Organic Fertilization of Growing Media: Response of N Mineralization to Temperature and Moisture
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design
2.3. Analysis
2.4. Data Treatments
2.4.1. Ammonium and Nitrate Concentrations
2.4.2. Abiotic Factors
2.5. Statistical Analyses
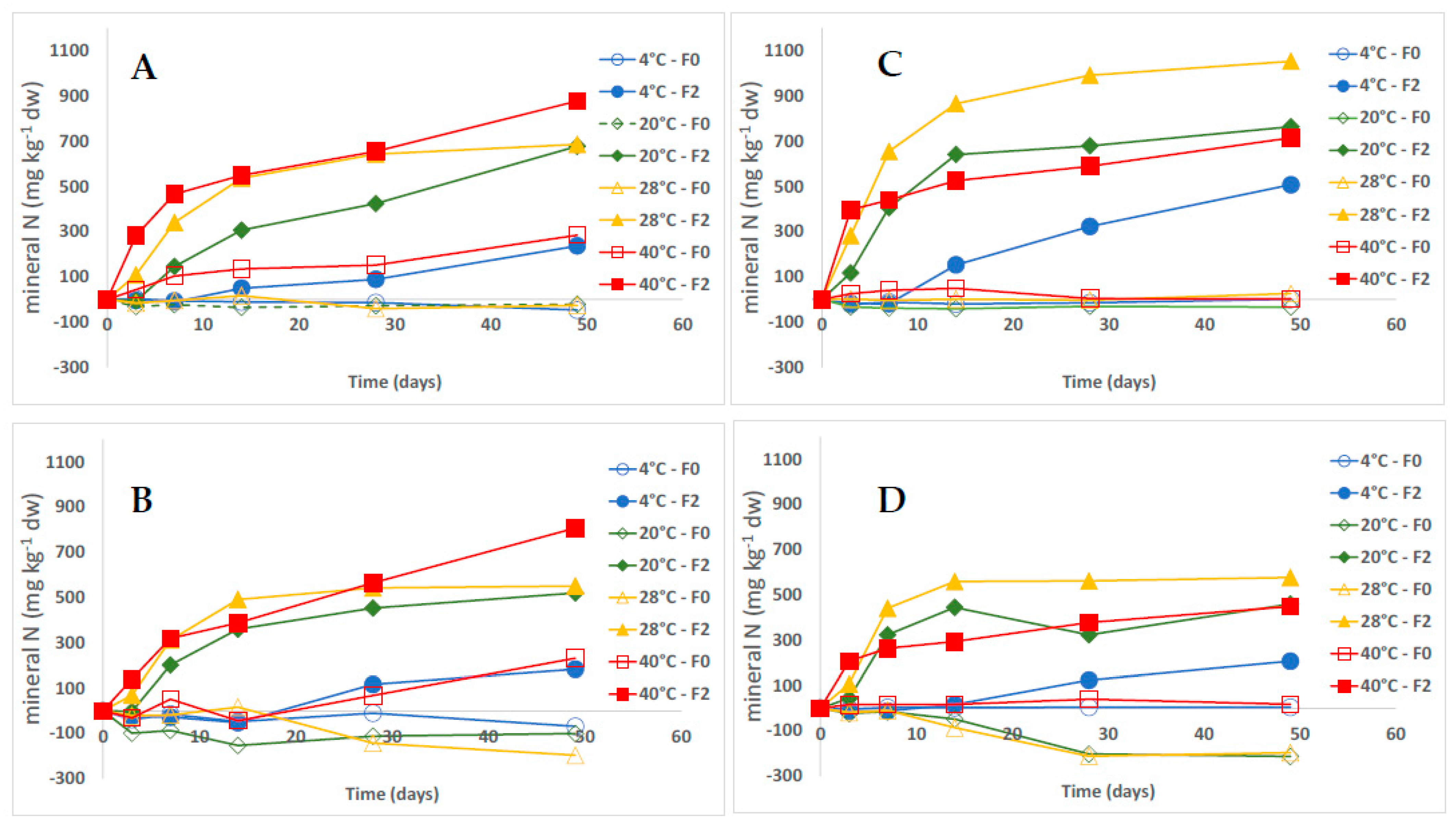
3. Results
3.1. Dynamics of Growing Media N Mineral Content
3.2. Fertilizer Mineralization
3.3. Relative Proportion of NO3− to Total Mineral N
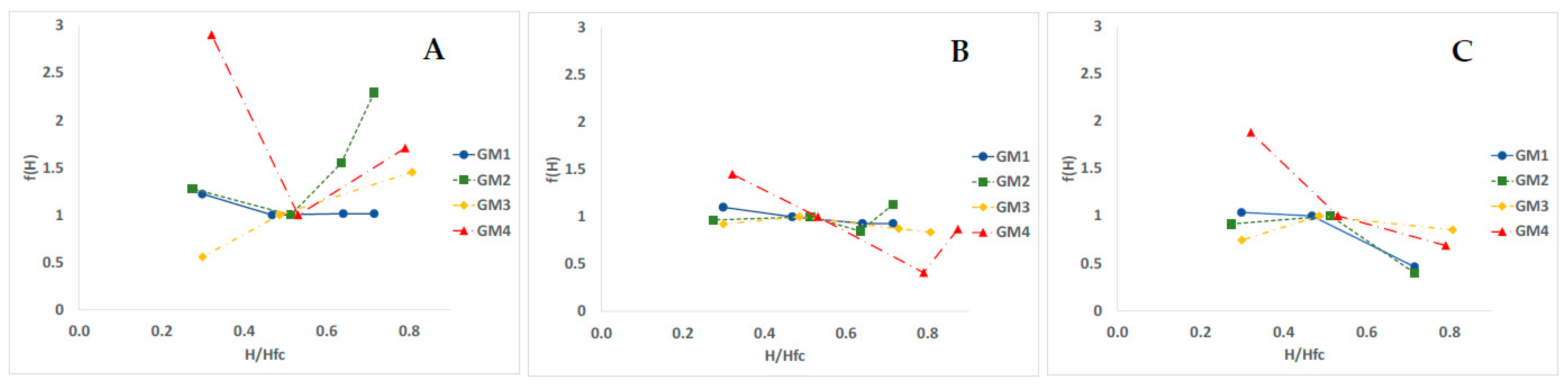
3.4. Temperature and Humidity Action Laws
4. Discussion
4.1. Growing Media Type
4.2. Fertilizer Type
4.3. Temperature Effects and Action Law
4.4. Humidity Effect and Action Law
4.5. Management Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burnett, S.E.; Mattson, N.S.; Williams, K.A. Substrates and fertilizers for organic container production of herbs, vegetables, and herbaceous ornamental plants grown in greenhouses in the United States. Scientia Hortic. 2016, 208, 111–119. [Google Scholar] [CrossRef]
- Raviv, M.; Wallach, R.; Silber, A.; Bar-Tal, A. Substrates and their analysis. In Hydroponic Production of Vegetables and Ornamentals; Savvas, D., Passam, H., Eds.; Embryo Publications: Athens, Greece, 2002; pp. 25–102. [Google Scholar]
- Barrett, G.E.; Alexander, P.D.; Robinson, J.S.; Bragg, N.C. Achieving environmentally sustainable growing media for soilless plant cultivation systems-A review. Scientia Hortic. 2016, 21, 220–234. [Google Scholar] [CrossRef]
- Caldwell, B.A. Enzyme activities as a component of soil biodiversity: A review. Pedobiologia 2005, 49, 637–644. [Google Scholar] [CrossRef]
- Treadwell, D.D.; Hochmuth, G.J.; Hochmuth, R.C.; Simonne, E.H.; Davis, L.L.; Laughlin, W.L.; Li, Y.; Olczyk, T.; Sprenkel, R.K.; Osborne, L.S. Nutrient management in organic greenhouse herb production: Where are we now? Hortic. Technol. 2007, 17, 461–466. [Google Scholar] [CrossRef]
- Montagne, V.; Capiaux, H.; Barret, M.; Cannavo, P.; Charpentier, S.; Grosbellet, C.; Lebeau, T. Bacterial and fungal communities vary with the type of organic substrate: Implications for biocontrol of soilless crops. Environ. Chem. Lett. 2017, 15, 537–545. [Google Scholar] [CrossRef]
