Effects of Elevated Temperature and Salicylic Acid on Heat Shock Response and Growth of Potato Microplants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. SA and Temperature Treatments
2.3. RNA Extraction and qRT-PCR Analysis
2.4. Potato Genomic Resources and Description of HSF and HSP Genes and Proteins
2.5. Statistical Analysis
3. Results
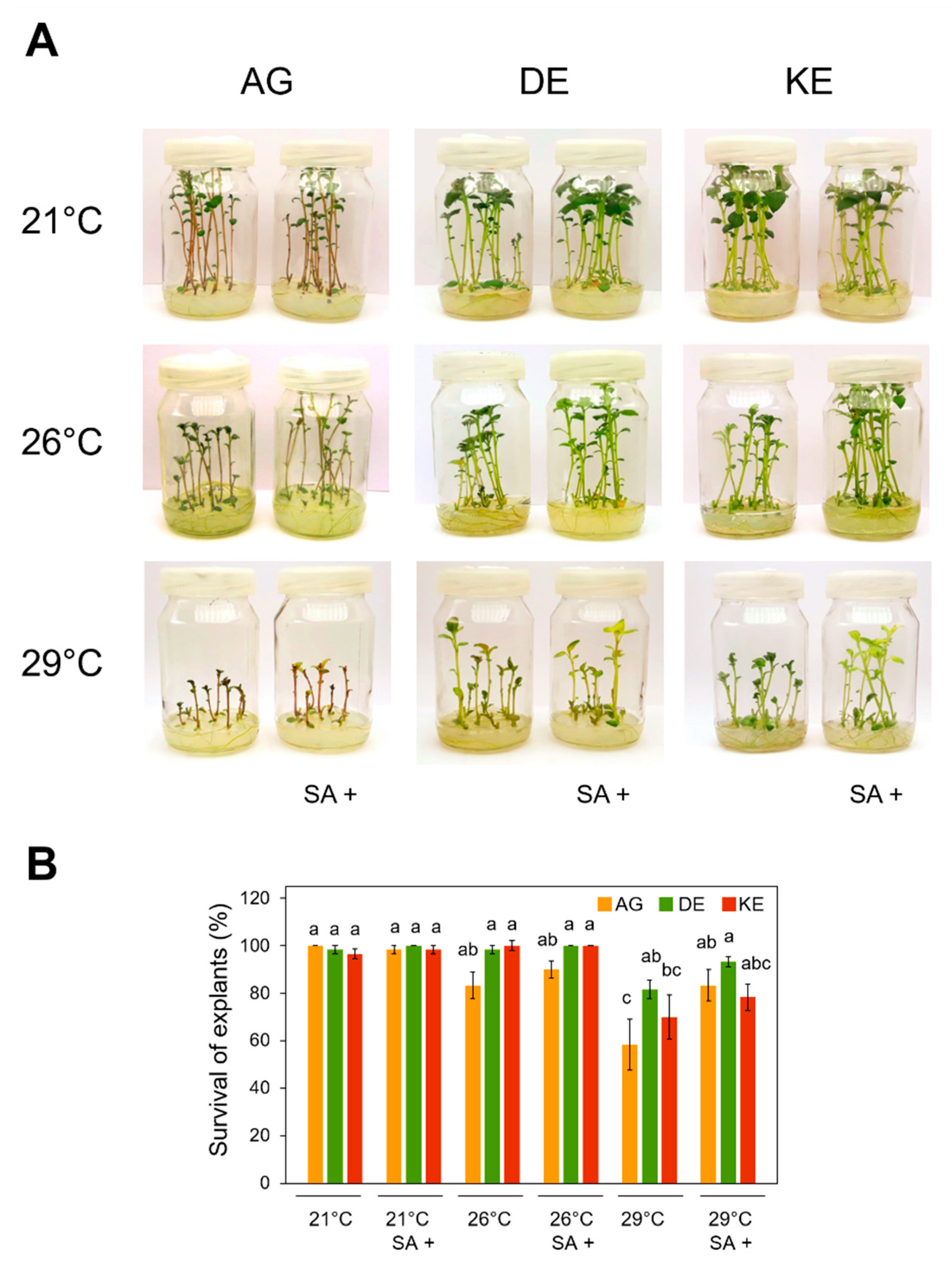
3.1. Effects of SA and Temperature Treatments on the Survival of Explants
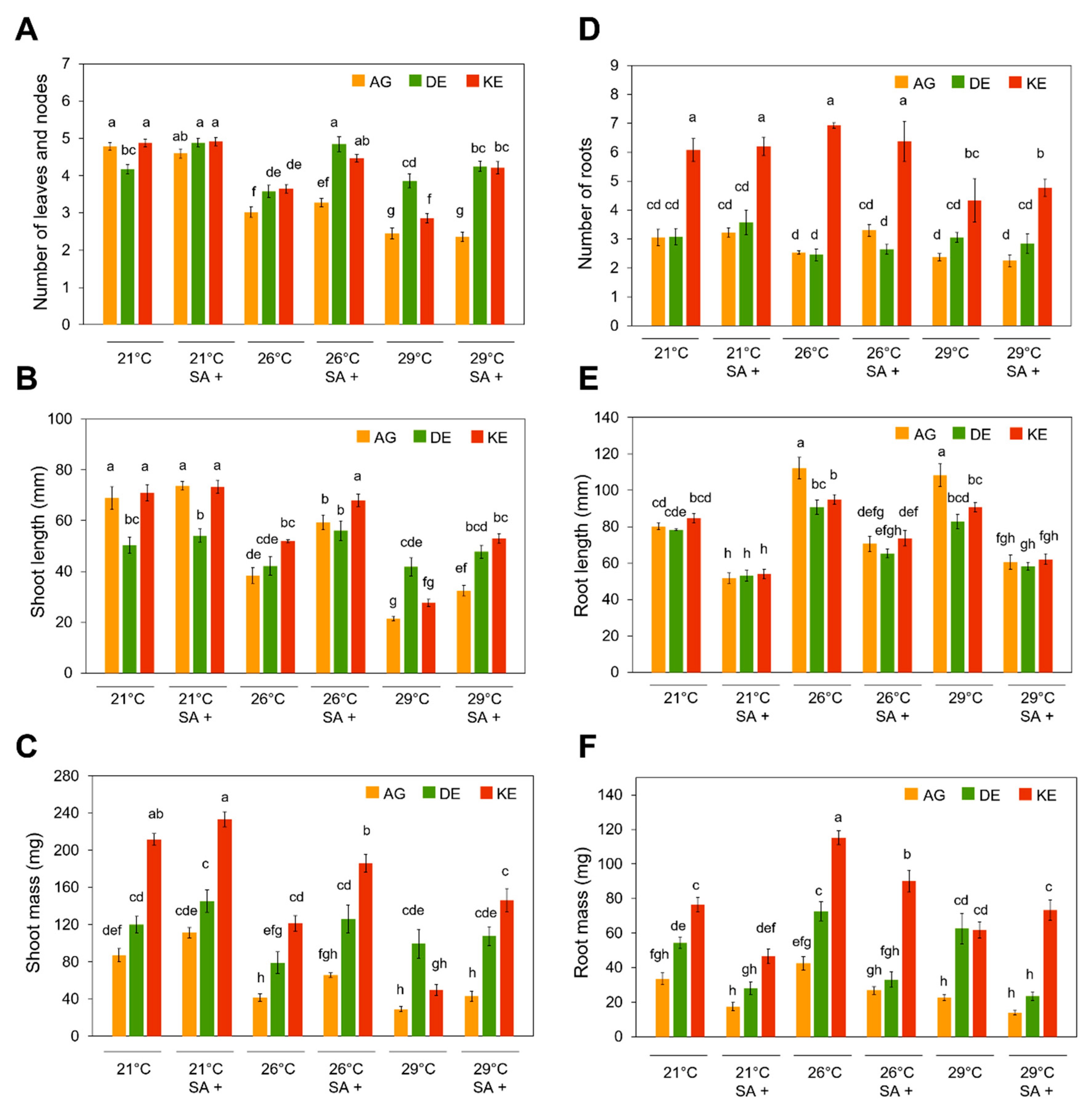
3.2. Effects of SA and Temperature on Growth and Development of Potato Plantlets
3.3. Potato HSFs and HSPs Selected for Expression Analysis
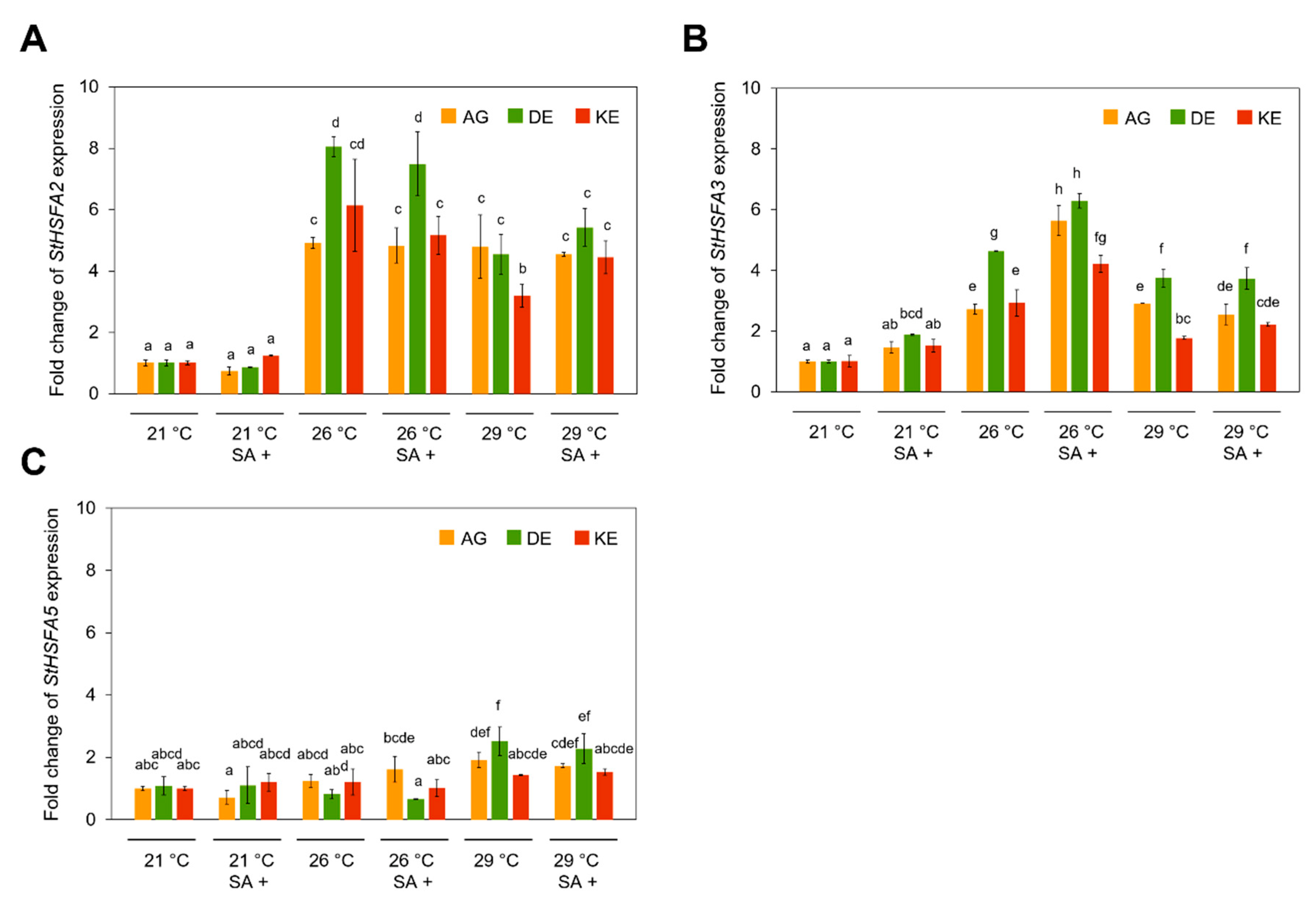
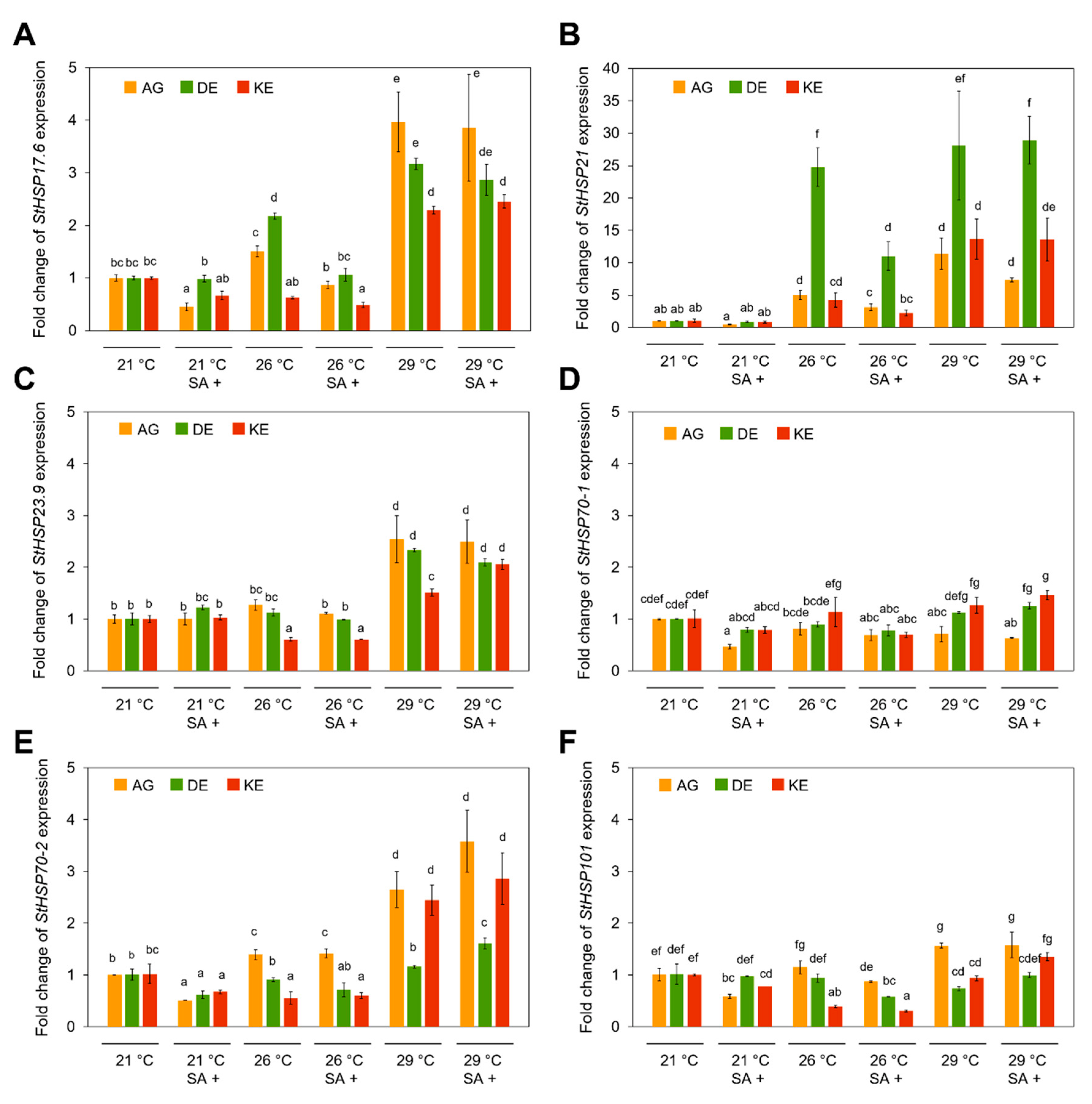
3.4. Effects of SA and Temperature on HSPs and HSFs Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Struik, P.C. Responses of the Potato Plant to Temperature. In Potato Biology and Biotechnology, 1st ed.; Vreugdenhil, D., Bradshaw, J., Gebhardt, C., Govers, F., Taylor, M., MacKerron, D., Ross, H., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2007; pp. 367–393. [Google Scholar] [CrossRef]
- Momčilović, I.; Fu, J.; Pantelić, D.; Rudić, J.; Broćić, Z. Impact of Heat Stress on Potato: Plant Responses and Approaches to Tolerance Improvement. In The Potato Crop: Management, Production, and Food Security; Villa, P.M., Ed.; Nova Science Publishers: New York, NY, USA, 2021; pp. 91–122. [Google Scholar] [CrossRef]
- Wolf, S.; Marani, A.; Rudich, J. Effect of temperature on carbohydrate metabolism in potato plants. J. Exp. Bot. 1991, 42, 619–625. [Google Scholar] [CrossRef]
- Hancock, R.D.; Morris, W.L.; Ducreux, L.J.; Morris, J.A.; Usman, M.; Verrall, S.R.; Fuller, J.; Simpson, C.G.; Zhang, R.; Hedley, P.E.; et al. Physiological, biochemical and molecular responses of the potato (Solanum tuberosum L.) plant to moderately elevated temperature. Plant Cell Environ. 2014, 37, 439–450. [Google Scholar] [CrossRef]
