Effects of Integrated and Organic Management on Strawberry (cv. Camarosa) Plant Growth, Nutrition, Fruit Yield, Quality, Nutraceutical Characteristics, and Soil Fertility Status
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Treatments and Cultivation Practices
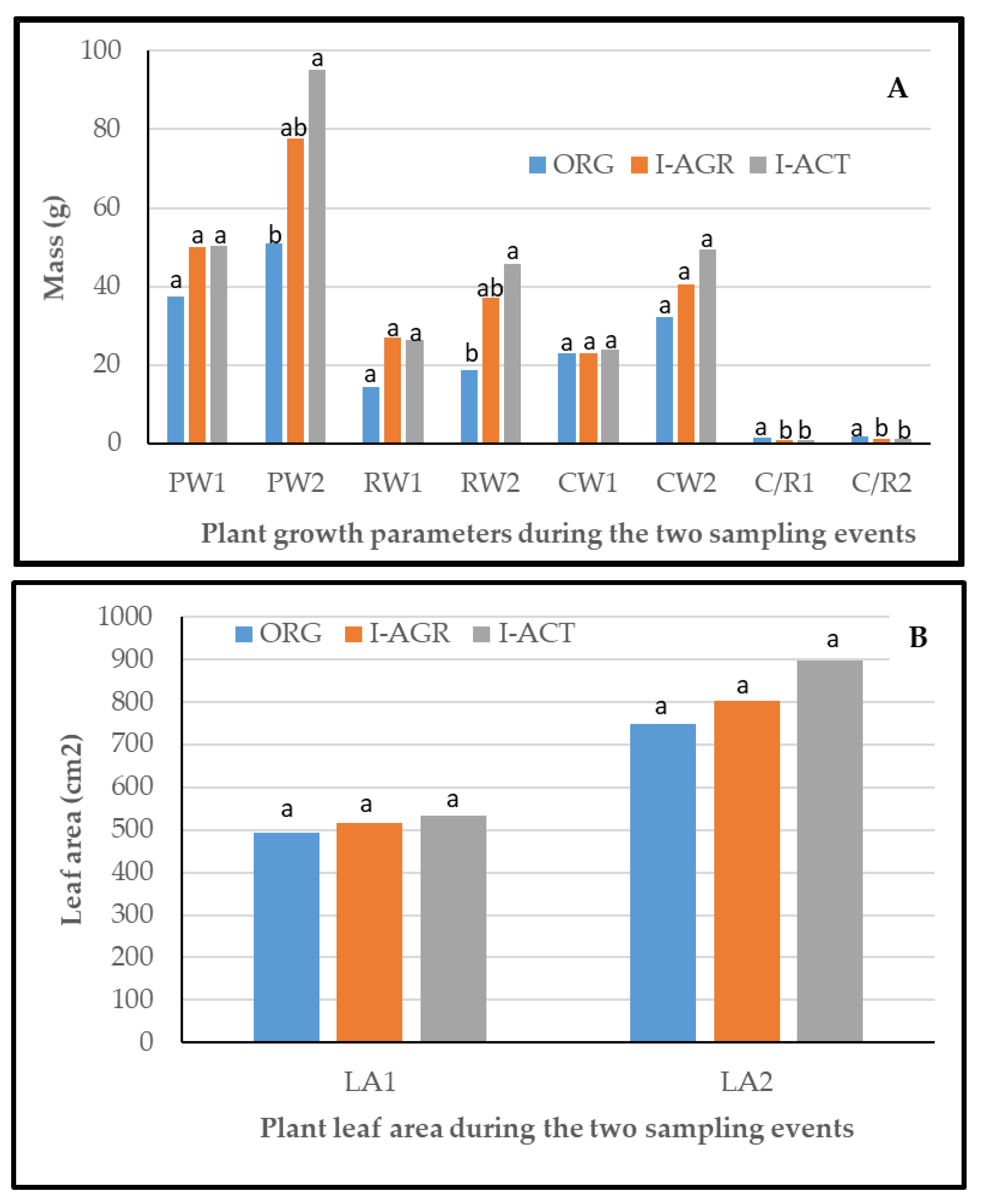
2.3. Samplings and Plant Weight Parameters
2.4. Evaluation of Fruit Physiological Attributes
2.5. Determination of Fruit Organoleptic Characteristics
2.6. Total Phenol Content, Total Anthocyanin Content, Individual Anthocyanin, and Antioxidant Capacity Determination
2.7. Carbohydrate and Organic Acid Determination
2.8. Soil and Plant Analysis
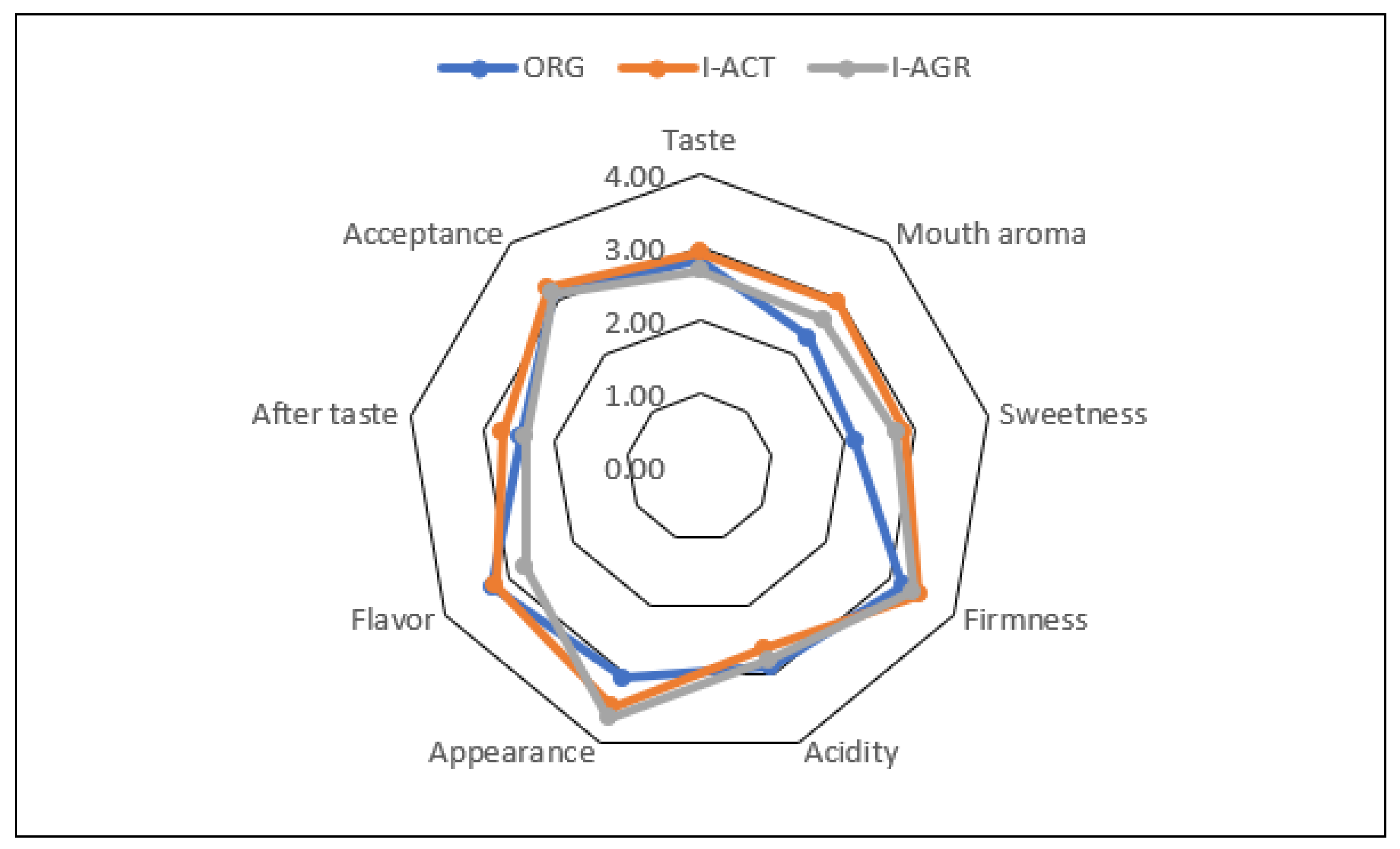
2.9. Taste Panel
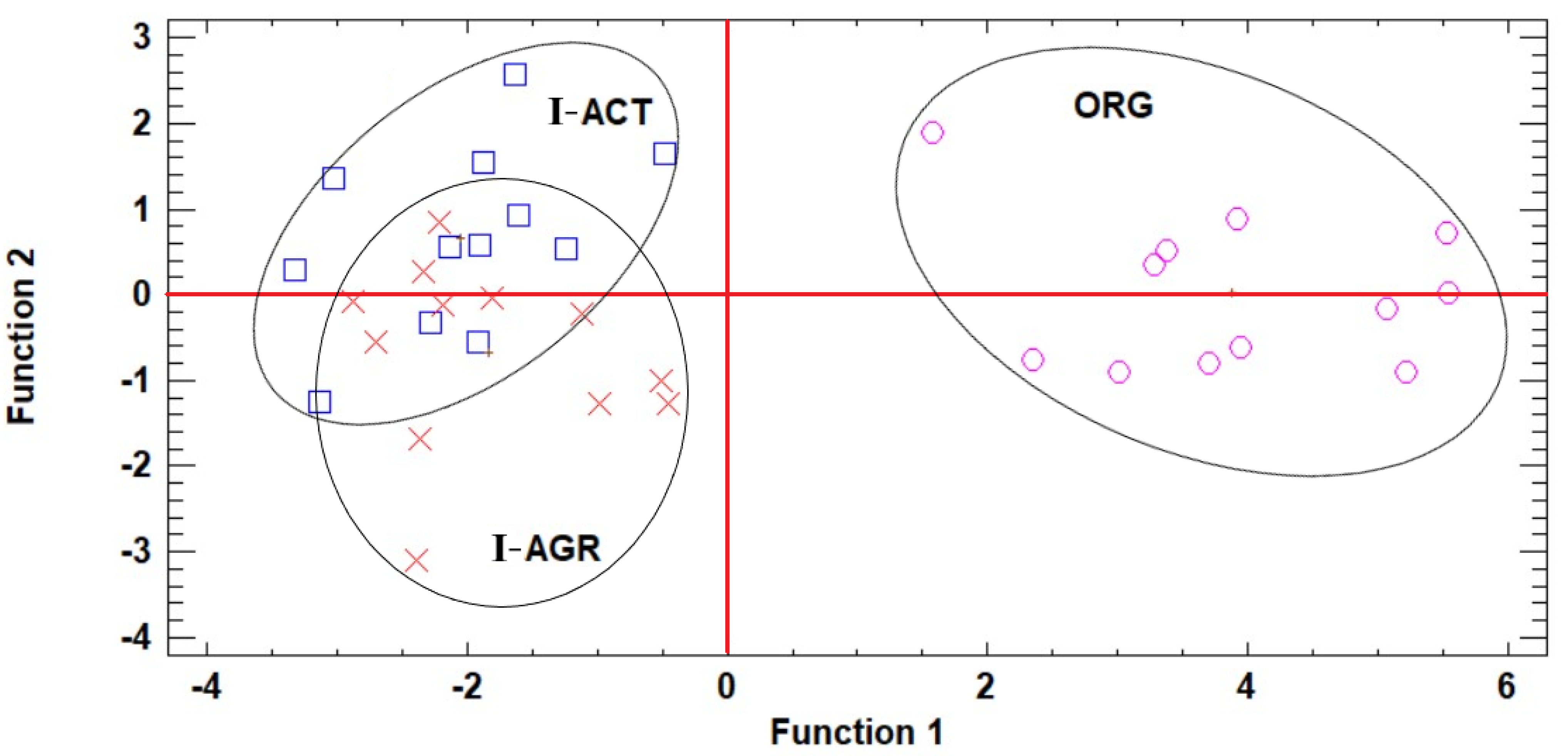
2.10. Trial Design and Statistical Analysis
3. Results
3.1. Effects of Cultivation Management on Yield and Fruit Physicochemical and Phytochemical Properties
3.2. Effects of Cultivation Management on Plant Growth and Nutrient Status
3.3. Effects of Cultivation Management on Soil Properties and Soil Nutrient Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- D’Urso, G.; d’Aquino, L.; Pizza, C.; Montoro, P. Integrated mass spectrometric and multivariate data analysis approaches for the discrimination of organic and conventional strawberry (Fragaria ananassa Duch.) crops. Food Res. Int. 2015, 77, 264–272. [Google Scholar] [CrossRef]
- Fernandes, V.C.; Domingues, V.F.; de Freitas, V.; Delerue-Matos, C.; Mateus, N. Strawberries from integrated pest management and organic farming: Phenolic composition and antioxidant properties. Food Chem. 2012, 134, 1926–1931. [Google Scholar] [CrossRef] [PubMed]
- Domingues, A.R.; Vidal, T.C.M.; Hata, F.T.; Ventura, M.U.; Gonçalves, L.S.A.; Silva, J.B. Postharvest quality, antioxidant activity and acceptability of strawberries grown in conventional and organic systems. Braz. J. Food Technol. 2018, 21, e2017154. [Google Scholar] [CrossRef]
- Conti, S.; Villari, G.; Faugno, S.; Melchionna, G.; Somma, S.; Caruso, G. Effects of organic vs. conventional farming system on yield and quality of strawberry grown as an annual or biennial crop in southern Italy. Sci. Hortic. 2014, 180, 63–71. [Google Scholar] [CrossRef]
- Crecente-Campo, J.; Nunes-Damaceno, M.; Romero-Rodríguez, M.A.; Vázquez-Odériz, M.L. Color, anthocyanin pigment, ascorbic acid and total phenolic compound determination in organic versus conventional strawberries (Fragaria × ananassa Duch, cv Selva). J. Food Compos. Anal. 2012, 28, 23–30. [Google Scholar] [CrossRef]
