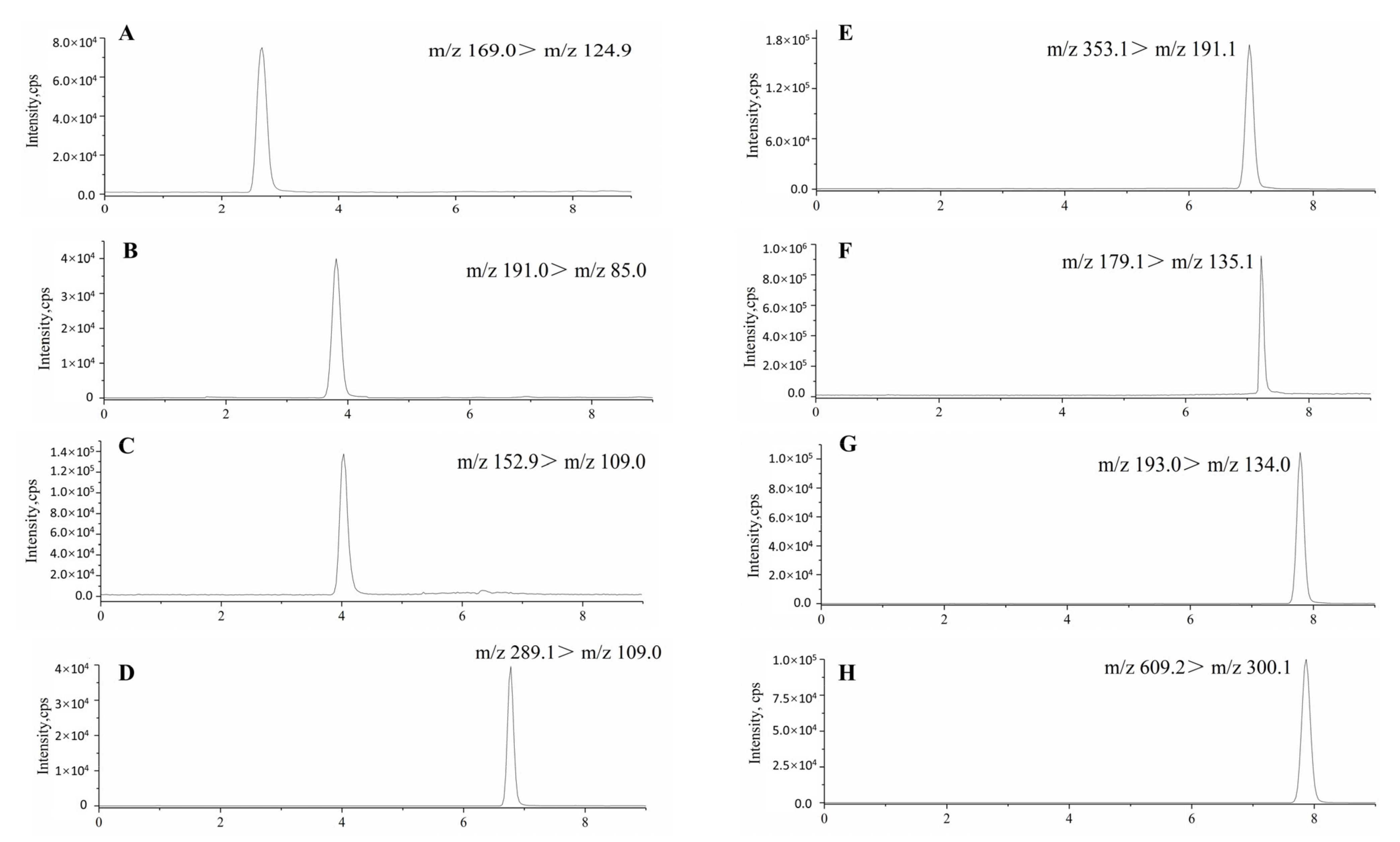
1. Introduction
Banana is an important tropical and subtropical fruit crop [
1], and is one of the most produced and consumed fruits in the world [
2,
3]. According to the latest data of Food and Agriculture Organization (FAO) in 2020, India was the world’s largest banana producer, followed by China, with annual production reaching 30.46 and 12 million tons, respectively [
4]. Banana peel accounts for about 40% of the total fresh quantity of ripe fruit [
5], which is largely discarded in nature and becomes household and industrial food waste [
5]. Usually, banana peel waste is indiscriminately dumped into the rivers, lakes, or low-lying landfills where it degrades slowly until it decays and forms methane and carbon dioxide, and other noxious gases such as hydrogen sulfide and ammonia that spread unpleasant odors, affect the nearby ecosystems [
6], and cause serious environmental damage [
6,
7,
8]. Thus, banana peel waste is needed to recycle or reuse instead of discarding anyway or incinerating.
