Low R/FR Ratio Affects Pakchoi’s Growth and Nitrate Content under Excess Nitrate Stress
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Under Different Nitrogen Levels, a Low R/FR Ratio Promoted the Growth of Pakchoi
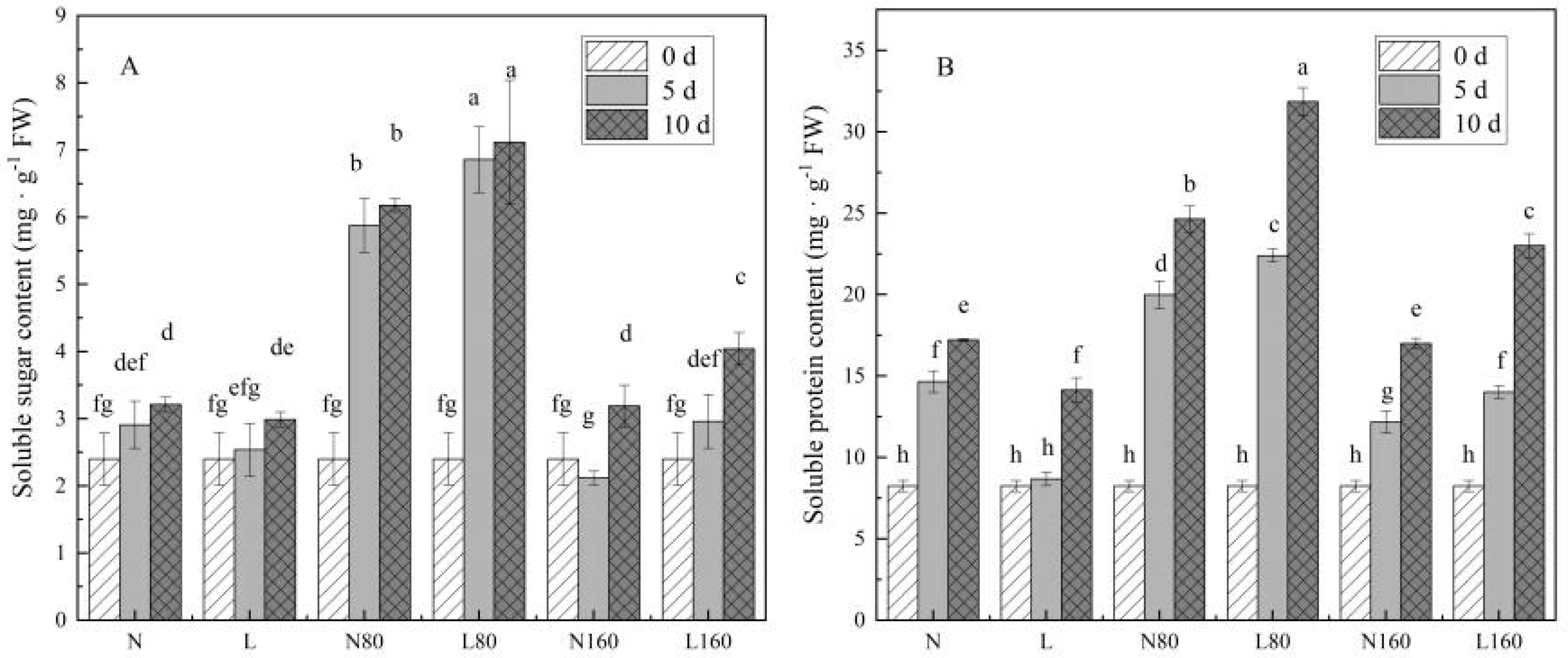
3.2. The R/FR Ratio Affects the Content of Osmotic Regulatory Substances in Pakchoi under Different Nitrogen Levels
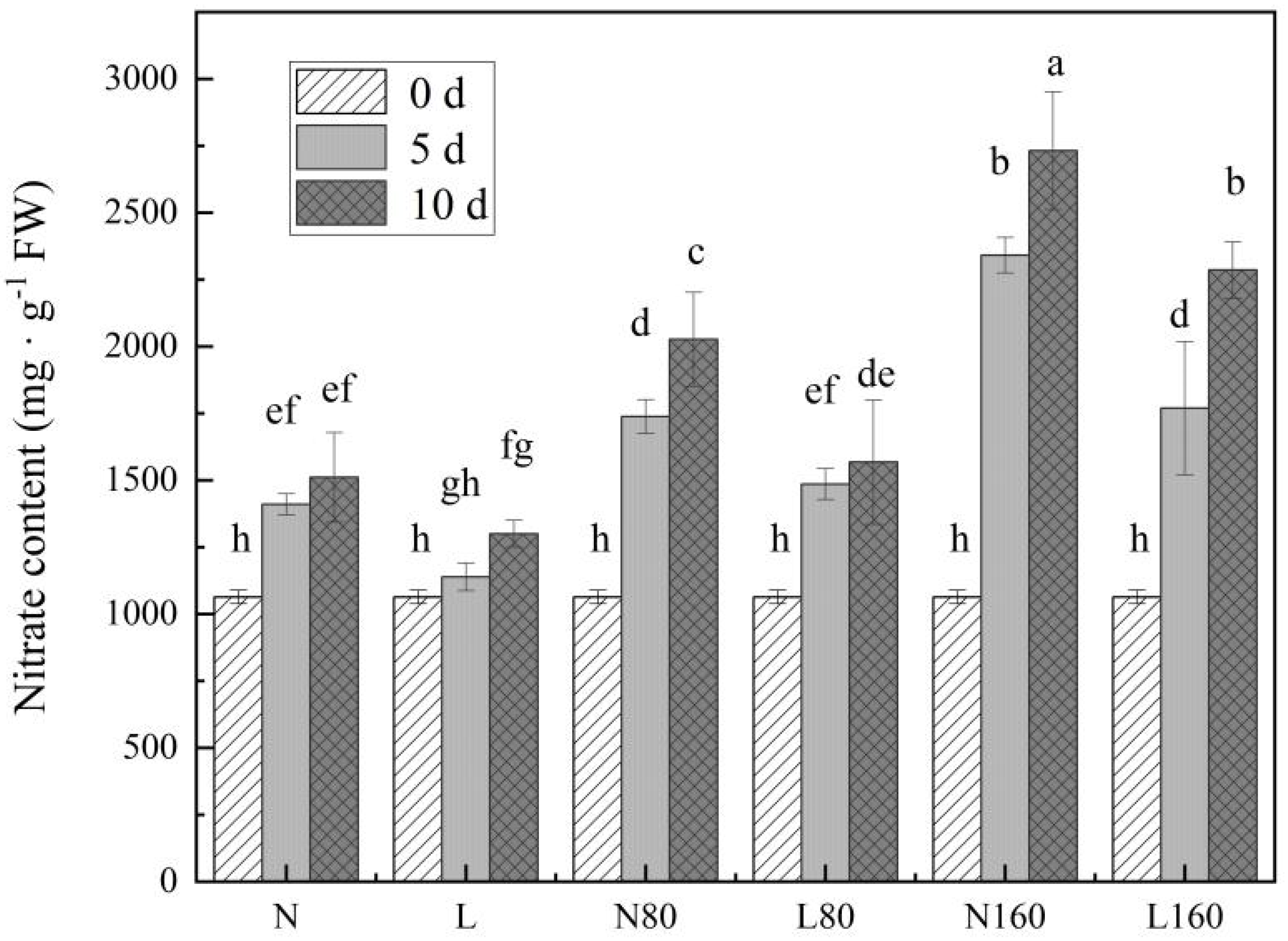
3.3. Nitrate Content in Pakchoi under Different Nitrogen Levels of the R/FR Ratio
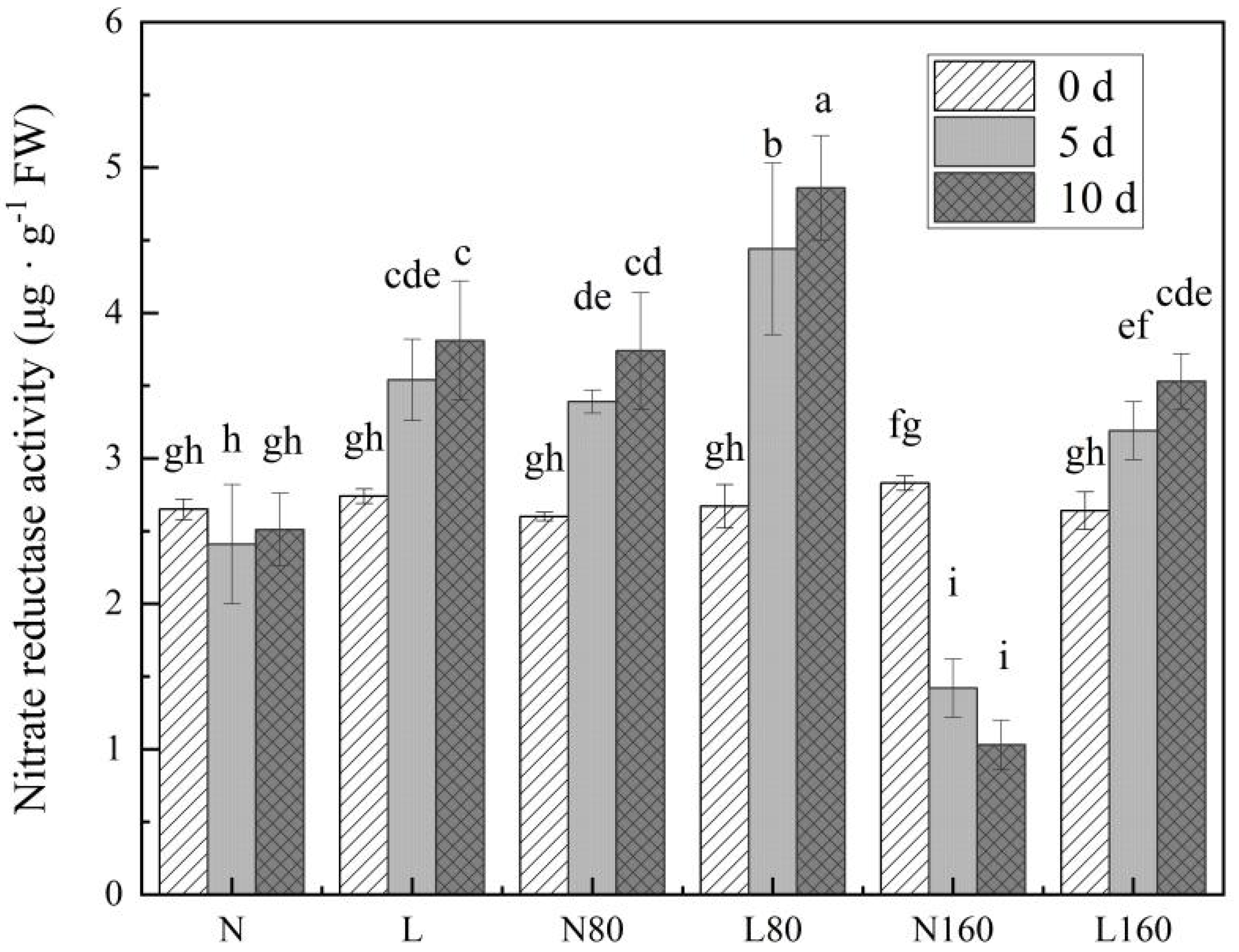
3.4. R/FR Ratio Affects the Activity of Nitrate Reductase in Pakchoi at Different Levels of Nitrogen
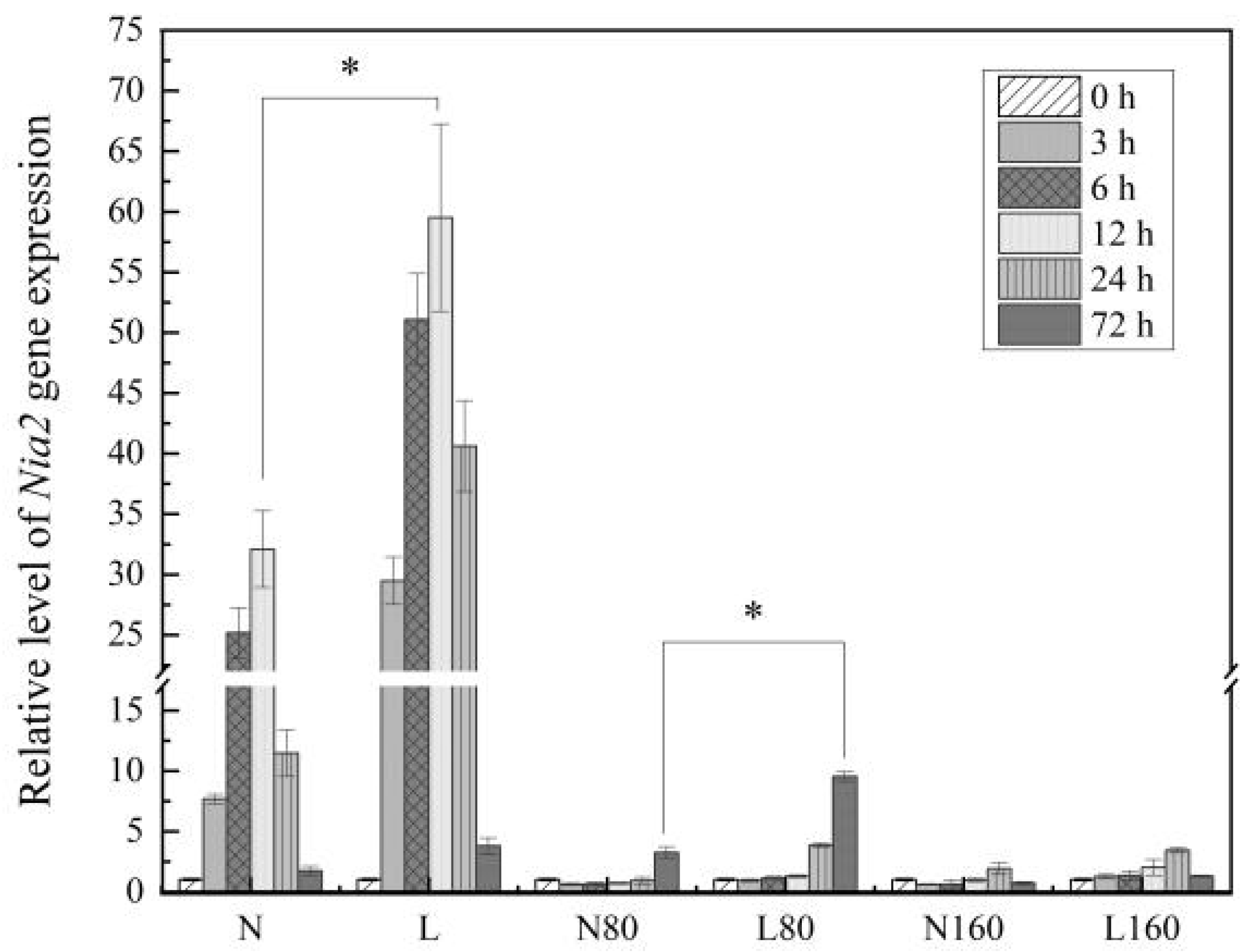
3.5. Levels of R/FR Have Different Effects on Nia2 Gene Expression under Different Nitrogen Levels in Pakchoi
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yuan, L.Y.; Shu, S.; Sun, J.; Guo, S.R.; Tezuka, T. Effects of 24-epibrassinolide on the photosynthetic characteristics, antioxidant system, and chloroplast ultrastructure in Cucumis sativus L. under Ca(NO3)2 stress. Photosynth. Res. 2012, 112, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.D.; Han, X.R.; Peng, J.; Fan, F.; Zhang, Q.G. Effect of Exogenous Nitric Oxide on PSII Function and Distribution and Utilization of Luminous Energy in Tomato Seedlings Under Stress of Ca(NO3)2. J. Nucl. Agric. Sci. 2016, 30, 2451–2459. [Google Scholar]
- Sheng, H.J.; Du, Y.; Shen, R.; Shan, Y.H. Effect of soluble salt content in secondary salinized soil from protected culture on seedling emergence and growth of pakchoi. J. Yangzhou Univ. 2015, 36, 100–104. [Google Scholar]