- Fu, J.; Momcilovic, I.; Prasad, P.V.V. Molecular bases and improvement of heat tolerance in crop plants. In Heat Stress: Causes, Prevention and Treatments; Josipovic, S., Ludwig, E., Eds.; Nova Science Publishers: New York, NY, USA, 2012; pp. 185–214. [Google Scholar]
- Bita, C.; Gerats, T. Plant tolerance to high temperature in a changing environment: Scientific fundamentals and production of heat stress-tolerant crops. Front. Plant Sci. 2013, 4, 273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, G.J.; Vierling, E. A small heat shock protein cooperates with heat shock protein 70 systems to reactivate a heat-denatured protein. Plant Physiol. 2000, 122, 189–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basha, E.; Lee, G.J.; Demeler, B.; Vierling, E. Chaperone activity of cytosolic small heat shock proteins from wheat. Eur. J. Biochem. 2004, 271, 1426–1436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doyle, S.M.; Genest, O.; Wickner, S. Protein rescue from aggregates by powerful molecular chaperone machines. Nat. Rev. Mol. Cell Biol. 2013, 14, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Wang, D.; Wang, R.; Kong, N.; Zhang, C.; Yang, C.; Wu, W.; Ma, H.; Chen, Q. Genome-wide analysis of the potato Hsp20 gene family: Identification, genomic organization and expression profiles in response to heat stress. BMC Genomics 2018, 19, 61. [Google Scholar] [CrossRef]
- Jacob, P.; Hirt, H.; Bendahmane, A. The heat-shock protein/chaperone network and multiple stress resistance. Plant Biotechnol. J. 2017, 15, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.R.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 2015, 6, 462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarke, S.M.; Mur, L.A.; Wood, J.E.; Scott, I.M. Salicylic acid dependent signaling promotes basal thermotolerance but is not essential for acquired thermotolerance in Arabidopsis thaliana. Plant J. 2004, 38, 432–447. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Zhan, J.; Liu, H.; Zhang, J.; Chen, J.; Wen, P.; Huang, W. Salicylic acid synthesized by benzoic acid 2-hydroxylase participates in the development of thermotolerance in pea plants. Plant Sci. 2006, 171, 226–233. [Google Scholar] [CrossRef]
- Rai, K.K.; Pandey, N.; Rai, S.P. Salicylic acid and nitric oxide signaling in plant heat stress. Physiol. Plant. 2020, 168, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Snyman, M.; Cronjé, M.J. Modulation of heat shock factors accompanies salicylic acid-mediated potentiation of Hsp70 in tomato seedlings. J. Exp. Bot. 2008, 59, 2125–2132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arif, Y.; Sami, F.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salicylic acid in relation to other phytohormones in plant: A study towards physiology and signal transduction under challenging environment. Environ. Exp. Bot. 2020, 175, 104040. [Google Scholar] [CrossRef]
- Pokotylo, I.; Kravets, V.; Ruelland, E. Salicylic acid binding proteins (SABPs): The hidden forefront of salicylic acid signalling. Int. J. Mol. Sci. 2019, 20, 4377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.I.R.; Iqbal, N.; Masood, A.; Per, T.S.; Khan, N.A. Salicylic acid alleviates adverse effects of heat stress on photosynthesis through changes in proline production and ethylene formation. Plant Signal. Behav. 2013, 8, e26374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Zhang, J.; Liu, H.; Huang, W. Salicylic acid or heat acclimation pre-treatment enhances the plasma membrane-associated ATPase activities in young grape plants under heat shock. Sci. Hortic. 2008, 119, 21–27. [Google Scholar] [CrossRef]