- Montagne, V.; Charpentier, S.; Cannavo, P.; Capiaux, H.; Grosbellet, C.; Lebeau, T. Structure and activity of spontaneous fungal communities in organic substrates used for soilless crops. Scientia Hortic. 2015, 192, 148–157. [Google Scholar] [CrossRef]
- Jack, A.L.H.; Rangarajan, A.; Culman, S.W.; Sooksa-Nguan, T.; Thies, J.E. Choice of organic amendments in tomato transplants has lasting effects on bacterial rhizosphere communities and crop performance in the field. Appl. Soil Ecol. 2011, 48, 94–101. [Google Scholar] [CrossRef]
- Voogt, W.; Cuijpers, W.J.M.; de Visser, P.H.E.; van de Burgt, G.J.H.M.; van Winkel, A. Nutrient management in organic greenhouse production; Navigation between constraint. Acta Hortic. 2011, 915, 75–82. [Google Scholar] [CrossRef]
- Paillat, L.; Cannavo, P.; Barraud, F.; Huché-Thélier, L.; Guénon, R. Growing Medium Type Acts Organic Fertilizer Mineralization and CNPS Microbial Enzyme Activities. Agronomy 2020, 10, 1955. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Müller, C.; Cai, Z. Ecological and practical significances of crop species preferential N uptake matching with soil N dynamics. Soil Biol. Biochem. 2016, 103, 63–70. [Google Scholar] [CrossRef]
- Houlton, B.Z.; Sigman, D.M.; Schuur, E.A.G.; Hedin, L.O. A climate-driven switch in plant nitrogen acquisition within tropical forest communities. Proc. Natl. Acad. Sci. USA 2007, 104, 8902–8906. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, B.J.; Robbins, S. pH affects ammonium, nitrate and proton fluxes in the apical region of conifer and soybean roots. Physiol. Plant. 2010, 138, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Schimel, J.P.; Jackson, L.E.; Firestone, M.K. Spatial and temporal effects on plant-microbial competition for inorganic nitrogen in a California annual grassland. Soil Biol. Biochem. 1989, 21, 1059–1066. [Google Scholar] [CrossRef]
- Benton Jones, J. Plant Nutrition Manual; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Gravel, V.; Dorais, M.; Pepin, S. Soil salinization of organically-grown greenhouse tomato. In Volume 1 Organic Crop Production, Proceedings of the 17th IFOAM Organic World Congress, Organic is Life: Knowledge for tomorrow, Third Scientific Conference of the International Society of Organic Agriculture Research (ISOFAR), Seoul, South Korea, 28 September–1 October 2011; International Society of Organic Agriculture Research (ISOFAR): Rennes, France, 2011; pp. 436–440. [Google Scholar]