- Abu-Zahra, T.R.; Al-Ismail, K.; Shatat, F. Effect of organic and conventional systems on fruit quality of strawberry (Fragaria × ananassa duch) grown under plastic house conditions in the jordan valley. Acta Hortic. 2007, 741, 159–171. [Google Scholar] [CrossRef]
- Asami, D.K.; Hong, Y.-J.; Barrett, D.M.; Mitchell, A.E. Comparison of the total phenolic and ascorbic acid content of freeze-dried and air-dried marionberry, strawberry, and corn grown using conventional, organic, and sustainable agricultural practices. J. Agric. Food Chem. 2003, 51, 1237–1241. [Google Scholar] [CrossRef]
- Mditshwa, A.; Magwaza, L.S.; Tesfay, S.Z.; Mbili, N. Postharvest quality and composition of organically and conventionally produced fruits: A review. Sci. Hortic. 2017, 216, 148–159. [Google Scholar] [CrossRef]
- Jin, P.; Wang, S.Y.; Wang, C.Y.; Zheng, Y. Effect of cultural system and storage temperature on antioxidant capacity and phenolic compounds in strawberries. Food Chem. 2011, 124, 262–270. [Google Scholar] [CrossRef]
- Akšić, M.F.; Tosti, T.; Sredojević, M.; Milivojević, J.; Meland, M.; Natić, M. Comparison of sugar profile between leaves and fruits of blueberry and strawberry cultivars grown in organic and integrated production system. Plants 2019, 8, 205. [Google Scholar] [CrossRef]
- Szeląg-Sikora, A.; Sikora, J.; Niemiec, M.; Gródek-Szostak, Z.; Kapusta-Duch, J.; Kuboń, M.; Komorowska, M.; Karcz, J. Impact of Integrated and Conventional Plant Production on Selected Soil Parameters in Carrot Production. Sustainability 2019, 11, 5621. [Google Scholar] [CrossRef]
- Kovačević, D.B.; Putnik, P.; Dragović-Uzelac, V.; Vahčić, N.; Babojelić, M.S.; Levaj, B. Influences of organically and conventionally grown strawberry cultivars on anthocyanins content and color in purees and low-sugar jams. Food Chem. 2015, 181, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Tittarelli, F.; Ceglie, F.G.; Ciaccia, C.; Mimiola, G.; Amodio, M.L.; Colelli, G. Organic strawberry in Mediterranean greenhouse: Effect of different production systems on soil fertility and fruit quality. Renew. Agric. Food Syst. 2016, 32, 485–497. [Google Scholar] [CrossRef]
- Gomiero, T. Food quality assessment in organic vs. conventional agricultural produce: Findings and issues. Appl. Soil Ecol. 2018, 123, 714–728. [Google Scholar] [CrossRef]