- Wang, L.J.; Li, S.H. Salicylic acid-induced heat or cold tolerance in relation to Ca2+ homeostasis and antioxidant systems in young grape plants. Plant Sci. 2006, 170, 685–694. [Google Scholar] [CrossRef]
- Nazar, R.; Iqbal, N.; Umar, S. Heat Stress Tolerance in Plants: Action of Salicylic Acid. In Salicylic Acid: A Multifaceted Hormone; Nazar, R., Iqbal, N., Khan, N., Eds.; Springer: Singapore, 2017; pp. 145–161. [Google Scholar] [CrossRef]
- Hijmans, R.J. The effect of climate change on global potato production. Am. J. Potato Res. 2003, 80, 271–279. [Google Scholar] [CrossRef]
- Rudić, J.; Dragićević, M.B.; Momčilović, I.; Simonović, A.D.; Pantelić, D. In Silico Study of Superoxide Dismutase Gene Family in Potato and Effects of Elevated Temperature and Salicylic Acid on Gene Expression. Antioxidants 2022, 11, 488. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ma, K.B.; Lu, Z.G.; Ren, S.X.; Jiang, H.R.; Cui, J.W.; Chen, G.; Teng, N.J.; Lam, H.M.; Jin, B. Differential physiological, transcriptomic and metabolomic responses of Arabidopsis leaves under prolonged warming and heat shock. BMC Plant Biol. 2020, 20, 86. [Google Scholar] [CrossRef] [PubMed]
- López-Delgado, H.; Mora-Herrera, M.E.; Zavaleta-Mancera, H.A.; Cadena-Hinojosa, M.; Scott, I.M. Salicylic acid enhances heat tolerance and potato virus X (PVX) elimination during thermotherapy of potato microplants. Am. J. Potato Res. 2004, 81, 171–176. [Google Scholar] [CrossRef]
- Makarova, S.; Makhotenko, A.; Spechenkova, N.; Love, A.J.; Kalinina, N.O.; Taliansky, M. Interactive responses of potato (Solanum tuberosum L.) plants to heat stress and infection with potato virus Y. Front. Microbiol. 2018, 9, 2582. [Google Scholar] [CrossRef] [Green Version]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Linsmaier, E.M.; Skoog, F. Organic growth factor requirements of tobacco tissue cultures. Physiol. Plant. 