In Vitro Growth Responses of Ornamental Bananas (Musa sp.) as Affected by Light Sources
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Culture Establishment
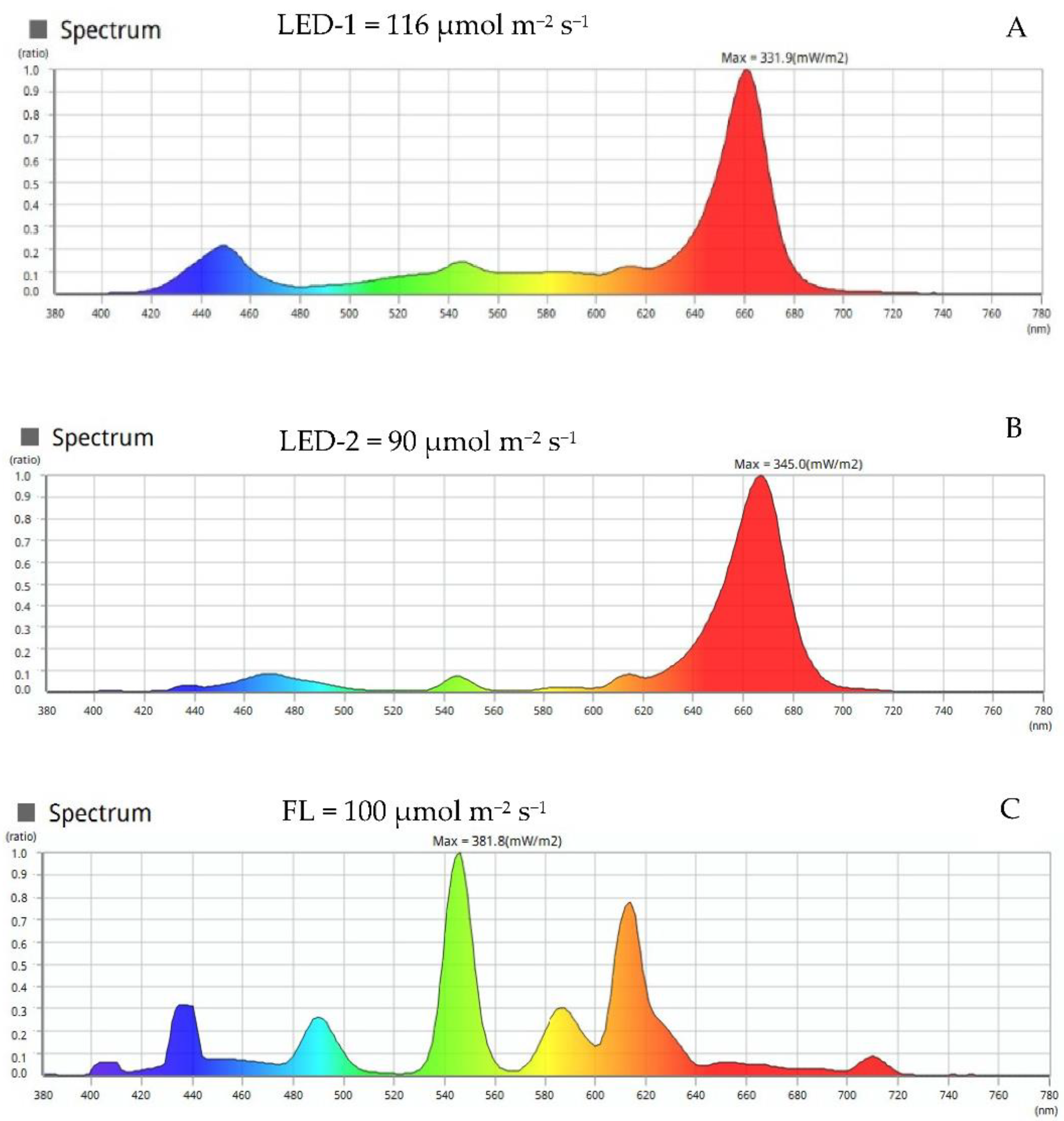
2.2. Light Sources
2.3. In Vitro Growth and Development
2.4. Relative Chlorophyll Content
2.5. Stomata Analysis
2.6. Anatomical Observations
2.7. Experimental Design and Statistical Analysis
3. Results
3.1. In Vitro Growth and Development
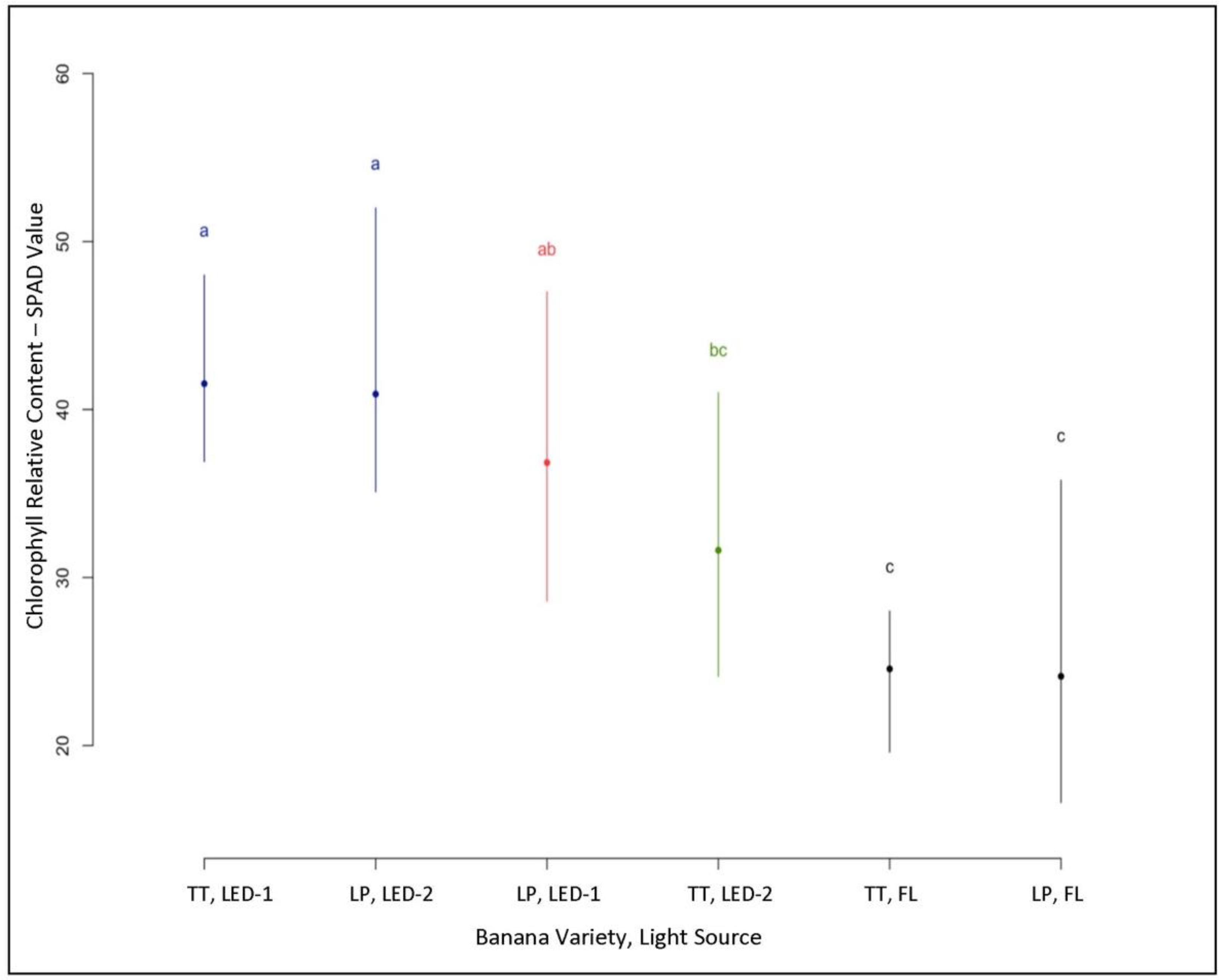
3.2. Relative Chlorophyll Content
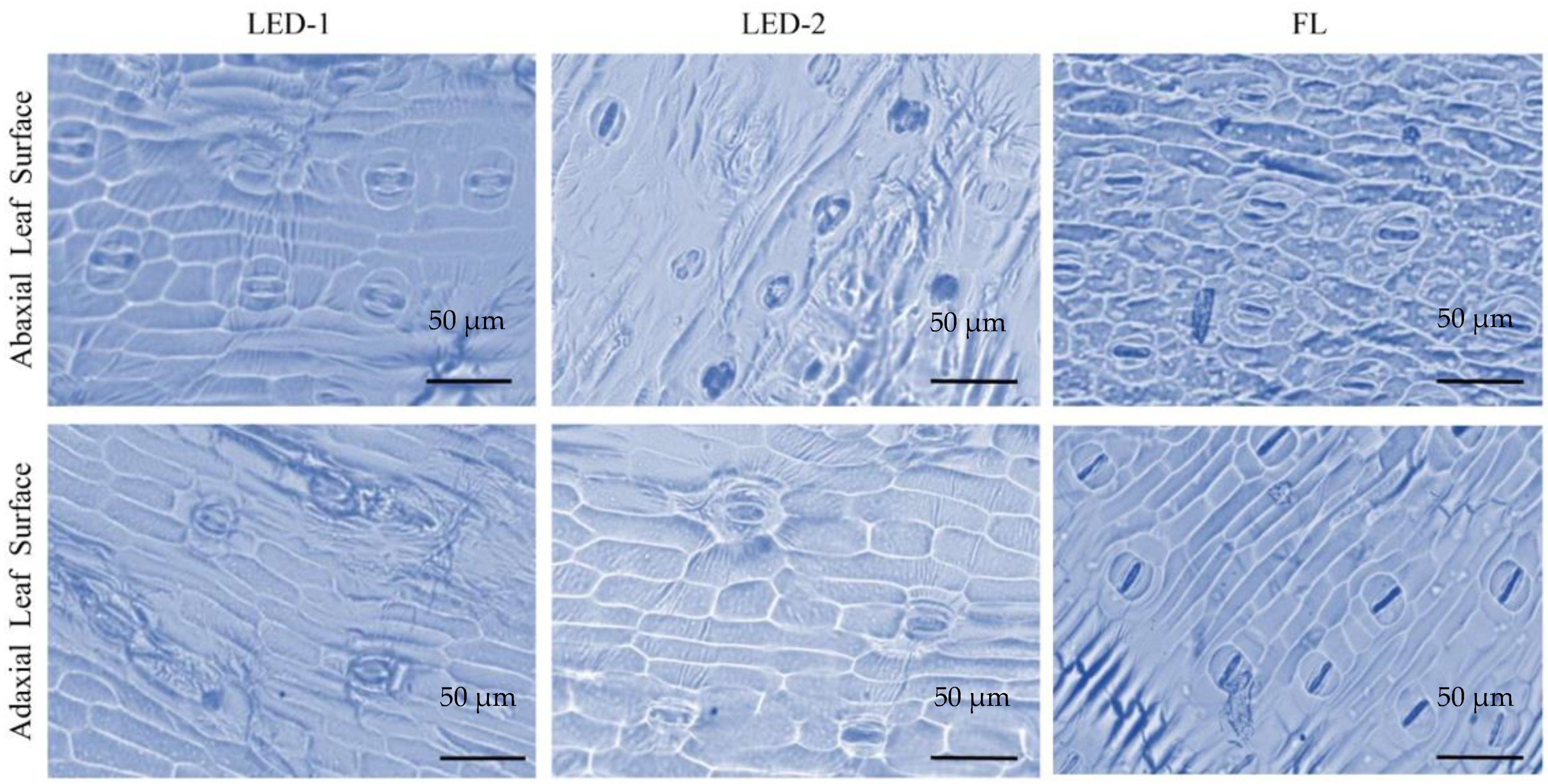
3.3. Stomata Analysis
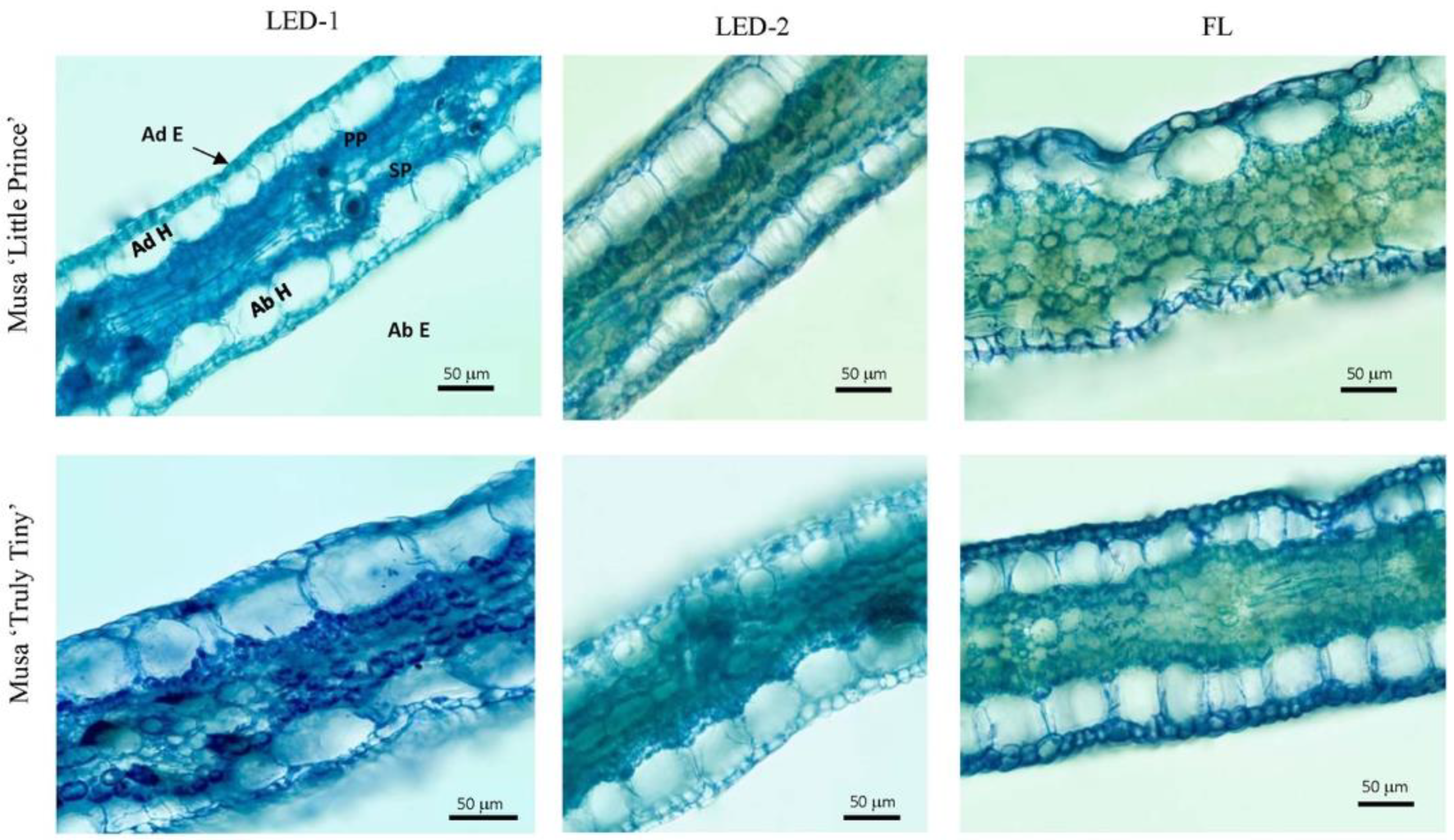
3.4. Anatomical Observations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rajapakse, N.C.; Shahak, Y. Light-quality manipulation by horticulture industry. Annual Plant Rev. Light Plant Dev. 2008, 30, 290. [Google Scholar]
- Faria, D.V.; Correia, L.N.F.; Souza, M.V.C.; Ríos-Ríos, A.M.; Vital, C.E. Irradiance and light quality affect two annatto (Bixa orellana L.) cultivars with contrasting bixin production. J. Photochem. Photobiol. 2019, 197, 111549. [Google Scholar] [CrossRef]
- Gupta, S.D.; Jatothu, B. Fundamentals and applications of light-emitting diodes (LEDs) in in vitro plant growth and morphogenesis. Plant Biotechnol. Rep. 2013, 7, 211–220. [Google Scholar] [CrossRef]
- Miyashita, Y.; Kitaya, Y.; Kozai, T.; Kimura, T. Effects of red and far-red light on the growth and morphology of potato plantlets in vitro: Using light emitting diode as a light source for micropropagation. Environ. Eff. Control. Plant Tissue Cult. 1994, 393, 189–194. [Google Scholar] [CrossRef]
- Kodym, A.; Zapata-Arias, F.J. Natural light as an alternative light source for the in vitro culture of banana (Musa acuminata cv.‘Grande Naine’). Plant Cell Tis. Org. Cult. 1998, 55, 141–145. [Google Scholar] [CrossRef]