- Gu, D.Y.; Wang, X.F.; Gao, J.J.; Jiao, J.; Liu, Z.L.; Zhang, Y.Y. Effects of purified humic acid on the growth and nitrogen metabolism of cucumber seedlings under nitrogen stress. Chin. J. Appl. Ecol. 2018, 29, 2575–2582. [Google Scholar]
- Park, Y.; Runkle, E.S. Far-red radiation promotes growth of seedlings by increasing leaf expansion and whole-plant net assimilation. Environ. Exp. Bot. 2017, 136, 41–49. [Google Scholar] [CrossRef] [Green Version]
- Zhen, S.Y.; van Iersel, M.W. Far-red light is needed for efficient photochemistry and photosynthesis. J. Plant Physiol. 2017, 209, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Liu, Q.L.; Cheng, Y.J.; Feng, L.Y.; Wu, X.L.; Fan, Y.F.; Raza, M.A.; Wang, X.C.; Yong, T.W.; Liu, W.G.; et al. Low red/far-red ratio as a signal promotes carbon assimilation of soybean seedlings by increasing the photosynthetic capacity. BMC Plant Biol. 2020, 20, 148. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.J.; Chen, J.X.; Wang, Z.L.; Fan, Y.F.; Chen, S.Y.; Li, Z.L.; Liu, Q.L.; Li, Z.C.; Yang, F.; Yang, W.Y. Effects of Light Intensity and Light Quality on Morphological and Photosynthetic Characteristics of Soybean Seedlings. Sci. Agric. Sin. 2018, 51, 2655–2663. [Google Scholar]
- Wang, F.; Wu, N.; Zhang, L.Y.; Ahammed, G.J.; Chen, X.X.; Xiang, X.; Zhou, J.; Xia, X.J.; Shi, K.; Yu, J.Q.; et al. Light Signaling-Dependent Regulation of Photoinhibition and Photoprotection in Tomato. Plant Physiol. 2018, 176, 1311–1326. [Google Scholar] [CrossRef]
- Boggs, J.Z.; Loewy, K.; Bibee, K.; Heschel, M.S. Phytochromes influence stomatal conductance plasticity in Arabidopsis thaliana. Plant Growth Regul. 2010, 60, 77–81. [Google Scholar] [CrossRef]