1965, 18, 100–127. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein Identification and Analysis Tools on the ExPASy Server. In The Proteomics Protocols Handbook; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2005; pp. 571–607. [Google Scholar] [CrossRef]
- Tang, R.; Zhu, W.; Song, X.; Lin, X.; Cai, J.; Wang, M.; Yang, Q. Genome-wide identification and function analyses of heat shock transcription factors in potato. Front. Plant Sci. 2016, 7, 490. [Google Scholar] [CrossRef] [Green Version]
- Hussey, G.; Stacey, N.J. In vitro propagation of potato (Solanum tuberosum L.). Ann. Bot. 1981, 48, 787–796. [Google Scholar] [CrossRef]
- Yaycili, O.; Alikamanoğlu, S. Induction of salt-tolerant potato (Solanum tuberosum L.) mutants with gamma irradiation and characterization of genetic variations via RAPD-PCR analysis. Turk. J. Biol. 2012, 36, 405–412. [Google Scholar] [CrossRef]
- Iqbal, A.; Rizwan, A.; Mukhtar, Z.; Mansoor, S.; Khalid, Z.M.; Asad, S. Establishment of an efficient and reproducible regeneration system for potato cultivars grown in Pakistan. Pak. J. Bot. 2016, 48, 258–290. [Google Scholar]
- Elwan, M.W.M.; Elhamahmy, M.A.M.; Mohamed, F.H. Physiological effect of potato genotypes and salicylic acid on plantlets growth and microtuber production under salt stress. Hortsci. J. Suez Canal Univ. 2018, 7, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Broćić, Z.; Momčilović, I.; Poštić, D.; Oljača, J.; Veljković, B. Production of High-Quality Seed Potato by Aeroponics. In The Potato Crop: Management, Production, and Food Security; Villa, P.M., Ed.; Nova Science Publishers: New York, NY, USA, 2021; pp. 25–59. [Google Scholar]
- López-Delgado, H.A.; Martínez-Gutiérrez, R.; Mora-Herrera, M.E.; Torres-Valdés, Y. Induction of freezing tolerance by the application of hydrogen peroxide and salicylic acid as tuber-dip or canopy spraying in Solanum tuberosum L. plants. Potato Res. 2018, 61, 195–206. [Google Scholar] [CrossRef]