- Häkkinen, S.H.; Törrönen, A.R. Content of flavonols and selected phenolic acids in strawberries and Vaccinium species: Influence of cultivar, cultivation site and technique. Food Res. Int. 2000, 33, 517–524. [Google Scholar] [CrossRef]
- Huber, M.; Rembiałkowska, E.; Średnicka, D.; Bügel, S.; van de Vijver, L.P.L. Organic food and impact on human health: Assessing the status quo and prospects of research. NJAS Wagening. J. Life Sci. 2011, 58, 103–109. [Google Scholar] [CrossRef]
- Looser, N.; Kostelac, D.; Scherbaum, E.; Anastassiades, M.; Zipper, H. Pesticide residues in strawberries sampled from the market of the Federal State of Baden-Württemberg in the period between 2002 and 2005. J. Verbraucherschutz Lebensmittelsicherheit 2006, 1, 135–141. [Google Scholar] [CrossRef]
- Olsson, M.E.; Andersson, C.S.; Oredsson, S.; Berglund, R.H.; Gustavsson, K.-E. Antioxidant levels and inhibition of cancer cell proliferation in vitro by extracts from organically and conventionally cultivated strawberries. J. Agric. Food Chem. 2006, 54, 1248–1255. [Google Scholar] [CrossRef]
- Cayuela, J.A.; Vidueira, J.M.; Albi, M.A.; Gutiérrez, F. Influence of the Ecological Cultivation of Strawberries (Fragaria × Ananassa Cv. Chandler) on the Quality of the Fruit and on Their Capacity for Conservation. J. Agric. Food Chem. 1997, 45, 1736–1740. [Google Scholar] [CrossRef]
- Cardoso, P.C.; Tomazini, A.P.B.; Stringheta, P.C.; Ribeiro, S.M.R.; Pinheiro-Sant’Ana, H.M. Vitamin C and carotenoids in organic and conventional fruits grown in Brazil. Food Chem. 2011, 126, 411–416. [Google Scholar] [CrossRef]
- Kahu, K.; Klaas, L.; Kikas, A. Effect of cultivars and different growing technologies on strawberry yield and fruit quality. Agron. Res. 2010, 8, 589–593. [Google Scholar]
- Roussos, P.A.; Denaxa, N.-K.; Ntanos, E.; Tsafouros, A.; Mavrikou, S.; Kintzios, S. Organoleptic, nutritional and anti-carcinogenic characteristics of the fruit and rooting performance of cuttings of black mulberry (Morus nigra L.) genotypes. J. Berry Res. 2020, 10, 77–93. [Google Scholar] [CrossRef]
- Keutgen, A.; Pawelzik, E. Modifications of taste-relevant compounds in strawberry fruit under NaCl salinity. Food Chem. 2007, 105, 1487–1494. [Google Scholar] [CrossRef]