Banana peel, as agricultural waste, contains a large number of oligosaccharides, cellulose, and mineral elements [
9], which is an ideal material for adsorbents and is widely used to reduce environmental pollutants, such as heavy metals, dyes, organic pollutants, nitrate, and arsenate in groundwater, pesticides, and various other gaseous pollutants [
7,
8]. Banana peel has recently been increasingly reported as a rich source of phenolic compounds [
10], with total phenolic content ranging from 4.95 to 47 mg garlic acid equivalent/g dry matter (mg GAE/g DM). This level was 1.5–3 times higher than that recorded in the pulp [
11]. These confer a wide range of biological and pharmacological properties with potential as antimutagenic, antiviral antithrombotic, hepatoprotective agents, as well as a food additive and signaling molecules because of strong natural antioxidant properties [
12,
13]. Many fruit peels’ phenolic compounds have now been studied [
14]. It is noted that gallic acid, quinic acid, protocatechuic acid, catechin, chlorogenic acid, caffeic acid, ferulic acid, and rutin are their main phenolic compounds [
15], which are mainly associated with their antioxidant [
16,
17,
18,
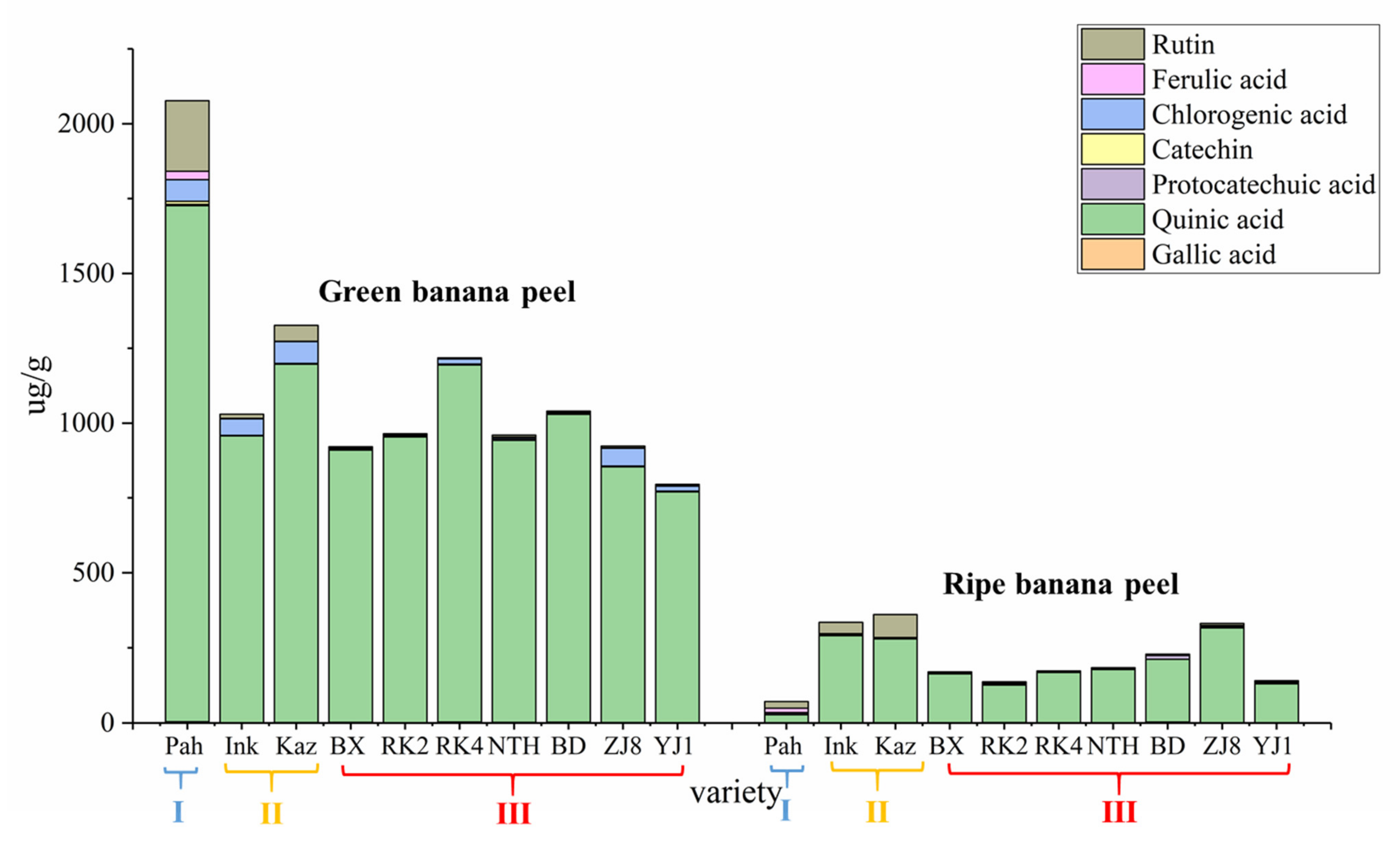
19]. However, little research is available on the natural contents of main phenolic compounds in the banana peel of different cultivars and maturities for picking out banana peel with rich phenolic compounds for further efficient utilization. Thus, an extraction and analytical method is urgently needed to determine main phenolic compound contents in fruit peel from different banana cultivars and subgroups over a range of maturities.
