Effects of Different Treatments on Physicochemical Characteristics of ‘Kyoho’ Grapes during Storage at Low Temperature
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Treatments
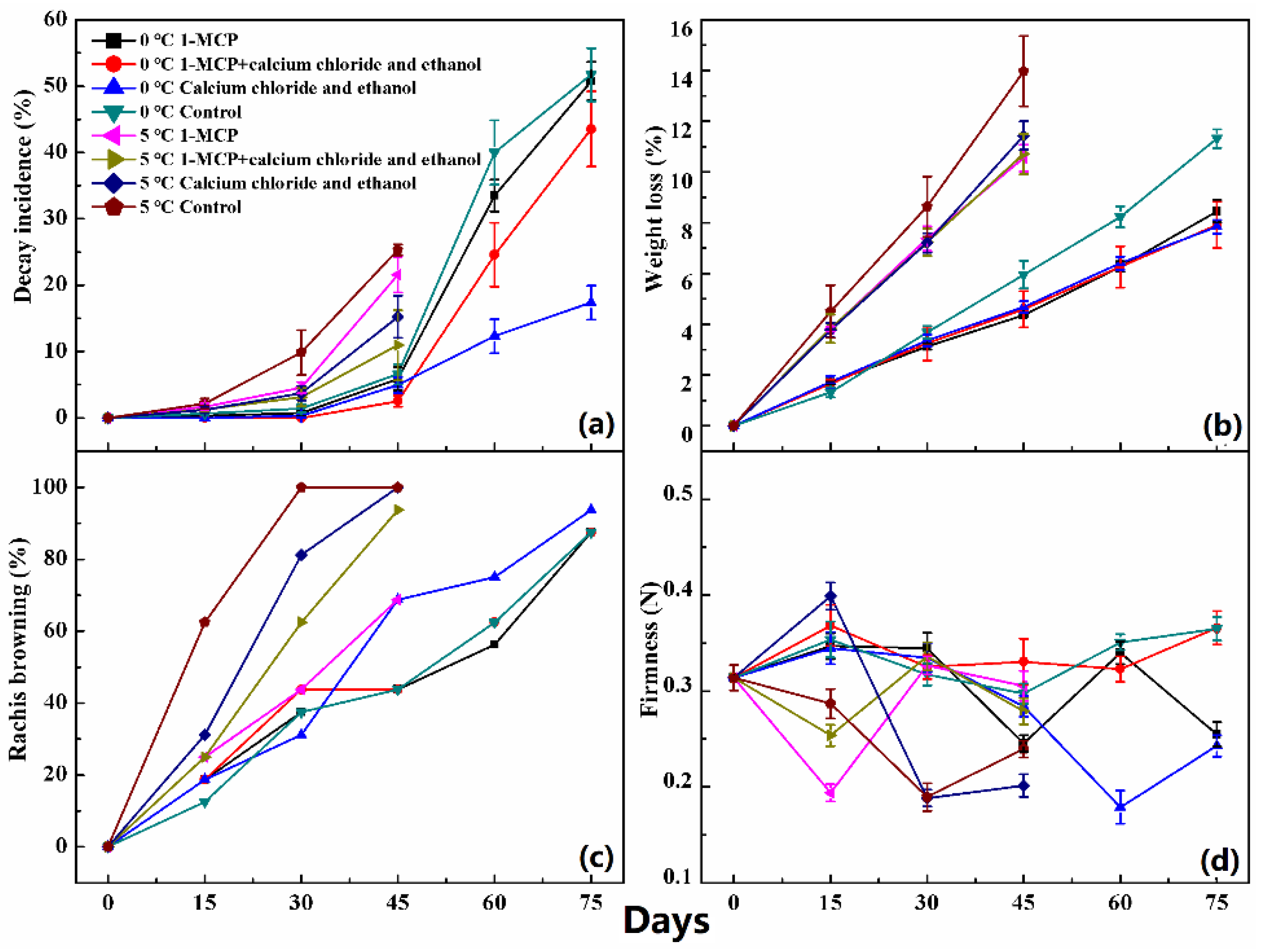
2.2. Measurement of Decay Incidence, Weight Loss, Rachis Browning and Firmness
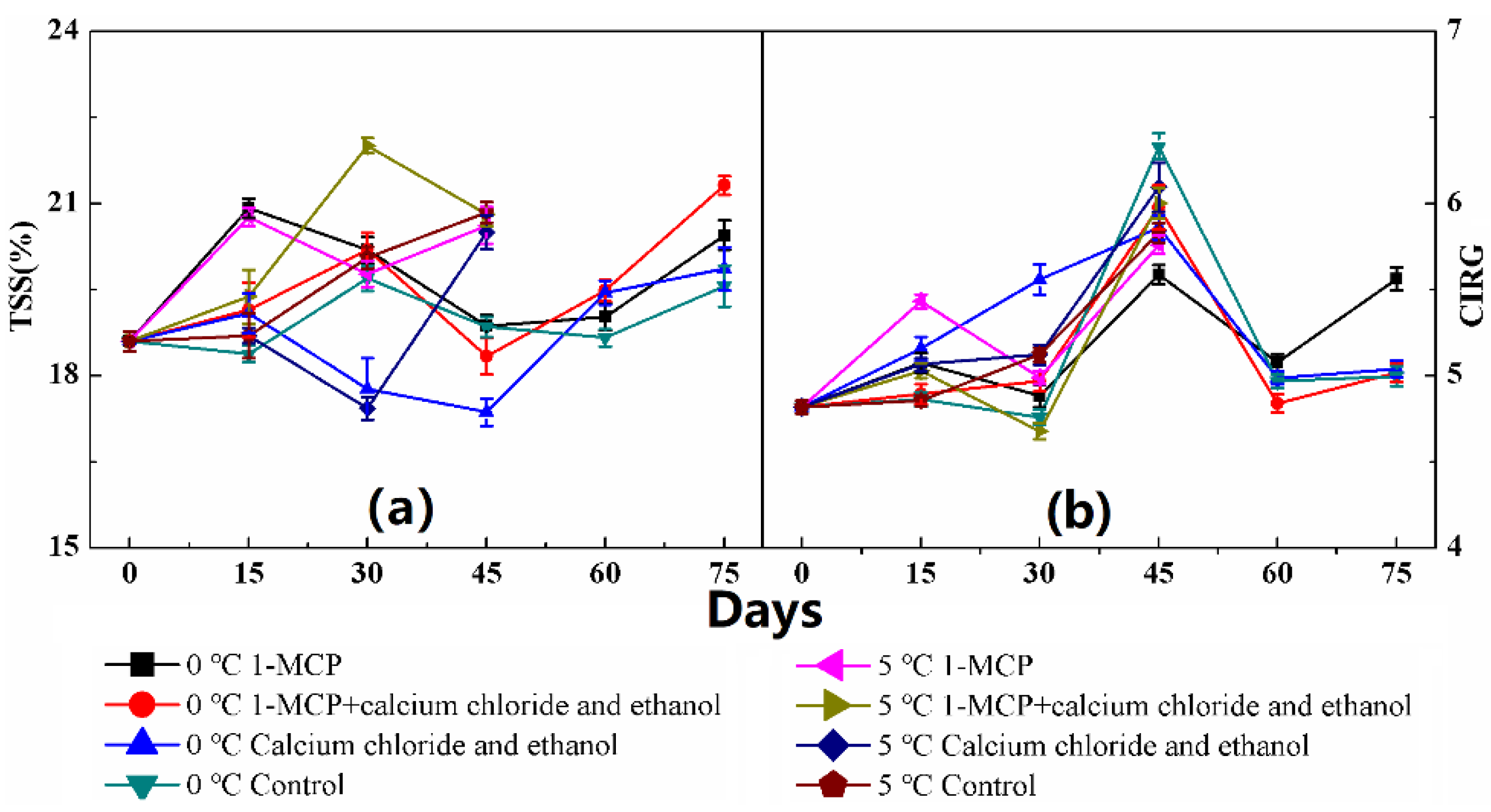
2.3. Color and Total Soluble Solids (TSS) Analysis
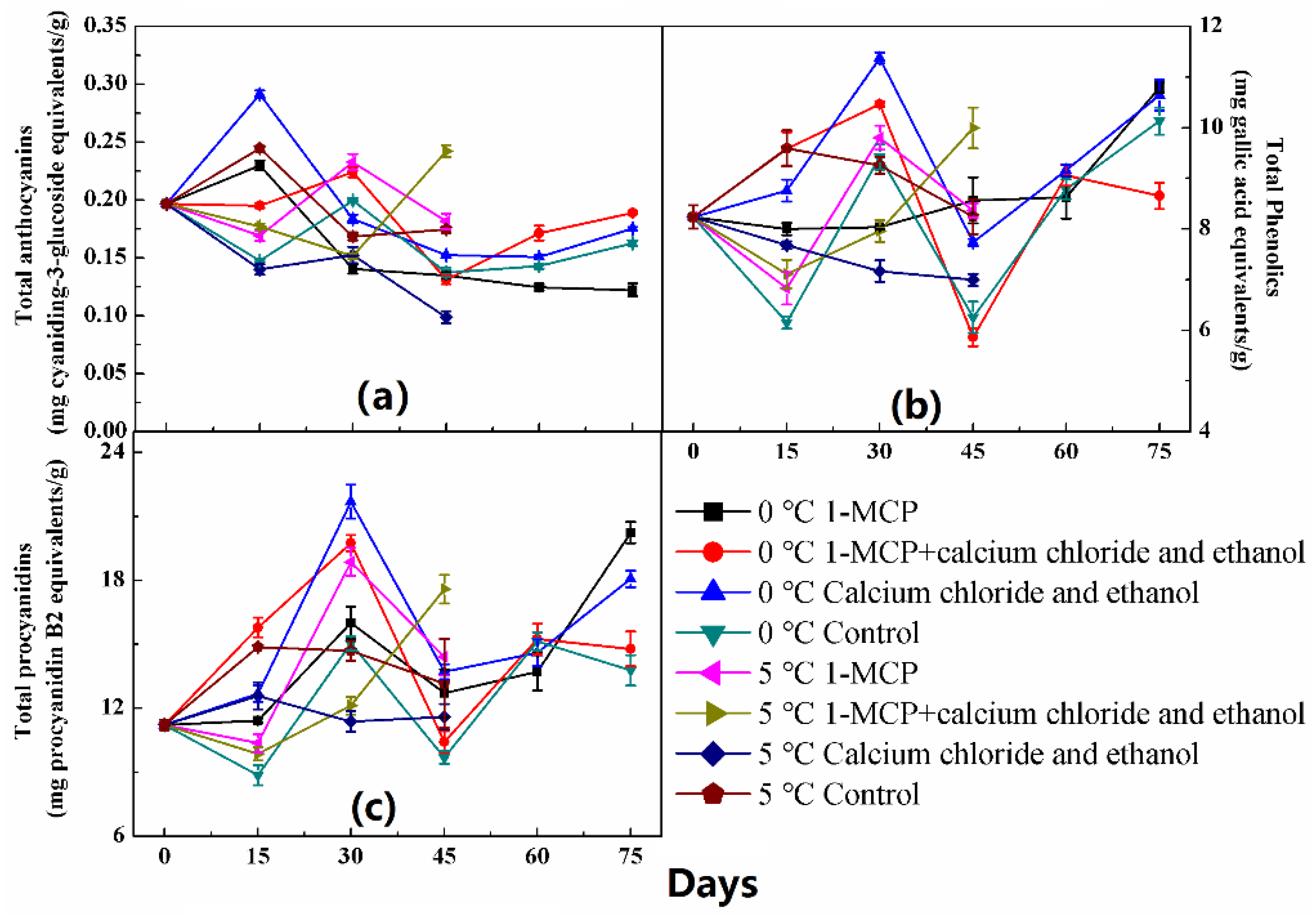
2.4. Total Phenolics, Total Anthocyanins and Total Procyanidins Analysis
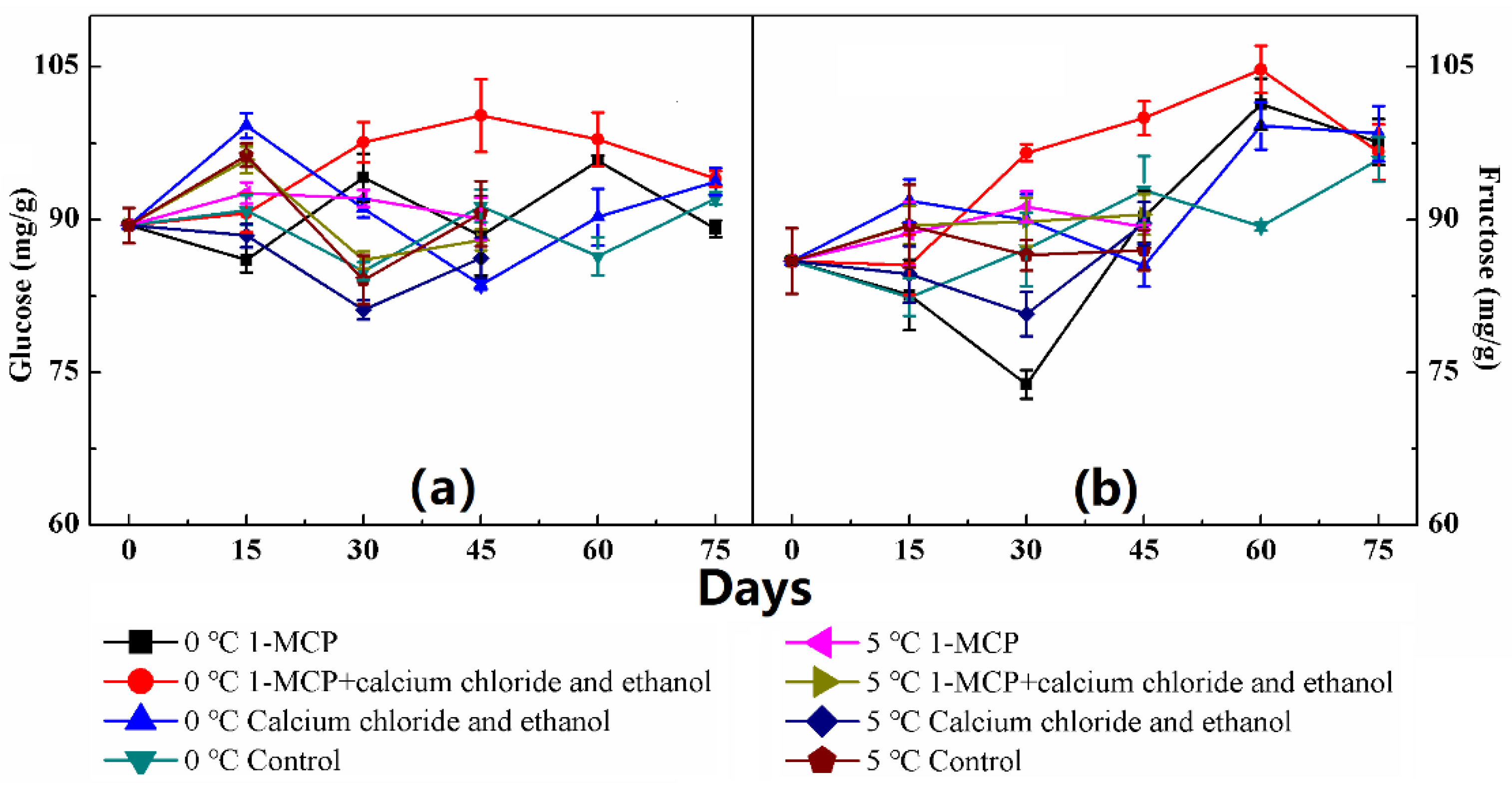
2.5. Quantification of Individual Sugars
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jin, Z.X.; Sun, T.Y.; Sun, H.; Yue, Q.Y.; Yao, Y.X. Modifications of ‘Summer Black’ grape berry quality as affected by the different rootstocks. Sci. Hortic. 2016, 210, 130–137. [Google Scholar] [CrossRef]
- Ciccarese, A.; Stellacci, A.M.; Gentilesco, G.; Rubino, P. Effectiveness of pre- and post-veraison calcium applications to control decay and maintain table grape fruit quality during storage. Postharvest Biol. Technol. 2013, 75, 135–141. [Google Scholar] [CrossRef]
- Meng, X.H.; Li, B.Q.; Liu, J.; Tian, S.P. Physiological responses and quality attributes of table grape fruit to chitosan preharvest spray and postharvest coating during storage. Food Chem. 2008, 106, 501–508. [Google Scholar] [CrossRef]
- Ngcobo, M.E.K.; Delele, M.A.; Pathare, P.B.; Chen, L.; Opara, U.L.; Meyer, C.J. Moisture loss characteristics of fresh table grapes packed in different film liners during cold storage. Biosyst. Eng. 2012, 113, 363–370. [Google Scholar] [CrossRef]