- Cao, K.; Yu, J.; Xu, D.W.; Ai, K.Q.; Bao, E.C.; Zou, Z.R. Exposure to lower red to far-red light ratios improve tomato tolerance to salt stress. BMC Plant Biol. 2018, 18, 92. [Google Scholar] [CrossRef] [PubMed]
- Franklin, K.A.; Quail, P.H. Phytochrome functions in Arabidopsis development. J. Exp. Bot. 2020, 61, 11–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fahad, S.; Hussain, S.; Matloob, A.; Khan, F.A.; Khaliq, A.; Saud, S.; Hassan, S.; Shan, D.; Khan, F.; Ullah, N.; et al. Phytohormones and plant responses to salinity stress: A review. Plant Growth Regul. 2015, 75, 391–404. [Google Scholar] [CrossRef]
- Liu, C.Y.; Li, Z.X.; Yu, D. Analysis and Determination on Nutrients of Chinese Cabbage. Chin. Hortic. Abstr. 2014, 30, 29–31. [Google Scholar] [CrossRef]
- Wang, J.; Ma, Q.X.; Liu, M.J.; Zhou, J.J.; Hu, Z.P.; Wu, L.H. Effects of different amino acids and different ratios of lysine and nitrate on the growth and quality of Chinese cabbage (Brassica pekinensis L.). Plant Nutr. Fertil. Sci. 2020, 26, 587–593. [Google Scholar]
- Zhang, J.L. Effects of different nitrogen fertilizer varieties on the yield and nitrate content of cabbage. Food Nutr. China 2006, 5, 25–26. [Google Scholar]
- Zhang, X.Q.; Guo, P.F.; Zhang, K.; Wan, W.L.; Diao, M. Effects of drip irrigation frequency and nitrogen application on tomato growth and nitrate reductase. J. Shihezi Univ. 2018, 36, 57–62. [Google Scholar]
- Yu, T.F.; Liu, X.J.; Zhang, X.L.; Hao, F. Effects of nitrogen on nitrogen content and nitrate reductase activity in root stem and leaf of alfalfa. Grassl. Turf 2017, 37, 14–20. [Google Scholar]