- Ntanos, E.; Kekelis, P.; Assimakopoulou, A.; Gasparatos, D.; Denaxa, N.-K.; Tsafouros, A.; Roussos, P.A. Amelioration Effects against salinity stress in strawberry by bentonite–zeolite mixture, glycine betaine, and bacillus amyloliquefaciens in terms of plant growth, nutrient content, soil properties, yield, and fruit quality characteristics. Appl. Sci. 2021, 11, 8796. [Google Scholar] [CrossRef]
- Demirsory, L.; Demirsoy, H.; Balci, G. Different growing conditions affect nutrient content, fruit yield and growth in strawberry. Pak. J. Bot. 2011, 44, 125–129. [Google Scholar]
- Barbieri, G.; Colonna, E.; Rouphael, Y.; Pascale, S.D. Effect of the farming system and postharvest frozen storage on quality attributes of two strawberry cultivars. Fruits 2015, 70, 351–360. [Google Scholar] [CrossRef]
- Gliessman, S.R.; Werner, M.R.; Allison, J.; Cochran, J. A comparison of strawberry plant development and yield under organic and conventional management on the central California coast. Biol. Agric. Hortic. 1996, 12, 327–338. [Google Scholar] [CrossRef]
- Reganold, J.P.; Andrews, P.K.; Reeve, J.R.; Carpenter-Boggs, L.; Schadt, C.W.; Alldredge, J.R.; Ross, C.F.; Davies, N.M.; Zhou, J. Fruit and soil quality of organic and conventional strawberry agroecosystems. PLoS ONE 2010, 5, e12346. [Google Scholar] [CrossRef]
- Kilic, N.; Burgut, A.; Gündesli, M.A.; Nogay, G.; Ercisli, S.; Kafkas, N.E.; Ekiert, H.; Elansary, H.O.; Szopa, A. The effect of organic, inorganic fertilizers and their combinations on fruit quality parameters in strawberry. Horticulturae 2021, 7, 354. [Google Scholar] [CrossRef]
- Hargreaves, J.C.; Adl, M.S.; Warman, P.R.; Rupasinghe, H.P.V. The effects of organic and conventional nutrient amendments on strawberry cultivation: Fruit yield and quality. J. Sci. Food Agric. 2008, 88, 2669–2675. [Google Scholar] [CrossRef]
- Khalil, H.A.; Hassan, S.M. Ascorbic acid, β-carotene, total phenolic compound and microbiological quality of organic and conventional citrus and strawberry grown in Egypt. Afr. J. Biotechnol. 2015, 14, 272–277. [Google Scholar] [CrossRef]
- Nunes, M.C.N.; Delgado, A. quality of organic compared to conventionally grown strawberries at the retail level. Acta Hortic. 2014, 1049, 723–730. [Google Scholar] [CrossRef]
- Kobi, H.; Martins, M.; Silva, P.; Souza, J.; Carneiro, J.; Heleno, F.; Queiroz, M.; Costa, N. Organic and conventional strawberries: Nutritional quality, antioxidant characteristics and pesticide residues. Fruits 2018, 73, 39–47. [Google Scholar] [CrossRef]