- Balic, I.; Moreno, A.; Sanhueza, D.; Huerta, C.; Orellana, A.; Defilippi, B.G.; Campos-Vargas, R. Molecular and physiological study of postharvest rachis browning of table grape cv Red Globe. Postharvest Biol. Technol. 2012, 72, 47–56. [Google Scholar] [CrossRef]
- Lado, J.; Gurrea, A.; Zacarias, L.; Rodrigo, M.J. Influence of the storage temperature on volatile emission, carotenoid content and chilling injury development in Star Ruby red grapefruit. Food Chem. 2019, 295, 72–81. [Google Scholar] [CrossRef]
- Yuan, X.Z.; Wu, Z.M.; Li, H.D.; Wang, Y.; Liu, F.; Cai, H.; Newlove, A.A.; Wang, Y. Biochemical and proteomic analysis of ‘Kyoho’ grape (Vitis labruscana) berries during cold storage. Postharvest Biol. Technol. 2014, 88, 79–87. [Google Scholar] [CrossRef]
- Chen, S.J.; Wang, H.O.; Wang, R.R.; Fu, Q.Q.; Zhang, W. Effect of gaseous chlorine dioxide (ClO2) with different concentrations and numbers of treatments on controlling berry decay and rachis browning of table grape. J. Food Processing Preserv. 2018, 42, e13662. [Google Scholar] [CrossRef]
- Rosales, R.; Fernandez-Caballero, C.; Romero, I.; Escribano, M.I.; Merodio, C.; Sanchez-Ballesta, M.T. Molecular analysis of the improvement in rachis quality by high CO2 levels in table grapes stored at low temperature. Postharvest Biol. Technol. 2013, 77, 50–58. [Google Scholar] [CrossRef]
- Sabir, F.K.; Sabir, A. Extending Postharvest Quality Attributesof Grapes (V. vinifera L. cv. ‘Thompson seedless’) by Preharvest Calcium Pulverizations. Acta Sci. Pol. Hortorum Cultus 2017, 16, 29–38. [Google Scholar] [CrossRef]