- Gaskell, M.; Smith, R. Nitrogen Sources for Organic Vegetable Crops. Hortic. Technol. 2007, 17, 431–441. [Google Scholar] [CrossRef]
- Casadesus, J.; Caceres, R.; Marfa, O. Dynamics of CO2 efflux from the substrate root system of container-grown plants associated with irrigation cycles. Plant Soil 2007, 300, 71–82. [Google Scholar] [CrossRef]
- Handreck, K.A. Rapid assessment of the rate of nitrogen immobilisation in organic components of potting media: I. Method development. Commun. Soil Sci. Plant Anal. 1992, 23, 201–215. [Google Scholar] [CrossRef]
- Harris, C.N.; Dickson, R.W.; Fisher, P.R.; Jackson, B.E.; Poleatewich, A.M. Evaluating Peat Substrates Amended with Pine Wood Fiber for Nitrogen Immobilization and Effects on Plant Performance with Container-grown Petunia. Hortic. Technol. 2020, 30, 107–116. [Google Scholar] [CrossRef]
- Boyer, C.R.; Fain, G.B.; Gilliam, C.H.; Gallagher, T.V.; Torbert, H.A.; Sibley, J. Description of cleanchip residual forest harvest and its availability for horticultural uses in the southeastern United States. Hortic. Technol. 2012, 22, 381–387. [Google Scholar]
- AFNOR Amendements du Sol et Supports de Culture—Détermination des Propriétés Physiques—Masse Volumique Apparente Sèche, Volume D’air, Volume d’eau, Valeur de Rétraction et Porosité Totale. NF EN 13040. U44-307. 2000. Available online: https://www.boutique.afnor.org/normes (accessed on 10 March 2017).
- AFNOR Amendements du Sol et Supports de Culture—Détermination du pH. NF EN 13037. U44-308. 2000. Available online: https://www.boutique.afnor.org/normes (accessed on 10 March 2017).
- AFNOR Amendements du Sol et Supports de Culture—Détermination de la Conductivité Electrique. NF EN 13038. U44-309. 2000. Available online: https://www.boutique.afnor.org/normes (accessed on 10 March 2017).
- AFNOR Qualité du Sol—Détermination de la Teneur Totale en Azote par Combustion Sèche. NF ISO 13878. X31-418. 1998. Available online: https://www.boutique.afnor.org/normes (accessed on 10 March 2017).