- Yu, X.; Guo, L.; Jiang, G.; Song, Y.; Muminov, M.A. Advances of organic products over conventional productions with respect to nutritional quality and food security. Acta Ecol. Sin. 2018, 38, 53–60. [Google Scholar] [CrossRef]
- Dixon, R.A.; Paiva, N.L. Stress-Induced Phenylpropanoid Metabolism. Plant Cell 1995, 7, 1085–1097. [Google Scholar] [CrossRef] [PubMed]
- Törrönen, R.; Määttä, K. Bioactive substances and health benefits of strawberries. Acta Hortic. 2002, 567, 797–803. [Google Scholar] [CrossRef]
- You, Q.; Wang, B.; Chen, F.; Huang, Z.; Wang, X.; Luo, P.G. Comparison of anthocyanins and phenolics in organically and conventionally grown blueberries in selected cultivars. Food Chem. 2011, 125, 201–208. [Google Scholar] [CrossRef]
- Vrček, I.V.; Bojić, M.; Žuntar, I.; Mendaš, G.; Medić-Šarić, M. Phenol content, antioxidant activity and metal composition of Croatian wines deriving from organically and conventionally grown grapes. Food Chem. 2011, 124, 354–361. [Google Scholar] [CrossRef]
- Wang, S.Y.; Chen, C.T.; Sciarappa, W.; Wang, C.Y.; Camp, M.J. Fruit quality, antioxidant capacity, and flavonoid content of organically and conventionally grown blueberries. J. Agric. Food Chem. 2008, 56, 5788–5794. [Google Scholar] [CrossRef]
- Hakala, M.; Lapveteläinen, A.; Huopalahti, R.; Kallio, H.; Tahvonen, R. Effects of varieties and cultivation conditions on the composition of strawberries. J. Food Compos. Anal. 2003, 16, 67–80. [Google Scholar] [CrossRef]
- Chivenge, P.; Vanlauwe, B.; Six, J. Does the combined application of organic and mineral nutrient sources influence maize productivity? A meta-analysis. Plant Soil 2011, 342, 1–30. [Google Scholar] [CrossRef]
- Abid, M.; Batool, T.; Siddique, G.; Ali, S.; Binyamin, R.; Shahid, M.J.; Rizwan, M.; Alsahli, A.A.; Alyemeni, M.N. Integrated nutrient management enhances soil quality and crop productivity in maize-based cropping system. Sustainability 2020, 12, 10214. [Google Scholar] [CrossRef]
- Gram, G.; Roobroeck, D.; Pypers, P.; Six, J.; Merckx, R.; Vanlauwe, B. Combining organic and mineral fertilizers as a climate-smart integrated soil fertility management practice in sub-Saharan Africa: A meta-analysis. PLoS ONE 2020, 15, e0239552. [Google Scholar] [CrossRef] [PubMed]