- Liang, L. Study of nitrate reductase activity on nitrate accumulation and related metabolic regulation in pakchoi. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2008. [Google Scholar]
- Guo, J.L.; Zhang, J.X. Review and Outlook of the Research on Nitrate Content of Vegetables. North. Hortic. 2011, 11, 183–188. [Google Scholar]
- Xiong, Q.E. A Experimental Course of Plant Physiology; Sichuan Science and Technology Press: Chengdu, China, 2003. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR. Methods 2002, 25, 402–408. [Google Scholar] [CrossRef]
- Hu, L.P.; Cao, K.; Zou, Z.R. Effects of different far-red light intensity and red light on tomato seedlings. In Proceedings of the 2013 Symposium on High Quality and Safe Production Technology of Vegetables and On-Site Observation Meeting of Facility Horticulture Branch of China Horticultural Society, Guangzhou, China, 21–24 November 2013. [Google Scholar]
- Possart, A.; Xu, T.; Paik, I.; Hanke, S.; Keim, S.; Hermann, H.M.; Wolf, L.; Hiß, M.; Becker, C.; Huq, E.; et al. Characterization of phytochrome interacting factors from the moss physcomitrella patentsillustrates conservation of phytochrome signaling modules in land plants. Plant Cell 2017, 29, 310–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheerin, D.J.; Menon, C.; zur Oven-Krockhaus, S.; Enderle, B.; Zhu, L.; Johnen, P.; Schleifenbaum, F.; Stierhof, Y.D.; Huq, E.; Hiltbrunner, A. Light-activated phytochrome A and B interact with members of the SPA family to promote photomorphogenesis in Arabidopsis by reorganizing the COP1/SPA complex. Plant Cell 2015, 27, 189–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, F.; Fan, Y.F.; Wu, X.L.; Cheng, Y.J.; Liu, Q.L.; Feng, L.Y.; Chen, J.X.; Wang, Z.L.; Wang, X.C.; Yong, T.W.; et