- Dooley, J.H. Influence of Lighting Spectra on Plant Tissue Culture; American Society of Association Executives: Washington, DC, USA, 1991; No. 7530. [Google Scholar]
- Standaert-de Metsenaere, R.E.A. Economic considerations. Micropropag. Technol. Appl. 1991, 123–140. [Google Scholar]
- Mitchell, C. Plant lighting in controlled environments for space and earth applications. Acta Hortic. 2012, 956, 23–36. [Google Scholar] [CrossRef]
- Olle, M.; Viršilė, A. The effects of light-emitting diode lighting on greenhouse plant growth and quality. Agric. Food Sci. 2013, 22, 223–234. [Google Scholar] [CrossRef]
- Nhut, D.T.; Nam, N.B. Light-emitting diodes (LEDs): An artificial lighting source for biological studies. In Proceedings of the Third International Conference on the Development of Biomedical Engineering in Vietnam, Ho Chi Minh City, Vietnam, 11–14 January 2010; IFMBE Proceedings. Van Toi, V., Khoa, T.Q.D., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 27, pp. 134–139. [Google Scholar]
- Kim, S.-J.; Hahn, E.-J.; Heo, J.-W.; Paek, K.-Y. Effects of LEDs on net photosynthetic rate, growth and leaf stomata of chrysanthemum plantlets in vitro. Sci. Hortic. 2003, 101, 143–151. [Google Scholar] [CrossRef]
- Silva, T.D.; Batista, D.; Fortini, E.A.; de Castro, K.M.; Felipe, S.H.S.; Fernandes, A.M.; Sousa, R.M.D.J.; Chagas, K.; da Silva, J.V.S.; Correia, L.N.D.F.; et al. Blue and red light affects morphogenesis and 20-hydroxyecdisone content of in vitro Pfaffia glomerata accessions. J. Photochem. Photobiol. B Biol. 2019, 203, 111761. [Google Scholar] [CrossRef]