- Sabir, A.; Sabir, F.K.; Kara, Z. Effects of modified atmosphere packing and honey dip treatments on quality maintenance of minimally processed grape cv. Razaki (V. vinifera L.) during cold storage. J. Food Sci. Technol. 2011, 48, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Luo, Z.S.; Li, J.H.; Yang, M.Y.; Yan, J.W.; Lu, H.Y.; Li, D.; Chen, C.K.; Aghdam, M.S.; Wu, B.; et al. Morphological and quality characterization of grape berry and rachis in response to postharvest 1-methylcyclopropene and elevated oxygen and carbon dioxide atmospheres. Postharvest Biol. Technol. 2019, 153, 107–117. [Google Scholar] [CrossRef]
- Zhu, X.Y.; Song, Z.Y.; Li, Q.M.; Li, J.; Chen, W.X.; Li, X.P. Physiological and transcriptomic analysis reveals the roles of 1-MCP in the ripening and fruit aroma quality of banana fruit (Fenjiao). Food Res. Int. 2020, 130, 108968. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.X.; Sun, D.W.; Pu, H.B.; Wei, Q.Y. Recent advances in detecting and regulating ethylene concentrations for shelf-life extension and maturity control of fruit: A review. Trends Food Sci. Technol. 2019, 91, 66–82. [Google Scholar] [CrossRef]
- Li, L.; Lichter, A.; Chalupowic, D.; Gamrasni, D.; Goldberg, T.; Nerya, O.; Ben-Arie, R.; Porat, R. Effects of the ethylene-action inhibitor 1-methylcyclopropene on postharvest quality of non-climacteric fruit crops. Postharvest Biol. Technol. 2016, 111, 322–329. [Google Scholar] [CrossRef]