al. Auxin-to-gibberellin ratio as a signal for light intensity and quality in regulating soybean growth and matter partitioning. Front. Plant Sci. 2018, 9, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.L.; Li, H.R.; Guo, S.Y.; Wang, S.M.; Shi, H.Z.; Han, Q.Q.; Bao, A.K.; Qing, M.A. Research advances in higher plant adaptation to salt stress. Acta Prataculturae Sin. 2015, 24, 220–236. [Google Scholar]
- Zhou, X.T.; Li, Z.L.; He, J.J.; Wang, X.Y.; Liu, Q.L.; Huang, J.; Xie, Y.D.; He, Z.Q. Effects of red to far-red light ratio on growth and photosynthetic characteristics of tomato seedlings under calcium nitrate stress. Photosynthetica 2021, 59, 625–632. [Google Scholar] [CrossRef]
- Zhu, M.; Li, X.Y.; Zhang, T.T.; Chen, L. Effects of far red light irradiation on the germination and seedling growth of rice under salt and drought stress. J. China Agric. Univ. 2021, 26, 21–32. [Google Scholar]
- Li, Z.L.; Chen, G.Y.; Gao, F.Q.; Luo, J.P.; Li, C.W.; He, Z.Q.; Zhou, X.T. Effects of different light quality ratio on growth and photosynthesis capacity in pakchoi under excess nitrate stress. IOP Conf. Ser. Earth Environ. Sci. 2021, 621, 012105. [Google Scholar] [CrossRef]
- Liu, J.X.; Ou, X.B.; Wang, J.C. Effects of exogenous hydrogen peroxide on growth and resistance physiology of naked oat seedlings under saline-alkali mixed stress. Bull. Bot. Res. 2019, 39, 181–191. [Google Scholar]
- Liu, N.; Song, B.Q.; Yan, Z.S.; Fan, Y.J.; Yang, J.S. Effect of nitrogen application on the content of soluble sugar and key enzyme activities in sugar metabolism of sugar beet. Chin. Agric. Sci. Bull. 2015, 31, 183–189. [Google Scholar]