- Jishi, T. LED lighting technique to control plant growth and morphology. In Smart Plant Factory; Kozai, T., Ed.; Springer: Singapore, 2018; pp. 211–222. [Google Scholar]
- Ortiz, R. Conventional banana and plantain breeding. In Proceedings of the VII International Symposium on Banana: ISHS-ProMusa Symposium on Bananas and Plantains: Towards Sustainable Global Production, Salvador, Brazil, 14 October 2011; International Society for Horticultural Science: Leuven, Belgium, 2011; Volume 986, pp. 177–194. [Google Scholar]
- Nguyen, Q.T.; Kozai, T. Growth of In vitro banana (Musa spp.) shoots under photomixotrophic and photoautotrophic conditions. Vitr. Cell. Dev. Biol.-Anim. 2001, 37, 824–829. [Google Scholar] [CrossRef]
- Wong, W.C. In vitro propagation of banana (Musa spp.): Initiation, proliferation and development of shoot-tip cultures on defined media. Plant Cell Tissue Organ Cult. 1986, 6, 159–166. [Google Scholar] [CrossRef]
- Bairu, M.W.; Stirk, W.A.; Doležal, K.; van Staden, J. The role of topolins in micropropagation and somaclonal variation of banana cultivars ‘Williams’ and ‘Grand Naine’ (Musa spp. AAA). Plant Cell Tis. Org. Cult. 2008, 95, 373–379. [Google Scholar] [CrossRef]
- Makara, A.M.; Rubaihayo, P.R.; Magambo, M.J.S. Carry-over effect of Thidiazuron on banana in vitro proliferation at different culture cycles and light incubation conditions. African J. Biotechnol. 2010, 9, 3079–3085. [Google Scholar]
- Ahirwar, M.K.; Sananda, M.; Singh, M.K.; Chaitali, S.; Singh, R.P. A high frequency plantlets regeneration protocol for banana (Musa paradisiaca L.) micropropagation. Asian J. Hort. 2012, 7, 397–401. [Google Scholar]
- Yusnita, Y.; Danial, E.; Hapsoro, D. In vitro shoot regeneration of indonesian bananas (Musa spp.) cv. ambon kuning and raja bulu, plantlet acclimatizationand field performance. AGRIVITA J. Agric. Sci. 2015, 37, 51–58. [Google Scholar] [CrossRef]