To add value to the industrial waste from banana processing, an effective extraction would be beneficial to recover functional ingredients sustainably [
19,
20]. In order to obtain phenolic-rich extracts, conventional extraction methods with organic solvents are commonly used [
21]. These extraction technologies are highly polluting, labor-intensive, solvent consuming, and time consuming, so it is necessary to develop green alternative methods [
22]. The ultrasound assisted extraction (UAE) could be one of the most suitable techniques [
23], as it is very simple to use, requires relatively inexpensive apparatus, reduces the volume of solvent [
24], uses acoustic energy and solvents to enhance the release and diffusion of phenolic compounds from various matrices [
25], and allows to use green extraction solvents with a high efficiency due to the mechanical effects that it produces in the cellular structure of the matrix [
26,
27]. Many analytical methods have been used to determine phenolic compound contents in fruit peel, but currently only UPLC-MS/MS could provide higher sensitivity, selectivity, shorter analysis time, and simultaneous determination of many phenolic compounds [
28]. Therefore, in the study, we first proposed to use UPLC-MS/MS combining with ultrasonic extraction to simultaneous determine the main phenolic compound contents in banana peel and further explored the difference of phenolic compound contents in banana peel of different cultivars and subgroups with different levels of maturity.
The plant cultivar and maturity are the important factors that influence the synthesis and accumulation of biologically active substances in fruit peel. Thus, our work aimed to develop a sensitive and effective UPLC-MS/MS method combined with ultrasonic extraction for simultaneous determination of eight main phenolic compounds in the fruit peels of different banana ecotypes and subgroups, with different degrees of maturity. Quantification of main phenolic compounds across the fruit peels with different banana ecotypes and maturity will also provide valuable data on their contents, and will demonstrate which cultivar and maturity peel is used to extract individual phenolic compound might be meaningful. Our study contributes to finding new sources of phenolic compounds and laying the foundation for future utilization of banana peel. We could use the fruit peel extracts for the production of functional food, dietary supplements, or other innovative preparations with a specific biological effect.
2. Materials and Methods
2.1. Chemicals and Reagents
Standards (gallic acid, quinic acid, protocatechuic acid, catechin, chlorogenic acid, caffeic acid, ferulic acid, and rutin) were purchased from Sigma Company (Ontario, CA, USA). HPLC-grade methanol and acetonitrile (99.95%) were purchased from Fisher Scientific (Loughborough, UK). Formic acid (HPLC grade) was purchased from Sigma–Aldrich (St. Quentin Fallavier, France). Dimethyl sulfoxide (DMSO, analytical-reagent grade) was purchased from Gaoyao Chemical Reagent Co., Ltd. (Shanghai, China). Deionized water used throughout the study was purified using a Milli-Q water-purification system (Millipore, Bedford, MA, USA).
2.2. Preparation of Stock and Standard Solutions
Individual stock standard solutions of gallic acid, quinic acid, protocatechuic acid, catechin, chlorogenic acid, caffeic acid, ferulic acid, and rutin were prepared on a weight basis in pure methanol at 1.0 mg/mL. Next, 0.1 mL of each individual standard stock solution was accurately measured in a 10 mL amber volumetric flask, and the mixture was diluted with pure methanol to prepare a 100.0 μg/mL mixed standard stock solution. The standard stock solutions were sealed and stored at −80 °C.
The individual standard working solutions (100.0, 10.0, 5.0, 1.0 μg/m, 100.0, 10.0, and 1.0 ng/mL) and mixed standard working solutions (1.0, 0.5 μg/mL, and 200.0, 100.0, 50.0, 20.0, and 10.0 ng/mL) were prepared by diluting the individual standard stock solutions and mixed standard stock solution with pure methanol, respectively. The individual standard working solutions and mixed standard working solution were stable for 1 week at −20 °C.