- Ding, G.Z.; Hou, J.; Chen, L.; Ma, F.M.; Chen, L.J. Cloning of Nia gene and its differential expression induced by different nitrogen forms in sugar beet (Beta vulgaris L.). Acta Agron. Sin. 2011, 37, 1949–1955. [Google Scholar] [CrossRef]
- Mesnard, F.; Ratcliffe, R.G. Nmr analysis of plant nitrogen metabolism. Photosynth. Res. 2005, 83, 163–180. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Wei, M.; Li, Y.; Wang, X.F.; Yang, F.J.; Shi, Q.H. Effects of light quality on carbon and nitrogen metabolism and enzyme activities in tomato seedlings. Acta Hortic. Sin. 2016, 43, 80–88. [Google Scholar]
- Tang, D.W.; Zhang, G.B.; Zhang, F.; Pan, X.M.; Yu, J.H. Effects of different LED light qualities on growth and physiological and biochemical characteristics of cucumber seedlings. J. Gansu Agric. Univ. 2011, 46, 44–48. [Google Scholar]
- Qin, Y.M.; Han, F.Y.; Yang, H.; Liu, M. Effects of light quality on morphogenesis and carbon and nitrogen metabolism of bitter gourd seedlings. China Cucurbits Veg. 2020, 33, 24–27. [Google Scholar]
- Wang, Z.R.; Wang, H.Y.; Deng, H.F.; Xu, C.Q. Regulation of transcription factor HY5 in plant photomorphogenesis and nitrogen metabolism. China Veg. 2018, 5, 20–27. [Google Scholar]
- Jonassen, E.M.; Lea, U.S.; Lillo, C. Hy5 and hyh are positive regulators of nitrate reductase in seedlings and rosette stage plants. Planta Berl. 2008, 227, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.B.; Yao, Q.F.; Gao, X.H.; Jiang, C.F.; Nicholas, P.H.; Fu, X.D. Shoot-to-root mobile transcription factor hy5 coordinates plant carbon and nitrogen acquisition. Curr. Biol. 2016, 26, 640–646. [Google Scholar] [CrossRef] [Green Version]