- Nhut, D.; Hong, L.; Watanabe, H.; Goi, M.; Tanaka, M. Growth of banana plantlets cultured in vitro under red and blue light-emitting diode (led) irradiation source. Acta Hortic. 2002, 575, 117–124. [Google Scholar] [CrossRef]
- Nhut, D.T.; Takamura, T.; Watanabe, H.; Tanaka, M. Efficiency of a novel culture system by using light-emitting diode (LED) on in vitro and subsequent growth of micropropagated banana plantlets. Acta Hort. 2003, 161, 121–127. [Google Scholar] [CrossRef]
- Trivedi, A.; Sengar, R.S. Effect of various light emittimg diodes on growth and photosynthetic pigments of banana (Musa acuminata) CV. Grande naine in vitro plantlets. Int. J. Chem. Stud. 2017, 5, 1819–1821. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Kraus, J.E.; Arduin, M. Manual Básico de Métodos em Morfologia Vegetal; EDUR: Seropédica, Brazil, 1997; p. 198. [Google Scholar]
- Chen, C. Fluorescent Lighting Distribution for Plant Micropropagation. Biosyst. Eng. 2005, 90, 295–306. [Google Scholar] [CrossRef]
- Aitken-Christie, J.; Kozai, T.; Smith, M.A.L. (Eds.) Automation and Environmental Control in Plant Tissue Culture; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; p. 586. [Google Scholar]
- Li, H.; Tang, C.; Xu, Z. The effects of different light qualities on rapeseed (Brassica napus L.) plantlet growth and morphogenesis in vitro. Sci. Hortic. 2013, 150, 117–124. [Google Scholar] [CrossRef]
- Tanaka, M.; Takamura, T.; Watanabe, H.; Endo, M.; Yanagi, T.; Okamoto, K. In vitro growth of Cymbidium plantlets cultured under super bright red and blue light-emitting diodes (LEDs). J. Hort. Sci. Biotechnol. 1998, 73, 39–44. [Google Scholar] [CrossRef]