- AFNOR Amendements du Sol et Supports de Culture—Détermination de la Teneur en Matieres Organiques. NF EN 13039. U44-304. 2000. Available online: https://www.boutique.afnor.org/normes (accessed on 10 March 2017).
- AFNOR Amendements du sol et Supports de Culture—Extraction D'éléments Solubles Dans L'eau Régale NF EN 13650. 2002. Available online: https://www.boutique.afnor.org/normes (accessed on 10 March 2017).
- AFNOR Qualité de L'eau-Dosage D'éléments Choisis Par Spectroscopie D'émission Optique Avec Plasma Induit Par Haute Fréquence (ICP-OES). NF EN ISO 11885. 2009. Available online: https://www.boutique.afnor.org/normes (accessed on 10 March 2017).
- De Boodt, M.; Verdonck, O.; Cappaert, I. Method for measuring the water-release curve of organic substrates. Acta Hortic. 1974, 37, 2054–2062. [Google Scholar] [CrossRef]
- Richards, L.A. A pressure-membrane extraction apparatus for soil solution. Soil Sci. 1941, 51, 377–386. [Google Scholar] [CrossRef]
- AFNOR Qualité du Sol— Détermination De La Caractéristique De La Rétention en eau-Méthodes De Laboratoire. NF ISO 11274. 2019. Available online: https://www.boutique.afnor.org/normes (accessed on 10 March 2017).
- Felske, A.; Akkermans, A.D.L. Spatial homogeneity of abundant bacterial 16S rRNA molecules in grassland soils. Microb. Ecol. 1998, 36, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Rotthauwe, J.H.; Witzel, K.P.; Liesack, W. The ammonia monooxygenase structural gene amoA as a functional marker: Molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl. Environ. Microbiol. 1997, 63, 4704–4712. [Google Scholar] [CrossRef] [PubMed]
- Brisson, N.; Launay, M.; Mary, B.; Beaudoin, N. Conceptual Basis, Formalisations and Parameterization of the STICS Crop Model; Editions Quae: Versailles, France, 2008; p. 301. [Google Scholar]
- Griffins, T.S.; Honeycutt, C.W.; He, Z. Effects of temperature, soil water status, and soil type on swine slurry nitrogen transformations. Biol. Fertil. Soils. 2001, 36, 442–446. [Google Scholar]
- Drury, K.F.; Zhang, T.Q.; Kay, B.D. The non-limiting and least limiting water ranges for soil nitrogen mineralization. Soil Sci. Soc. Am. J. 2003, 67, 1388–1404. [Google Scholar] [CrossRef]
- Sørensen, P.; Jensen, E.S. Mineralization of carbon and nitrogen from fresh and anaerobically stored sheep manure in soils with different texture and water contents. Biol. Fertil. Soils 1995, 19, 29–35. [Google Scholar] [CrossRef]
- Thomsen, I.K.; Schoenning, P.; Christensen, B.T. Mineralization of 15N-labelled sheep manure in soils of different texture and water contents. Biol. Fertil. Soils 2003, 37, 295–301. [Google Scholar] [CrossRef]
- Depardieu, C.; Prémont, V.; Boily, C.; Caron, J. Sawdust and bark-based substrates for soilless strawberry production: Irrigation and electrical conductivity management. PLoS ONE 2016, 11, e0154104. [Google Scholar] [CrossRef]
- Savvas, D.; Gruda, N. Application of soilless culture technologies in the modern greenhouse industry—A review. Eur. J. Hortic. Sci. 2018, 83, 280–293. [Google Scholar] [CrossRef]
- Schulker, B.A.; Jackson, B.E.; Fonteno, W.C.; Heitman, J.L.; Albano, J.P. Comparison of Water Capture Efficiency through Two Irrigation Techniques of Three Common Greenhouse Soilless Substrate Components. Agronomy 2020, 10, 1389. [Google Scholar] [CrossRef]