- Yaldız, G.; Çamlıca, M.; Özen, F.; Eratalar, S.A. Effect of poultry manure on yield and nutrient composition of sweet basil (Ocimum basilicum L.). Commun. Soil Sci. Plant Anal. 2019, 50, 838–852. [Google Scholar] [CrossRef]
- Aliyu, L. Effect of organic and mineral fertilizers on growth, yield and composition of pepper (Capsicum annuum L.). Biol. Agric. Hortic. 2000, 18, 29–36. [Google Scholar] [CrossRef]
- Antoniadis, V.; Koliniati, R.; Efstratiou, E.; Golia, E.; Petropoulos, S. Effect of soils with varying degree of weathering and pH values on phosphorus sorption. CATENA 2016, 139, 214–219. [Google Scholar] [CrossRef]
- Hu, Z.; Ding, Z.; Al-Yasi, H.M.; Ali, E.F.; Eissa, M.A.; Abou-Elwafa, S.F.; Sayed, M.A.; Said, M.T.; Said, A.A.; Ibrahim, K.A.M.; et al. Modeling of phosphorus nutrition to obtain maximum yield, high p use efficiency and low P-loss risk for wheat grown in sandy calcareous soils. Agronomy 2021, 11, 1950. [Google Scholar] [CrossRef]
- Hargraves, J.C.; Adl, M.S.; Warman, P.R. Are compost teas an effective nutrient amendment in the cultivation of strawberries? Soil and plant tissue effects. J. Sci. Food Agric. 2009, 89, 390–397. [Google Scholar] [CrossRef]
- Niskanen, R.; Dris, R. Nutritional status of strawberry fields. Acta Hortic. 2002, 567, 439–442. [Google Scholar] [CrossRef]
- Watanabe, H.; Hoshino, K.; Adachi, Y. Effects of poultry manure on soil solution electrical conductivity and early growth of Monochoria vaginalis. Plant Prod. Sci. 2017, 20, 67–71. [Google Scholar] [CrossRef][Green Version]
- Kelderer, M.; Topp, A.; Lardschneider, E.; Rainer, A.; Matteazzi, A. Organic apple tree nutrition: Comparison of different organic fertilizers, application timing and rate, and soil management techniques: Results of a 5 year field study. In Proceedings of the 16th International Congress on Organic Fruit Growing, Hohenheim, Germany, 17–19 February 2014; pp. 116–126. [Google Scholar]
- Wang, B.; Liu, H.; Cai, C.; Thabit, M.; Wang, P.; Li, G.; Duan, Z. Effect of dry mycelium of Penicillium chrysogenum fertilizer on soil microbial community composition, enzyme activities and snap bean growth. Environ. Sci. Pollut. Res. 2016, 23, 20728–20738. [Google Scholar] [CrossRef] [PubMed]
- Nardi, S.; Morari, F.; Berti, A.; Tosoni, M.; Giardini, L. Soil organic matter properties after 40 years of different use of organic and mineral fertilizers. Eur. J. Agron. 2004, 21, 357–367. [Google Scholar] [CrossRef]