- Nhut, D.T.; Don, N.T.; Tanaka, M. Light-emitting diodes as an effective lighting source for in vitro banana culture. In Protocols for Micropropagation of Woody Trees and Fruits; Springer: Dordrecht, The Netherlands, 2007; pp. 527–541. [Google Scholar]
- Shin, K.S.; Murthy, H.N.; Heo, J.W.; Hahn, E.J.; Paek, K.Y. The effect of light quality on the growth and development of in vitro cultured Doritaenopsis plants. Acta Physiol. Plant. 2008, 30, 339–343. [Google Scholar] [CrossRef]
- Goins, G.D.; Yorio, N.C.; Sanwo, M.M.; Brown, C.S. Photomorphogenesis, photosynthesis, and seed yield of wheat plants grown under red light-emitting diodes (LEDs) with and without supplemental blue lighting. J. Exp. Bot. 1997, 48, 1407–1413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoenecke, M.; Bula, R.; Tibbitts, T. Importance of ‘Blue’ Photon Levels for Lettuce Seedlings Grown under Red-light-emitting Diodes. HortScience 1992, 27, 427–430. [Google Scholar] [CrossRef] [Green Version]
- Tripathy, B.C.; Brown, C.S. Root-Shoot Interaction in the Greening of Wheat Seedlings Grown under Red Light. Plant Physiol. 1995, 107, 407–411. [Google Scholar] [CrossRef] [Green Version]
- Nascimento Vieira, L.; de Freitas Fraga, H.P.; dos Anjos, K.G.; Puttkammer, C.C.; Scherer, R.F.; da Silva, D.A.; Guerra, M.P. Light-emitting diodes (LED) increase the stomata formation and chlorophyll content in Musa acuminata (AAA) ‘Nanicão Corupá’ in vitro plantlets. Theor. Experim. Plant Physiol. 2015, 27, 91–98. [Google Scholar] [CrossRef]
- Rosa, M.; Neto, A.R.; Marques, V.D.O.; Silva, F.G.; De Assis, E.S.; Costa, A.C.; Dantas, L.A.; Pereira, P.S. Variations in photon flux density alter the morphophysiological and chemical characteristics of Anacardium othonianum Rizz. in vitro. Plant Cell Tissue Organ Cult. 2019, 140, 523–537. [Google Scholar] [CrossRef]