- Xu, F.X.; Liu, S.Y. Control of Postharvest Quality in Blueberry Fruit by Combined 1-Methylcyclopropene (1-MCP) and UV-C Irradiation. Food Bioprocess Technol. 2017, 10, 1695–1703. [Google Scholar] [CrossRef]
- Song, C.C.; Li, A.; Chai, Y.F.; Li, Q.P.; Lin, Q.; Duan, Y.Q. Effects of 1-Methylcyclopropene Combined with Modified Atmosphere on Quality of Fig (Ficus carica L.) during Postharvest Storage. J. Food Qual. 2019, 4, 1–9. [Google Scholar] [CrossRef]
- Lv, J.Y.; Zhang, M.Y.; Bai, L.; Han, X.Z.; Ge, Y.H.; Wang, W.H.; Li, J.R. Effects of 1-methylcyclopropene (1-MCP) on the expression of genes involved in the chlorophyll degradation pathway of apple fruit during storage. Food Chem. 2020, 308, 125707. [Google Scholar] [CrossRef]
- Kou, J.J.; Wei, C.Q.; Zhao, Z.H.; Guan, J.F.; Wang, W.J. Effects of ethylene and 1-methylcyclopropene treatments on physiological changes and ripening-related gene expression of ‘Mopan’ persimmon fruit during storage. Postharvest Biol. Technol. 2020, 166, 111185. [Google Scholar] [CrossRef]
- Wang, K.T.; Jin, P.; Tang, S.S.; Shang, H.T.; Rui, H.J.; Di, H.T.; Cai, Y.; Zheng, Y.H. Improved control of postharvest decay in Chinese bayberries by a combination treatment of ethanol vapor with hot air. Food Control. 2011, 22, 82–87. [Google Scholar] [CrossRef]
- Romanazzi, G.; Karabulut, O.A.; Smilanick, J.L. Combination of chitosan and ethanol to control postharvest gray mold of table grapes. Postharvest Biol. Technol. 2007, 45, 134–140. [Google Scholar] [CrossRef]