- Nardi, S.; Pizzeghello, D.; Ertani, A. Hormone-like activity of the soil organic matter. Appl. Soil Ecol. 2018, 123, 517–520. [Google Scholar] [CrossRef]

| Treatment | Yield per Plant (g) | Mean Fruit Weight (g) | Diameter (mm) | Length (mm) | D/L | Firmness (kg) | DW (%) |
|---|---|---|---|---|---|---|---|
| ORG | 256.8 b | 16.27 a | 34.75 a | 41.54 a | 0.83 a | 0.47 a | 7.54 a |
| I-AGR | 294.8 ab | 16.68 a | 33.99 a | 39.89 a | 0.85 a | 0.46 a | 7.33 a |
| I-ACT | 344.1 a | 16.07 a | 31.01 a | 36.82 a | 0.84 a | 0.46 a | 7.76 a |
| Treatment | Hue | Chroma | L* | Fruit Number per Plant | Extra (%) | I&II (%) |
|---|---|---|---|---|---|---|
| ORG | 30.01 a | 40.35 a | 35.17 a | 16.29 b | 89.63 a | 10.01 a |
| I-AGR | 29.26 a | 40.25 a | 34.93 a | 17.41 b | 91.14 a | 8.85 a |
| I-ACT | 29.52 a | 41.10 a | 35.44 a | 22.58 a | 85.22 a | 14.77 a |
| Treatment | TA (% w/w Citric Acid) | TSS (oBrix) | pH | TSS/TA |
|---|---|---|---|---|
| ORG | 0.85 a | 7.35 a | 3.54 a | 8.79 a |
| I-AGR | 0.86 a | 6.99 a | 3.61 a | 8.28 a |
| I-ACT | 0.85 a | 7.40 a | 3.60 a | 8.80 a |
| Treatment | Total Phenols | Total o-Diphenols | Total Flavonoids | Total Flavanols | DPPH | FRAP |
|---|---|---|---|---|---|---|
| ORG | 174.4 a | 27.28 a | 249.2 a | 28.28 a | 53.06 a | 19.05 a |
| I-AGR | 171.9 a | 23.63 a | 210.5 a | 17.62 b | 52.02 a | 13.89 b |
| I-ACT | 167.7 a | 22.75 a | 209.6 a | 17.29 b | 56.41 a | 14.22 b |
| Treatment | TANTHO | Cy-3-gluc | Pg-3-gluc | Pg-3-rut |
|---|---|---|---|---|
| ORG | 16.478 a | 50.14 a | 488.0 a | 75.65 a |
| I-AGR | 16.67 a | 57.95 a | 541.5 a | 80.97 a |
| I-ACT | 15.63 a | 55.31 a | 491.4 a | 80.90 a |
| Treatment | SUC | GLUC | FRUCT | TSUGS | SI |
|---|---|---|---|---|---|
| ORG | 1.78 a | 1.75 a | 1.89 a | 5.43 a | 8.52 a |
| I-AGR | 1.53 a | 1.45 b | 1.62 b | 4.60 b | 7.24 b |
| I-ACT | 1.81 a | 1.58 ab | 1.68 ab | 5.08 ab | 7.91 ab |
| Treatment | Malic Acid | Citric Acid | Ascorbic Acid | Sum of Acids Determined | SOURI |
|---|---|---|---|---|---|
| ORG | 2.62 a | 10.81 a | 0.13 a | 13.57 a | 5.04 ab |
| I-AGR | 2.30 a | 9.77 a | 0.09 a | 12.17 b | 4.77 b |
| I-ACT | 2.58 a | 9.81 a | 0.10 a | 12.49 ab | 5.30 a |
| Treatment | N | P | K | Na | Ca | Mg | Fe | Mn | Cu | Zn |
|---|---|---|---|---|---|---|---|---|---|---|
| (%) | (mg kg−1) | |||||||||
| ORG | 1.88 b | 0.13 a | 0.86 b | 0.10 a | 1.75 a | 0.26 a | 67.84 b | 35.24 b | 17.2 a | 24.32 a |