- Nhut, D.T.; Takamura, T.; Watanabe, H.; Okamoto, K.; Tanaka, M. Responses of strawberry plantlets cultured in vitro under superbright red and blue light-emitting diodes (LEDs). Plant Cell Tissue Organ Cult. 2003, 73, 43–52. [Google Scholar] [CrossRef]
- Costa, F.D.S.; de Castro, E.M.; Pasqual, M.; Pereira, J.E.S.; Oliveira, C.D. Alterações anatômicas de bananeiras micropropagadas em resposta a aclimatização ex vitro. Ciência Rural. 2009, 39, 386–392. [Google Scholar] [CrossRef]
- Schuerger, A.C.; Brown, C.S.; Stryjewski, E.C. Anatomical Features of Pepper Plants (Capsicum annuum L.) Grown under Red Light-emitting Diodes Supplemented with Blue or Far-red Light. Ann. Bot. 1997, 79, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Neto, A.R.; Chagas, E.A.; Costa, B.N.S.; Chagas, P.C.; Vendrame, W.A. Photomixotrophic growth response of sugarcane plantlets with increased light intensity and design of culture vessel. Vitr. Cell Dev. Biol. Plant 2020, 56, 504–514. [Google Scholar] [CrossRef]
| Banana Variety | Light Source | Stem Diameter * (mm) | Plant Fresh Weight (g) | Shoot Fresh Weight (g) | Root Fresh Weight (g) | Number of Leaves | Shoot Length (cm) | Root Length (cm) | Number of Roots | Shoot Dry Weight (g) | Root Dry Weight (g) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Little Prince | LED-1 | 5.32 ± 0.50 a | 2.84 ± 0.14 a | 1.63 ± 0.25 a | 0.19 ± 0.08 a | 3.6 ± 0.89 a | 7.45 ± 0.67 a | 3.02 ± 0.30 a | 6.4 ± 2.07 a | 0.24 ± 0.03 a | 0.01 ± 0.01 a |
| LED-2 | 5.24 ± 0.44 a | 2.62 ± 0.42 ab | 1.94 ± 0.54 a | 0.34 ± 0.09 a | 4.0 ± 1.00 a | 7.33 ± 0.76 a | 3.70 ± 0.82 a | 8.2 ± 2.17 a | 0.28 ± 0.07 a | 0.03 ± 0.01 a | |