- Liu, H.R.; Meng, F.L.; Chen, S.S.; Yin, T.T.; Hu, S.S.; Shao, Z.Y.; Liu, Y.Y.; Zhu, C.Q.; Ye, H.X.; Wang, Q.M. Ethanol treatment improves the sensory quality of cherry tomatoes stored at room temperature. Food Chem. 2019, 298, 125069. [Google Scholar] [CrossRef] [PubMed]
- Chervin, C.; Westercamp, P.; Monteils, G. Ethanol vapours limit Botrytis development over the postharvest life of table grapes. Postharvest Biol. Technol. 2005, 36, 319–322. [Google Scholar] [CrossRef]
- Martins, V.; Garcia, A.; Costa, C.; Sottomayor, M.; Geros, H. Calcium- and hormone-driven regulation of secondary metabolism and cell wall enzymes in grape berry cells. J. Plant Physiol. 2018, 231, 57–67. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C. Effect of calcium chloride in combination with salicylic acid on post-harvest freshness of apples. Food Sci. Biotechnol. 2015, 24, 1139–1146. [Google Scholar] [CrossRef]
- Nigro, F.; Schena, L.; Ligorio, A.; Pentimone, I.; Ippolito, A.; Salerno, M.G. Control of table grape storage rots by pre-harvest applications of salts. Postharvest Biol. Technol. 2006, 42, 142–149. [Google Scholar] [CrossRef]
- Deng, Y.; Wu, Y.; Li, Y.F. Changes in firmness, cell wall composition and cell wall hydrolases of grapes stored in high oxygen atmospheres. Food Res. Int. 2005, 38, 769–776. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Garner, D.; Crisosto, G. Carbon dioxide-enriched atmospheres during cold storage limit losses from Botrytis but accelerate rachis browning of ‘Redglobe’ table grapes. Postharvest Biol. Technol. 2002, 26, 181–189. [Google Scholar] [CrossRef]