- Mostafa, A.A.; Sarwat, M.I.; Salama, D.A.; Sallam, H.A. Effect of ascorbate, salicylate and silicate on potato plant under water deficit stress conditions. Arab Univ. J. Agric. Sci. 2019, 27, 1–15. [Google Scholar] [CrossRef]
- Lopez-Delgado, H.; Dat, J.F.; Foyer, C.H.; Scott, I.M. Induction of thermotolerance in potato microplants by acetylsalicylic acid and H2O2. J. Exp. Bot. 1998, 49, 713–720. [Google Scholar] [CrossRef]
- Pasternak, T.; Groot, E.P.; Kazantsev, F.V.; Teale, W.; Omelyanchuk, N.; Kovrizhnykh, V.; Palme, K.; Mironova, V.V. Salicylic acid affects root meristem patterning via auxin distribution in a concentration-dependent manner. Plant Physiol. 2019, 180, 1725–1739. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Abas, M.; Verstraeten, I.; Glanc, M.; Molnár, G.; Hajný, J.; Lasák, P.; Petřík, I.; Russinova, E.; Petrášek, P.; et al. Salicylic acid targets protein phosphatase 2A to attenuate growth in plants. Curr. Biol. 2020, 30, 381–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armengot, L.; Marquès-Bueno, M.M.; Soria-Garcia, A.; Müller, M.; Munné-Bosch, S.; Martínez, M.C. Functional interplay between protein kinase CK 2 and salicylic acid sustains PIN transcriptional expression and root development. Plant J. 2014, 78, 411–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waters, E.R. The evolution, function, structure, and expression of the plant sHSPs. J. Exp. Bot. 2013, 64, 391–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Whaibi, M.H. Plant heat-shock proteins: A mini review. J. King Saud Univ. Sci. 2011, 23, 139–150. [Google Scholar] [CrossRef] [Green Version]
- Tang, R.; Gupta, S.K.; Niu, S.; Li, X.Q.; Yang, Q.; Chen, G.; Zhu, W.; Haroon, M. Transcriptome analysis of heat stress response genes in potato leaves. Mol. Biol. Rep. 2020, 47, 4311–4321. [Google Scholar] [CrossRef]