| FL | 5.08 ± 0.71 a | 2.21 ± 0.39 c | 1.44 ± 0.05 a | 0.16 ± 0.07 a | 4.2 ± 0.84 a | 5.87 ± 0.82 b | 3.21 ± 0.25 a | 7.8 ± 3.19 a | 0.24 ± 0.02 a | 0.02 ± 0.01 a | |
| Truly Tiny | LED-1 | 4.28 ± 0.55 a | 2.31 ± 0.16 bc | 1.27 ± 0.19 a | 0.25 ± 0.10 a | 3.6 ± 0.89 a | 6.67 ± 0.30 ab | 4.21 ± 0.34 a | 5.6 ± 1.52 a | 0.18 ± 0.03 a | 0.02 ± 0.01 a |
| LED-2 | 3.94 ± 0.37 a | 1.79 ±0.21 d | 1.01 ± 0.27 a | 0.27 ± 0.07 a | 3.6 ± 1.14 a | 6.46 ± 0.67 b | 4.38 ± 0.92 a | 5.2 ± 1.79 a | 0.12 ± 0.03 a | 0.02 ± 0.01 a | |
| FL | 4.36 ± 0.92 a | 1.91 ± 0.38 cd | 1.39 ± 0.62 a | 0.34 ± 0.16 a | 4.0 ± 1.00 a | 6.02 ± 0.47 b | 4.60 ± 0.36 a | 6.0 ± 2.12 a | 0.21 ± 0.10 a | 0.02 ± 0.01 a |
| Banana Variety | Light Source | AdSN * (per mm2) | AbSN (per mm2) | AdSL (µm) | AbSL (µm) | AdSW (µm) | AbSW (µm) |
|---|---|---|---|---|---|---|---|
| Little Prince | LED-1 | 22.8 ± 7.40 b | 75.4 ± 14.08 cd | 25.18 ± 2.57 b | 21.41 ± 3.19 c | 10.13 ± 0.94 c | 10.35 ± 1.25 a |
| LED-2 | 28.4 ± 7.67 ab | 67.6 ± 12.03 d | 25.63 ± 1.11 b | 27.08 ± 2.47 ab | 11.13 ± 0.91 bc | 11.35 ± 1.08 a | |
| FL | 30.0 ± 6.20 ab | 83.6 ± 11.35 bcd | 28.51 ± 3.08 a | 27.16 ± 1.15 ab | 12.87 ± 0.97 a | 11.47 ± 1.58 a | |
| Truly Tiny | LED-1 | 24.4 ± 2.51 b | 92.0 ± 5.74 abc | 25.00 ± 1.89 b | 24.81 ± 1.65 b | 10.16 ± 0.61 b | 10.11 ± 1.72 a |
| LED-2 | 25.8 ± 4.66 ab | 94.2 ± 4.32 ab | 27.72 ± 2.19 ab | 27.68 ± 2.46 a | 10.00 ± 1.79 c | 11.05 ± 0.68 a | |
| FL | 32.0 ± 3.54 a | 101.6 ± 21.55 a | 28.45 ± 0.98 a | 25.64 ± 1.44 ab | 12.02 ± 1.20 ab | 9.97 ± 1.18 a |
| Banana Variety | Light Source | AbE (µm) | AdE (µm) | AbH (µm) | AdH (µm) | PP (µm) | SP (µm) |
|---|---|---|---|---|---|---|---|