- Leng, F.; Lin, Q.; Wu, D.; Wang, S.P.; Wang, D.L.; Sun, C.D. Comparative Transcriptomic Analysis of Grape Berry in Response to Root Restriction during Developmental Stages. Molecules 2016, 21, 1431. [Google Scholar] [CrossRef]
- Cao, J.P.; Jiang, Q.; Lin, J.Y.; Li, X.; Sun, C.D.; Chen, K.S. Physicochemical characterisation of four cherry species (Prunus spp.) grown in China. Food Chem. 2015, 173, 855–863. [Google Scholar] [CrossRef]
- Broeckling, C.D.; Huhman, D.V.; Farag, M.A.; Smith, J.T.; May, G.D.; Mendes, P.; Dixon, R.A.; Sumner, L.W. Metabolic profiling of Medicago truncatula cell cultures reveals the effects of biotic and abiotic elicitors on metabolism. J. Exp. Bot. 2005, 56, 323–336. [Google Scholar] [CrossRef]
- Ejsmentewicz, T.; Balic, I.; Sanhueza, D.; Barria, R.; Meneses, C.; Orellana, A.; Prieto, H.; Defilippi, B.G.; Campos-Vargas, R. Comparative Study of Two Table Grape Varieties with Contrasting Texture during Cold Storage. Molecules 2015, 20, 3667–3680. [Google Scholar] [CrossRef]
- Jiang, L.L.; Jin, P.; Wang, L.; Yu, X.; Wang, H.Y.; Zheng, Y.H. Methyl jasmonate primes defense responses against Botrytis cinerea and reduces disease development in harvested table grapes. Sci. Hortic. 2015, 192, 218–223. [Google Scholar] [CrossRef]
- Blankenship, S.M.; Dole, J.M. 1-methylcyclopropene: A review. Postharvest Biol. Technol. 2003, 28, 1–25. [Google Scholar] [CrossRef]
- Deng, Y.; Wu, Y.; Li, Y.F. Effects of high CO2 and low O2 atmospheres on the berry drop of ‘Kyoho’ grapes. Food Chem. 2007, 100, 768–773. [Google Scholar] [CrossRef]