| I-AGR | 1.47 c | 0.16 a | 1.42 ab | 0.19 a | 2.56 a | 0.43 a | 128.9 a | 99.82 a | 21.2 a | 24.02 a |
| I-ACT | 2.29 a | 0.19 a | 1.98 a | 0.19 a | 2.00 a | 0.42 a | 125.8 a | 101.56 a | 20.7 a | 27.66 a |
| Treatment | pH | CaCO3 (%) | EC (mS cm−1) | OM (%) | CEC (meq 100 g−1) |
|---|---|---|---|---|---|
| ORG | 7.52 a | 26.36 a | 3.96 b | 4.77 a | 21.06 a |
| I-AGR | 7.52 a | 26.46 a | 4.46 ab | 5.07 a | 21.08 a |
| I-ACT | 7.47 a | 25.64 a | 5.2 a | 4.62 a | 21.38 a |
| Treatments | P | K | Na | Ca | Mg | Fe | Mn | Cu | Zn |
|---|---|---|---|---|---|---|---|---|---|
| (mg kg−1) | (meq 100 g−1) | (mg kg−1) | |||||||
| ORG | 109.6 a | 0.79 a | 0.35 b | 26.83 a | 2.23 a | 3.89 a | 21.08 a | 1.17 a | 3.15 ab |
| I-AGR | 56.9 b | 0.78 a | 0.46 a | 26.97 a | 2.06 a | 4.08 a | 26.64 a | 0.99 a | 2.62 b |
| I-ACT | 67.5 b | 1.04 a | 0.38 ab | 30.62 a | 2.19 a | 3.93 a | 27.00 a | 0.99 a | 3.44 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roussos, P.A.; Triantafillidis, A.; Kepolas, E.; Peppas, P.; Piou, A.; Zoti, M.; Gasparatos, D. Effects of Integrated and Organic Management on Strawberry (cv. Camarosa) Plant Growth, Nutrition, Fruit Yield, Quality, Nutraceutical Characteristics, and Soil Fertility Status. Horticulturae 2022, 8, 184. https://doi.org/10.3390/horticulturae8020184
Roussos PA, Triantafillidis A, Kepolas E, Peppas P, Piou A, Zoti M, Gasparatos D. Effects of Integrated and Organic Management on Strawberry (cv. Camarosa) Plant Growth, Nutrition, Fruit Yield, Quality, Nutraceutical Characteristics, and Soil Fertility Status. Horticulturae. 2022; 8(2):184. https://doi.org/10.3390/horticulturae8020184
Chicago/Turabian StyleRoussos, Peter Anargyrou, Athanassios Triantafillidis, Evaggelos Kepolas, Panagiotis Peppas, Anastassia Piou, Maria Zoti, and Dionisios Gasparatos. 2022. "Effects of Integrated and Organic Management on Strawberry (cv. Camarosa) Plant Growth, Nutrition, Fruit Yield, Quality, Nutraceutical Characteristics, and Soil Fertility Status" Horticulturae 8, no. 2: 184. https://doi.org/10.3390/horticulturae8020184
APA StyleRoussos, P. A., Triantafillidis, A., Kepolas, E., Peppas, P., Piou, A., Zoti, M., & Gasparatos, D. (2022). Effects of Integrated and Organic Management on Strawberry (cv. Camarosa) Plant Growth, Nutrition, Fruit Yield, Quality, Nutraceutical Characteristics, and Soil Fertility Status. Horticulturae, 8(2), 184. https://doi.org/10.3390/horticulturae8020184








