0.29 ± 0.01 | MUFA |
| 5 | Heptadecanoic acid | Margaric acid | C17:0 | 0.49 ± 0.03 | Saturated |
| 6 | Octadecanoic acid | Stearic acid | C18:0 | 15.15 ± 0.46 | Saturated |
| 7 | 9-Octadecenoic acid (Z)- | Oleic acid | C18:1n9 | 18.02 ± 0.36 | MUFA |
| 8 | 9,12-Octadecadienoic acid (Z,Z)- | Linoleic acid | C18:2n6 | 50.31 ± 0.33 | PUFA |
| 9 | 9,12,15-Octadecatrienoic acid | α-Linolenic acid | C18:3n3 | 0.50 ± 0.05 | PUFA |
| 10 | Eicosanoic acid | Arachidic acid | C20:0 | 1.08 ± 0.03 | Saturated |
| 11 | 11-Eicosenoic acid | Gadoleic acid | C20:1n9 | 0.52 ± 0.03 | MUFA |
| 12 | Docosanoic acid | Behenic acid | C22:0 | 0.45 ± 0.02 | Saturated |
| 13 | Tricosanoic acid | Tricosanoic acid | C23:0 | 0.09 ± 0.02 | Saturated |
| 14 | Tetracosanoic acid | Lignoceric acid | C24:0 | 0.44 ± 0.04 | Saturated |
| Fatty Acids | India a | India b | India c | India d | Malaysia e | Israel f | Jordan g | Algeria h | Current Study |
|---|---|---|---|---|---|---|---|---|---|
| wt % | |||||||||
| Palmitic acid (C16:0) | 9.38 | 10.43 | 11.70 | 10.30 | 10.48 | 10.10 | 8.35 | 10.22 | 12.41 |
| Stearic acid (C18:0) | 7.34 | 9.84 | 9.70 | 8.00 | 9.72 | 6.70 | 5.36 | 8.98 | 15.15 |
| Oleic acid (C18:1) | 17.04 | 15.90 | 11.40 | 24.50 | 17.95 | 13.10 | 9.04 | 9.36 | 18.02 |
| Linoleic acid (C18:2) | 61.05 | 62.81 | 66.10 | 55.90 | 61.41 | 70.10 | 74.77 | 68.49 | 50.31 |
| Parameters | Content |
|---|---|
| Total phenolic content (mg/100 g of oil gallic acid Eq.) | 5.39 ± 0.73 |
| Total flavonoids content (mg/100 g of oil catechin Eq.) | 938.0 ± 18.0 |
| Oryzanol (%) | 0.066 ± 0.003 |
| Lignans (%) | 0.012 ±0.002 |
| Carotenoids (mg/kg) | 79.5 ± 16.1 |
| Total antioxidant activity by phosphomolybdate method (mg/100 g oil ascorbic acid Eq.) | 70.83 ± 2.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berwal, M.K.; Ram, C.; Gurjar, P.S.; Gora, J.S.; Kumar, R.; Verma, A.K.; Singh, D.; Basile, B.; Rouphael, Y.; Kumar, P. The Bioactive Compounds and Fatty Acid Profile of Bitter Apple Seed Oil Obtained in Hot, Arid Environments. Horticulturae 2022, 8, 259. https://doi.org/10.3390/horticulturae8030259
Berwal MK, Ram C, Gurjar PS, Gora JS, Kumar R, Verma AK, Singh D, Basile B, Rouphael Y, Kumar P. The Bioactive Compounds and Fatty Acid Profile of Bitter Apple Seed Oil Obtained in Hot, Arid Environments. Horticulturae. 2022; 8(3):259. https://doi.org/10.3390/horticulturae8030259
Chicago/Turabian StyleBerwal, Mukesh Kumar, Chet Ram, Pawan Singh Gurjar, Jagan Singh Gora, Ramesh Kumar, Ajay Kumar Verma, Dhurendra Singh, Boris Basile, Youssef Rouphael, and Pradeep Kumar. 2022. "The Bioactive Compounds and Fatty Acid Profile of Bitter Apple Seed Oil Obtained in Hot, Arid Environments" Horticulturae 8, no. 3: 259. https://doi.org/10.3390/horticulturae8030259
APA StyleBerwal, M. K., Ram, C., Gurjar, P. S., Gora, J. S., Kumar, R., Verma, A. K., Singh, D., Basile, B., Rouphael, Y., & Kumar, P. (2022). The Bioactive Compounds and Fatty Acid Profile of Bitter Apple Seed Oil Obtained in Hot, Arid Environments. Horticulturae, 8(3), 259. https://doi.org/10.3390/horticulturae8030259