- Durand, S.; Jackson, B.E.; Fonteno, W.C.; Michel, J.-C. The Use of Wood Fiber for Reducing Risks of Hydrophobicity in Peat-Based Substrates. Agronomy 2021, 11, 907. [Google Scholar] [CrossRef]
- Naasz, R.; Michel, J.C.; Charpentier, S. Water repellency of organic growing media related to hysteretic water retention properties. Eur. J. Soil Sci. 2008, 59, 156–165. [Google Scholar] [CrossRef]
- Cannavo, P.; Michel, J.C. Effect of growing medium particle size on spatial root distribution: Consequences on the evolution of hydraulic properties in the medium. Sci. Hortic. 2013, 151, 11–21. [Google Scholar] [CrossRef]
- Grunert, O.; Reheul, D.; Van Labeke, M.; Perneel, M.; Hernandez-Sanabria, E.; Vlaeminck, S.E.; Boon, N. Growing media constituents determine the microbial nitrogen conversions in organic growing media for horticulture. Microb. Biotechnol. 2016, 9, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, C.C.; Liptzin, D. C: N: P stoichiometry in soil: Is there a “Redfield ratio” for the microbial biomass? Biogeochemistry 2007, 85, 235–252. [Google Scholar] [CrossRef]
- Xu, X.; Thornton, P.E.; Post, W.M. A global analysis of soil microbial biomass carbon, nitrogen and phosphorus in terrestrial ecosystems. Glob. Ecol. Biogeogr. 2013, 22, 737–749. [Google Scholar] [CrossRef]
- Bunt, A.C. Some physical and chemical characteristics of loam-less pot-plant substrates and their relation to plant growth. In Proceedings of the Ist Symposium on Artificial Media in Horticulture, Gent, Belgium, 10 September 1973; Acta Horticulturae. International Society for Horticultural Science (ISHS): Leuven, Belgium, 1973; Volume 37, pp. 1954–1965. [Google Scholar]
- Taylor, A.; Giguere, A.; Zoebelein, C.; Myrold, D.D.; Bottomley, P.J. Modeling of soil nitrification responses to temperature reveals thermodynamic differences between ammonia-oxidizing activity of archaea and bacteria. ISME J. 2017, 11, 896–908. [Google Scholar] [CrossRef]
- Enwezor, W.O. The mineralization of nitrogen and phosphorus in organic materials of varying C: N and C: P ratios. Plant Soil 1976, 44, 237–240. [Google Scholar] [CrossRef]
- Nannipieri, P.; Paul, E.A. The chemical and functional characterization of soil N and its biotic components. Soil Biol. Biochem. 2009, 41, 2357–2369. [Google Scholar] [CrossRef]
- Allison, S.D.; Weintraub, M.N.; Gartner, T.B.; Waldrop, M.P. Evolutionary-economic principles as regulators of soil enzyme production and ecosystem function. In Soil Enzymology, Soil Biology; Shukla, G., Varma, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 229–243. [Google Scholar]
- Brookes, P. The soil microbial biomass: Concept, measurement and applications in soil ecosystem research. Microbes Environ. 2001, 16, 131–140. [Google Scholar] [CrossRef]
- Hamer, U.; Marschner, B. Priming effects of sugars, amino acids, organic acids and catechol on the mineralization of lignin and peat. J. Plant Nutr. Soil Sci. 2002, 165, 261–268. [Google Scholar] [CrossRef]
- Lang, H.J.; Elliott, G.C. Influence of Ammonium: Nitrate Ratio and Nitrogen Concentration on Nitrification Activity in Soilless Potting Media. J. Am. Soc. Hortic. Sci. 1991, 116, 642–645. [Google Scholar] [CrossRef]