- Oljača, J. The Effects of Cultivar and Cultivation Technology on Potato Stress Tolerance. Ph.D. Thesis, University of Belgrade, Belgrade, Serbia, 2016. [Google Scholar]
| Name | Gene ID | Chromosomal Localization | Transcript ID | Transcript Length (bp) | Protein Length (aa) | pI | Instability Index | Aliphatic Index | MW (kDa) | Protein Localization |
|---|---|---|---|---|---|---|---|---|---|---|
| StHSP17.6 | PGSC0003DMG400030426 | Chr. 6: 56,893,292–56,894,077 | PGSC0003DMT400078201 | 786 | 154 | 5.83 | 44.31 | 65.71 | 17.6 | cytosol |
| StHSP17.6 | PGSC0003DMG400030339 | Chr. 6: 56,900,911–56,901,677 | PGSC0003DMT400078006 | 767 | 154 | 6.20 | 50.37 | 65.06 | 17.6 | cytosol |
| StHSP17.6 | PGSC0003DMG400030340 | Chr. 6: 56,905,147–56,905,872 | PGSC0003DMT400078007 | 726 | 154 | 7.91 | 44.85 | 65.71 | 17.6 | cytosol |
| StHSP21 * | PGSC0003DMG400003219 | Chr. 3: 47,576,728–47,579,687 | PGSC0003DMT400008351 | 2776 | 233 | 6.98 | 39.66 | 70.21 | 25.8 | chloroplast |
| StHSP23.9 | PGSC0003DMG400004808 | Chr. 8: 52,375,001–52,376,265 | PGSC0003DMT400012249 | 1028 | 211 | 6.45 | 57.07 | 71.61 | 23.9 | mitochondria |
| StHSP70-1 | PGSC0003DMG400024887 | Chr. 1: 80,158,423–80,162,913 | PGSC0003DMT400064031 | 2644 | 706 | 5.16 | 27.82 | 85.21 | 75.1 | chloroplast |
| StHSP70-2 | 102600309 (NCBI) PGSC0003DMG400027611 | Chr. 11:12,761,196–12,763,127 | XM_006361313.2 PGSC0003DMT400070986 | 2577 1032 | 696 257 | 5.26 - | 29.14 - | 84.31 - | 74.8 - | chloroplast |
| StHSP101 | PGSC0003DMG400024644 | Chr. 3: 55,148,140–55,152,302 | PGSC0003DMT400063352 | 3139 | 912 | 5.82 | 36.29 | 96.57 | 101.1 | cytosol |
| StHSFA2 | PGSC0003DMG400008223 | Chr. 8: 35,415,712–35,418,551 | PGSC0003DMT400021232 | 2521 | 254 | 5.12 | 64.12 | 69.84 | 29.3 | cytosol/nucleus |