- Nguyen, V.B.; Nguyen, D.H.H.; Nguyen, H.H. Combination effects of calcium chloride and nano-chitosan on the postharvest quality of strawberry (Fragaria x ananassa Duch.). Postharvest Biol. Technol. 2020, 162, 111103. [Google Scholar] [CrossRef]
- Lurie, S.; Pesis, E.; Gadiyeva, O.; Feygenberg, O.; Ben-Arie, R.; Kaplunov, T.; Zutahy, Y.; Lichter, A. Modified ethanol atmosphere to control decay of table grapes during storage. Postharvest Biol. Technol. 2006, 42, 222–227. [Google Scholar] [CrossRef]
- Chervin, C.; Lavigne, D.; Westercamp, P. Reduction of gray mold development in table grapes by preharvest sprays with ethanol and calcium chloride. Postharvest Biol. Technol. 2009, 54, 115–117. [Google Scholar] [CrossRef][Green Version]
- Sogvar, O.B.; Saba, M.K.; Emamifar, A. Aloe vera and ascorbic acid coatings maintain postharvest quality and reduce microbial load of strawberry fruit. Postharvest Biol. Technol. 2016, 114, 29–35. [Google Scholar] [CrossRef]
- Wang, D.D.; Yeats, T.H.; Uluisik, S.; Rose, J.K.C.; Seymour, G.B. Fruit Softening: Revisiting the Role of Pectin. Trends Plant Sci. 2018, 23, 302–310. [Google Scholar] [CrossRef]
- Cao, J.P.; Kang, C.; Chen, Y.Y.; Karim, N.; Wang, Y.; Sun, C.D. Physiochemical changes in Citrus reticulata cv. Shatangju fruit during vesicle collapse. Postharvest Biol. Technol. 2020, 165, 111180. [Google Scholar] [CrossRef]
- Deluc, L.G.; Grimplet, J.; Wheatley, M.D.; Tillett, R.L.; Quilici, D.R.; Osborne, C.; Schooley, D.A.; Schlauch, K.A.; Cushman, J.C.; Cramer, G.R. Transcriptomic and metabolite analyses of Cabernet Sauvignon grape berry development. BMC Genom. 2007, 8, 429. [Google Scholar] [CrossRef] [PubMed]