- Fisk, L.M.; Maccarone, L.D.; Barton, L.; Murphy, D.V. Nitrapyrin decreased nitrification of nitrogen released from soil organic matter but not amoA gene abundance at high soil temperature. Soil Biol. Biochem. 2015, 88, 214–223. [Google Scholar] [CrossRef]
- Dessureault-Rompré, J.; Zebarth, B.J.; Georgallas, A.; Burton, D.L.; Grant, C.A.; Drury, C.F. Temperature dependence of soil nitrogen mineralization rate: Comparison of mathematical models, reference temperatures and origin of the soils. Geodermatology 2010, 157, 97–108. [Google Scholar] [CrossRef]
- Kirschbaum, M.U.F. The temperature-dependence of soil organic matter decomposition, and the effect of global warming on soil organic C storage. Soil Biol. Biochem. 1995, 27, 753–760. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, C.; He, N.; Wen, X.; Gao, Y.; Li, S.; Niu, S.; Butterbach-Bahl, K.; Luo, Y.; Yu, G. A global synthesis of the rate and temperature sensitivity of soil nitrogen mineralization: Latitudinal patterns and mechanisms. Glob. Chang. Biol. 2017, 23, 455–464. [Google Scholar] [CrossRef]
- Kämäräinen, A.; Simojoki, A.; Lindén, L.; Jokinen, K.; Silvan, N. Physical growing media characteristics of Sphagnum biomass dominated by Sphagnum fuscum (Schimp.) Klinggr. Mires Peat 2018, 21, 1–16. [Google Scholar]
- Tiemann, L.K.; Billings, S.A. Changes in variability of soil moisture alter microbial community C and N resource use. Soil Biol. Biochem. 2011, 43, 1837–1847. [Google Scholar] [CrossRef]
- Harris, R.F. Effect of water potential on microbial growth and activity. In Water Potential Relations in Soil Microbiology; Parr, J.F., Gardner, W.R., Elliott, L.F., Eds.; American Society of Agronomy: Madison, WI, USA, 1981; pp. 23–95. [Google Scholar]
- Devêvre, O.C.; Horwáth, W.R. Decomposition of rice straw and microbial carbon use efficiency under different soil temperatures and moistures. Soil Biol. Biochem. 2000, 32, 1773–1785. [Google Scholar] [CrossRef]
- Linn, D.M.; Doran, J.W. Effect of water-filled pore space on carbon dioxide and nitrous oxide production in tilled and non-tilled soils. Soil Sci. Soc. Am. J. 1984, 48, 1267–1272. [Google Scholar] [CrossRef]
- Robertson, G.P.; Groffman, P.M. Nitrogen transformations. In Soil Microbiology, Ecology, and Biochemistry, 4th ed.; Paul, E.A., Ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 341–364. [Google Scholar]
- Van Gerrewey, T.; Ameloot, N.; Navarrete, O.; Vandecruys, M.; Perneel, M.; Boon, N.; Geelen, D. Microbial activity in peat-reduced plant growing media: Identifying influential growing medium constituents and physicochemical properties using fractional factorial design of experiments. J. Clean. Prod. 2020, 256, 120323. [Google Scholar] [CrossRef]
- Lellei-Kovács, E.; Kovács-Láng, E.; Botta-Dukát, Z.; Kalapos, T.; Emmett, B.; Beier, C. Thresholds and interactive effects of soil moisture on the temperature response of soil respiration. Eur. J. Soil Biol. 2011, 47, 247–255. [Google Scholar] [CrossRef]
- Zhou, W.P.; Shen, W.J.; Li, Y.E.; Hui, D.F. Interactive effects of temperature and moisture on composition of the soil microbial community. Eur. J. Soil Sci. 2017, 68, 909–918. [Google Scholar] [CrossRef]