2.3. Conditions of Ultra-High-Performance Liquid Chromatography–Tandem Mass Spectrometry
The analytical system was UPLC-MS/MS analyses. The model of mass spectrometer (MS) consisted of a Qtrap 6500+ (Applied Biosystems, New York, NY, USA) with an electrospray ionization (ESI) source for molecule ionization. The optimized ESI temperature was set at 500 °C, ionspray voltage at −4500 V for negative mode. The curtain gas and collision gas pressure were 36 and 10 psi, respectively. All qualitative and quantitative data in this study were acquired using MRM mode. A UPLC model 20A (Shimadzu, Kyoto, Japanese) consisted of a binary pump, degasser, and auto-sampler. Peak areas of each constituent were automatically integrated using analyst software. The analytical column was Agilent Zorbax Extend-C
18 (2.1 × 100 mm, 3.5 µm; Agilent Technologies, Santa Clara, CA, USA). The binary mobile phase contained both methanol (solvent A) and 0.2% (
v/
v) aqueous formic acid (solvent B), which was pumped at a flow rate of 0.3 mL/min for a total run time of 9 min [
29]. The injection volume was 5.0 μL, and the column temperature was 35 °C.
2.4. Samples Collection
Banana samples were imported from the Alliance of Bioversity International and CIAT, the Agrobiodiversity and Banana Research Team of Agricultural Environment and Resources Institute, Yunnan Academy of Agricultural Sciences, then tissue culture and rapid propagation were performed. Afterwards, 10 banana germplasm resources were planted in the experimental base of Xishuangbanna, Yunnan (21°54′13″ N; 100°48′25″ E). The 10 banana ecotypes were, respectively, Pahang, Inkira, Kazirakwe, Brazilian, Reke No. 2, Reke No. 4, Nantianhuang, Baodaojiao, Zhongjiao No. 8, and Yunjiao No. 1. During sampling, the third comb of banana was collected from top to bottom, with 3 repeating bases, one for each cultivar in each base, and the middle 4–6 fruit fingers of each comb of banana shall be continuously taken for measurement and statistics.
Banana germplasms are classified into different subgroups according to clusters of phenotypically similar populations of a particular species. The 10 banana ecotype cultivars in the study were divided into three subgroups based on their origin and genetic relationship: the Malaccensis subgroup, the Mutika–Lujμgira subgroup, and the Cavendish subgroup. Pahang in the Malaccensis subgroup was the wild banana species from Malaysia, Inkira, and Kazirakwe in the Mutika–Lujμgira subgroup were the cultivated cooked bananas from Africa, and the other seven banana cultivars, in the Cavendish subgroup, were all the cultivated and domesticated cultivars which were commonly grown in China with high yields and the highest degree of commercialization. The first two subgroups belong to non-dessert banana, which includes non-edible banana and boiled banana, and the last subgroup is the dessert banana which includes fresh banana.
2.5. Sample Extraction
According to the color chart of banana maturity, seven ripe-green bananas and full-ripe bananas accelerated with Ethrel were harvested. A knife was used to separate the peel from the pulp to obtain a weighed sample of 100.0 g of fresh fruit peel. After washing and slicing, we put the slices in an oven at 70 °C and dried them for 9–10 h until they were dry. They were then weighed and pulverized, and sieved through a 60-mesh sieve to make a powder for use. Samples of accurately weighed 1.0 g of banana peel powder were placed in a 50 mL centrifuge tube, and 45 mL of 30% acetonitrile was added, and vortexed for 10 s. Then ultrasonic extraction was conducted for 40 min, centrifugation at 5000 rpm/min for 10 min, and then 15 mL of supernatant in a new centrifuge tube was taken. We repeated the above operation 3 times. Finally, 45 mL of the supernatant was obtained, vortexed to mix, and passed through 0.22 μm microporous membrane to obtain the test solution.
2.6. Determination of Fruit Agronomic Traits, the Proportion and the Moisture Content of the Peel in Different Green/Ripe Banana Ecotypes
Six fruit agronomic characters of green and ripe banana were measured, including fruit finger girth, fruit finger length, peel thickness, fresh weight of pulp, fresh weight of peel, and fruit finger fresh weight. The girth of fruit finger refers to the circumference of the middle position of fruit finger. The fruit finger length refers to the length from the top of the fruit along the outer edge of the curved surface to the base of the fruit handle. Then, we determined the proportion of the fresh green fruit peel and dry weight of fruit peel of 10 banana cultivars. The proportion of fresh green fruit peel was the percentage of fresh green fruit peel fresh weight/green banana fresh weight, the dry weight ratio of banana peel refers to the percentage of dried banana peel weight/fresh banana peel weight. The proportion of dry weight of the peel can be used to explain the water content of the peel. The smaller the dry weight is, the higher the water content is. The data indexes of banana samples were determined six times.
2.7. Statistical Analysis
For the quantitative analysis of phenolic compounds in banana peel, we measured 10 samples for each banana cultivar and calculated the average value. The validation experiment was performed in six replicates. Experimental data are represented as means ± SD and p < 0.05 was considered to be statistically significant. Data were calculated by one-way analysis of variance (ANOVA) and analyses were performed using SPSS 26.0. Probability values of less than 5% were considered to be significant.