| Little Prince | LED-1 | 15.53 ± 4.89 b | 17.63 ± 5.89 a | 59.02 ± 14.25 ab | 59.17 ± 5.69 c | 35.87 ± 9.23 a | 38.91 ± 9.38 ab |
| LED-2 | 17.70 ± 2.79 b | 16.40 ± 5.92 a | 52.38 ± 39.51 b | 69.31 ± 13.52 abc | 39.63 ± 10.75 a | 41.12 ± 10.29 b | |
| FL | 20.66 ± 6.31 ab | 20.55 ± 5.28 a | 59.82 ± 4.46 ab | 64.33 ± 7.75 bc | 42.71 ± 11.53 a | 38.40 ± 11.25 ab | |
| Truly Tiny | LED-1 | 19.68 ± 7.05 ab | 15.07 ± 2.39 a | 61.61 ± 7.71 ab | 82.90 ± 14.51 a | 43.71 ± 11.57 a | 42.10 ± 8.66 ab |
| LED-2 | 17.73 ± 5.80 b | 15.08 ± 4.37 a | 69.01 ± 18.48 ab | 78.79 ± 20.14 ab | 35.39 ± 7.94 a | 33.88 ± 9.39 ab | |
| FL | 25.10 ± 5.54 a | 18.15 ± 3.82 a | 62.76 ± 14.47 a | 69.96 ± 15.37 abc | 47.95 ± 13.36 a | 52.33 ± 17.81 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vendrame, W.A.; Feuille, C.; Beleski, D.; Bueno, P.M.C. In Vitro Growth Responses of Ornamental Bananas (Musa sp.) as Affected by Light Sources. Horticulturae 2022, 8, 92. https://doi.org/10.3390/horticulturae8020092
Vendrame WA, Feuille C, Beleski D, Bueno PMC. In Vitro Growth Responses of Ornamental Bananas (Musa sp.) as Affected by Light Sources. Horticulturae. 2022; 8(2):92. https://doi.org/10.3390/horticulturae8020092
Chicago/Turabian StyleVendrame, Wagner A., Cassandre Feuille, David Beleski, and Paulo Mauricio Centenaro Bueno. 2022. "In Vitro Growth Responses of Ornamental Bananas (Musa sp.) as Affected by Light Sources" Horticulturae 8, no. 2: 92. https://doi.org/10.3390/horticulturae8020092
APA StyleVendrame, W. A., Feuille, C., Beleski, D., & Bueno, P. M. C. (2022). In Vitro Growth Responses of Ornamental Bananas (Musa sp.) as Affected by Light Sources. Horticulturae, 8(2), 92. https://doi.org/10.3390/horticulturae8020092