- Leng, F.; Cao, J.; Ge, Z.; Wang, Y.; Zhao, C.; Wang, S.; Li, X.; Zhang, Y.; Sun, C. Transcriptomic Analysis of Root Restriction Effects on Phenolic Metabolites during Grape Berry Development and Ripening. J. Agric. Food Chem. 2020, 68, 9090–9099. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leng, F.; Wang, C.; Sun, L.; Li, P.; Cao, J.; Wang, Y.; Zhang, C.; Sun, C. Effects of Different Treatments on Physicochemical Characteristics of ‘Kyoho’ Grapes during Storage at Low Temperature. Horticulturae 2022, 8, 94. https://doi.org/10.3390/horticulturae8020094
Leng F, Wang C, Sun L, Li P, Cao J, Wang Y, Zhang C, Sun C. Effects of Different Treatments on Physicochemical Characteristics of ‘Kyoho’ Grapes during Storage at Low Temperature. Horticulturae. 2022; 8(2):94. https://doi.org/10.3390/horticulturae8020094
Chicago/Turabian StyleLeng, Feng, Chengyang Wang, Liping Sun, Pei Li, Jinping Cao, Yue Wang, Changfeng Zhang, and Chongde Sun. 2022. "Effects of Different Treatments on Physicochemical Characteristics of ‘Kyoho’ Grapes during Storage at Low Temperature" Horticulturae 8, no. 2: 94. https://doi.org/10.3390/horticulturae8020094
APA StyleLeng, F., Wang, C., Sun, L., Li, P., Cao, J., Wang, Y., Zhang, C., & Sun, C. (2022). Effects of Different Treatments on Physicochemical Characteristics of ‘Kyoho’ Grapes during Storage at Low Temperature. Horticulturae, 8(2), 94. https://doi.org/10.3390/horticulturae8020094