- Rodrigo, A.; Recous, S.; Neel, C.; Mary, B. Modelling temperature and moisture effects on C–N transformations in soils: Comparison of nine models. Ecol. Model. 1997, 102, 325–339. [Google Scholar] [CrossRef]
- Moyano, F.E.; Vasilyeva, N.; Bouckaert, L.; Cook, F.; Craine, F.; Curiel Yuste, J.; Don, A.; Epron, D.; Formanek, P.; Franzluebbers, A.; et al. The moisture response of soil heterotrophic respiration: Interaction with soil properties. Biogeosciences 2012, 9, 1173–1182. [Google Scholar] [CrossRef]
- Reich, M. Chapter 4-The significance of nutrient interactions for crop yield and nutrient use efficiency. In Plant Macronutrient Use Efficiency; Hossain, M.A., Kamiya, T., Burritt, D.J., Tran, L.-S.P., Fujiwara, T., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 65–82. [Google Scholar]
- Succop, C.E.; Newman, S.E. Organic Fertilization of Fresh Market Sweet Basil in a Greenhouse. Hortic. Technol. 2004, 14, 235–239. [Google Scholar] [CrossRef]
- Bufalo, J.; Cantrell, C.L.; Astatkie, T.; Zheljazkov, V.D.; Gawde, A.; Boaro, C.S.F. Organic versus conventional fertilization effects on sweet basil (Ocimum basilicum L.) growth in a greenhouse system. Ind. Crop. Prod. 2015, 74, 249–254. [Google Scholar] [CrossRef][Green Version]
- Kiferle, C.; Maggini, R.; Pardossi, A. Influence of nitrogen nutrition on growth and accumulation of rosmarinic acid in sweet basil (Ocimum basilicum L.) grown in hydroponic culture. Aust. J. Crop. Sci. 2013, 7, 321–327. [Google Scholar]
- Frerichs, C.; Daum, D.; Pacholski, A.S. Ammonia and Ammonium Exposure of Basil (Ocimum basilicum L.) Growing in an Organically Fertilized Peat Substrate and Strategies to Mitigate Related Harmful Impacts on Plant Growth. Front. Plant Sci. 2020, 10, 1696. [Google Scholar] [CrossRef]




| GM1 | GM2 | GM3 | GM4 | |
|---|---|---|---|---|
| Professional Use | Container/Aromatic and Flowering Plants | Market Garden Plants in Plugs or Trays | Container/Tree and Shrub | Market Garden Plants in Plugs or Trays |
| Particle size distribution | ||||
| Size fraction >4 mm (%) | 6.1 | 4.7 | 43.3 | 5.4 |
| Size fraction 2–4 mm (%) | 10.1 | 12.7 | 11.6 | 9.3 |
| Size fraction <2 mm (%) | 83.8 | 82.6 | 45.1 | 85.3 |
| Bulk density (g·cm−3) 1 | 0.18 | 0.20 | 0.12 | 0.18 |
| Total porosity (v/v) 2 | 89.1 | 88.3 | 92.0 | 88.0 |
| EAW (v/v) 3 | 0.38 | 0.41 | 0.23 | 0.40 |
| AFP (v/v) 4 | 0.17 | 0.03 | 0.47 | 0.04 |
| pH water 5 | 6.8 | 6.7 | 7.3 | 6.5 |
| EC (mS·cm−1) 6 | 0.7 | 0.7 | 0.6 | 0.6 |
| OM (g dw·kg−1) 7 | 687.3 | 690.6 | 908.5 | 712.6 |
| Org N (g dw·kg−1) | 11.1 | 11.1 | 6.9 | 13.1 |
| C:N ratio | 30.9 | 31.0 | 65.9 | 27.2 |
| Total N (g dw·kg−1) 8 | 11.5 | 11.5 | 6.9 | 13.4 |
| AmoA (log nb_seq·g−1) | 8.06 | 7.96 | 6.60 | 8.02 |
| Basal respiration (µg C-CO2·g−1 dw·h−1) 9 | 0.30 ± 0.1 | 0.80 ± 0.32 | 0.85 ± 0.1 | 0.99 ± 0.2 |
| F1 | F2 | |
|---|---|---|
| OM (g·kg−1) | 559.5 | 636.7 |
| C (g·kg−1) | 279.8 | 318.4 |
| Org N (g·kg−1) | 59.5 | 54.1 |
| Total N (g·kg−1) | 67.4 | 55.1 |
| Total P (mg·kg−1) | 35.4 | 18.6 |
| Total K (mg·kg−1) | 52.3 | 40.8 |
| Total Mg (mg·kg−1) | 6.4 | 5.2 |
| Total Mn (mg·kg−1) | 50.8 | 258.8 |
| C:N ratio | 3.8 | 5.1 |
| C:P ratio | 7.9 | 17.1 |
| N:P ratio | 1.9 | 3.0 |
| pH | 6.8 | 6.9 |
| GM1 | GM2 | GM3 | GM4 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F1 | F2 | F1 | F2 | F1 | F2 | ||||||||||