| Treatments | Fresh Weight (g) | Root Length (cm) | Leaf Width (cm) | Leaf Length (cm) |
|---|---|---|---|---|
| N | 122.60 α± 0.88b β | 39.00 ± 1.00b | 14.10 ± 0.78b | 19.20 ± 0.46b |
| L | 215.60 ± 0.26a | 45.50 ± 1.63a | 17.20 ± 0.46a | 22.00 ± 0.61a |
| N80 | 71.00 ± 2.65d | 30.33 ± 5.41c | 10.80 ± 1.31c | 18.40 ± 1.45b |
| L80 | 84.47 ± 0.40c | 37.50 ± 5.72b | 14.50 ± 0.17b | 20.80 ± 0.36a |
| N160 | 15.36 ± 0.49f | 15.86 ± 1.05e | 8.40 ± 0.62d | 8.30 ± 0.78d |
| L160 | 21.07 ± 0.60e | 19.93 ± 0.96d | 9.76 ± 0.75cd | 12.16 ± 0.80c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Huang, J.; Liu, Q.; Li, Z.; Chen, X.; Han, J.; Gan, Y.; He, Y.; Jiang, C.; Tang, Y.; et al. Low R/FR Ratio Affects Pakchoi’s Growth and Nitrate Content under Excess Nitrate Stress. Horticulturae 2022, 8, 186. https://doi.org/10.3390/horticulturae8030186
Chen L, Huang J, Liu Q, Li Z, Chen X, Han J, Gan Y, He Y, Jiang C, Tang Y, et al. Low R/FR Ratio Affects Pakchoi’s Growth and Nitrate Content under Excess Nitrate Stress. Horticulturae. 2022; 8(3):186. https://doi.org/10.3390/horticulturae8030186
Chicago/Turabian StyleChen, Libang, Jia Huang, Qinglin Liu, Zelin Li, Xu Chen, Jiaxi Han, Yirong Gan, Yuexuan He, Chenxiang Jiang, Yunxin Tang, and et al. 2022. "Low R/FR Ratio Affects Pakchoi’s Growth and Nitrate Content under Excess Nitrate Stress" Horticulturae 8, no. 3: 186. https://doi.org/10.3390/horticulturae8030186
APA StyleChen, L., Huang, J., Liu, Q., Li, Z., Chen, X., Han, J., Gan, Y., He, Y., Jiang, C., Tang, Y., & Zhou, X. (2022). Low R/FR Ratio Affects Pakchoi’s Growth and Nitrate Content under Excess Nitrate Stress. Horticulturae, 8(3), 186. https://doi.org/10.3390/horticulturae8030186