| PGSC0003DMT400021233 | 2744 | 226 | 4.58 | 64.32 | 76.77 | 26.0 | ||||
| PGSC0003DMT400021234 | 2350 | 353 | 4.92 | 56.69 | 74.22 | 40.7 | ||||
| PGSC0003DMT400021235 | 1817 | 340 | 5.14 | 56.88 | 71.62 | 39.1 | ||||
| StHSFA3 | PGSC0003DMG401002683 | Chr. 9: 3,849,902–3,856,182 | PGSC0003DMT400006917 | 1494 | 497 | 4.78 | 54.25 | 68.07 | 54.7 | cytosol/nucleus |
| PGSC0003DMT400006919 | 3530 | 380 | 4.98 | 52.01 | 66.97 | 41.7 | ||||
| PGSC0003DMT400006920 | 3276 | 357 | 4.97 | 56.23 | 60.39 | 39.2 | ||||
| PGSC0003DMT400006921 | 2645 | 480 | 4.81 | 55.73 | 64.60 | 52.9 | ||||
| PGSC0003DMT400006922 | 3013 | 480 | 4.81 | 55.73 | 64.60 | 52.9 | ||||
| StHSFA5 | PGSC0003DMG400004662 | Chr. 12:59,294,378–59,298,391 | PGSC0003DMT400011871 | 1700 | 478 | 5.48 | 57.97 | 70.98 | 53.4 | cytosol/nucleus |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudić, J.; Pantelić, D.; Oljača, J.; Momčilović, I. Effects of Elevated Temperature and Salicylic Acid on Heat Shock Response and Growth of Potato Microplants. Horticulturae 2022, 8, 372. https://doi.org/10.3390/horticulturae8050372
Rudić J, Pantelić D, Oljača J, Momčilović I. Effects of Elevated Temperature and Salicylic Acid on Heat Shock Response and Growth of Potato Microplants. Horticulturae. 2022; 8(5):372. https://doi.org/10.3390/horticulturae8050372
Chicago/Turabian StyleRudić, Jelena, Danijel Pantelić, Jasmina Oljača, and Ivana Momčilović. 2022. "Effects of Elevated Temperature and Salicylic Acid on Heat Shock Response and Growth of Potato Microplants" Horticulturae 8, no. 5: 372. https://doi.org/10.3390/horticulturae8050372
APA StyleRudić, J., Pantelić, D., Oljača, J., & Momčilović, I. (2022). Effects of Elevated Temperature and Salicylic Acid on Heat Shock Response and Growth of Potato Microplants. Horticulturae, 8(5), 372. https://doi.org/10.3390/horticulturae8050372