| suction (kPa) | T | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| 4 | 10.7 | 1.2 | 14.7 | 1.3 | 31.7 | 2.7 | 36.5 | 3.3 | 20.3 | 1.4 | 35.0 | 0.5 | 14.5 | 0.8 | 35.0 | 8.2 | |
| −3.2 | 20 | 28.2 | 0.9 | 43.1 | 3.7 | 32.3 | 1.6 | 42.5 | 0.6 | 29.2 | 2.7 | 37.5 | 0.5 | 26.1 | 1.4 | 43.0 | 2.5 |
| 28 | 25.0 | 0.3 | 42.7 | 0.7 | 24.2 | 1.1 | 39.9 | 0.6 | 32.7 | 1.4 | 40.0 | 1.3 | 39.7 | 0.8 | 27.8 | 1.4 | |
| 40 | 30.9 | 1.0 | 28.8 | 0.8 | 17.0 | 1.8 | 29.7 | 0.9 | 22.3 | 1.1 | 31.0 | 0.8 | 38.1 | 1.6 | 45.6 | 1.4 | |
| 4 | 22.3 | 1.8 | 22.6 | 1.9 | 18.5 | 0.8 | 23.1 | 1.4 | 17.1 | 3.5 | 25.2 | 0.7 | 12.7 | 1.6 | 17.0 | 1.2 | |
| −10 | 20 | 44.9 | 0.9 | 55.7 | 1.1 | 56.9 | 1.2 | 57.0 | 0.3 | 36.2 | 0.7 | 39.7 | 1.3 | 46.2 | 1.4 | 56.3 | 0.6 |
| 28 | 45.2 | 0.4 | 56.6 | 0.4 | 52.5 | 0.2 | 68.5 | 0.4 | 32.4 | 2.2 | 51.2 | 0.3 | 44.2 | 2.6 | 64.7 | 0.3 | |
| 40 | 39.1 | 1.6 | 47.1 | 0.8 | 42.5 | 0.4 | 52.7 | 0.3 | 26.8 | 2.0 | 35.4 | 1.0 | 28.3 | 1.0 | 36.2 | 1.4 | |
| 4 | 19.1 | 2.4 | 21.1 | 0.4 | 16.6 | 3.3 | 32.1 | 2.7 | 13.8 | 0.8 | 17.8 | 0.5 | 26.2 | 2.0 | 34.3 | 0.7 | |
| −31.6 | 20 | 34.0 | 1.2 | 45.5 | 2.2 | 40.8 | 1.8 | 48.4 | 1.4 | 29.2 | 2.5 | 35.8 | 4.6 | 69.2 | 0.9 | 71.3 | 0.8 |
| 28 | 50.5 | 1.2 | 57.4 | 1.5 | 29.2 | 1.1 | 35.6 | 1.3 | 36.9 | 4.8 | 43.5 | 0.5 | 59.5 | 1.0 | 61.8 | 0.5 | |
| 40 | 36.3 | 1.8 | 59.8 | 2.0 | 50.7 | 1.3 | 57.5 | 0.7 | 20.3 | 0.9 | 25.3 | 0.7 | 47.4 | 1.8 | 50.7 | 2.4 | |
| −3.2 kPa | −10 kPa | −31.6 kPa | All Suction Treatments | |
|---|---|---|---|---|
| B | 1.00 | 1.20 | 1.16 | 1.35 |
| k | 0.25 | 0.12 | 0.12 | 0.12 |
| RMSE | 0.21 | 0.14 | 0.26 | 0.24 |
| R2 | 0.30 ns | 0.91 *** | 0.70 *** | 0.66 *** |
| GM1 | GM2 | GM3 | GM4 | |
|---|---|---|---|---|
| θ at 0 kPa (v/v) | 0.891 | 0.883 | 0.920 | 0.880 |
| θ at −3.2 kPa (v/v) | 0.515 | 0.608 | 0.364 | 0.667 |
| θ at −10 kPa (v/v) | 0.337 | 0.436 | 0.219 | 0.448 |
| θ at −31.6 kPa (v/v) | 0.215 | 0.233 | 0.135 | 0.271 |
| WFPS −3.2 kPa (%) | 57.8 | 68.9 | 39.6 | 75.8 |
| WFPS −10 kPa (%) | 37.8 | 49.4 | 23.8 | 50.9 |
| WFPS −31.6 kPa (%) | 24.1 | 26.4 | 14.7 | 30.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cannavo, P.; Recous, S.; Valé, M.; Bresch, S.; Paillat, L.; Benbrahim, M.; Guénon, R. Organic Fertilization of Growing Media: Response of N Mineralization to Temperature and Moisture. Horticulturae 2022, 8, 152. https://doi.org/10.3390/horticulturae8020152
Cannavo P, Recous S, Valé M, Bresch S, Paillat L, Benbrahim M, Guénon R. Organic Fertilization of Growing Media: Response of N Mineralization to Temperature and Moisture. Horticulturae. 2022; 8(2):152. https://doi.org/10.3390/horticulturae8020152
Chicago/Turabian StyleCannavo, Patrice, Sylvie Recous, Matthieu Valé, Sophie Bresch, Louise Paillat, Mohammed Benbrahim, and René Guénon. 2022. "Organic Fertilization of Growing Media: Response of N Mineralization to Temperature and Moisture" Horticulturae 8, no. 2: 152. https://doi.org/10.3390/horticulturae8020152
APA StyleCannavo, P., Recous, S., Valé, M., Bresch, S., Paillat, L., Benbrahim, M., & Guénon, R. (2022). Organic Fertilization of Growing Media: Response of N Mineralization to Temperature and Moisture. Horticulturae, 8(2), 152. https://doi.org/10.3390/horticulturae8020152






