Slight Changes in Fruit Firmness at Harvest Determine the Storage Potential of the ‘Rojo Brillante’ Persimmon Treated with Gibberellic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fruit Material
2.2. Determinations
2.3. Statistical Analysis
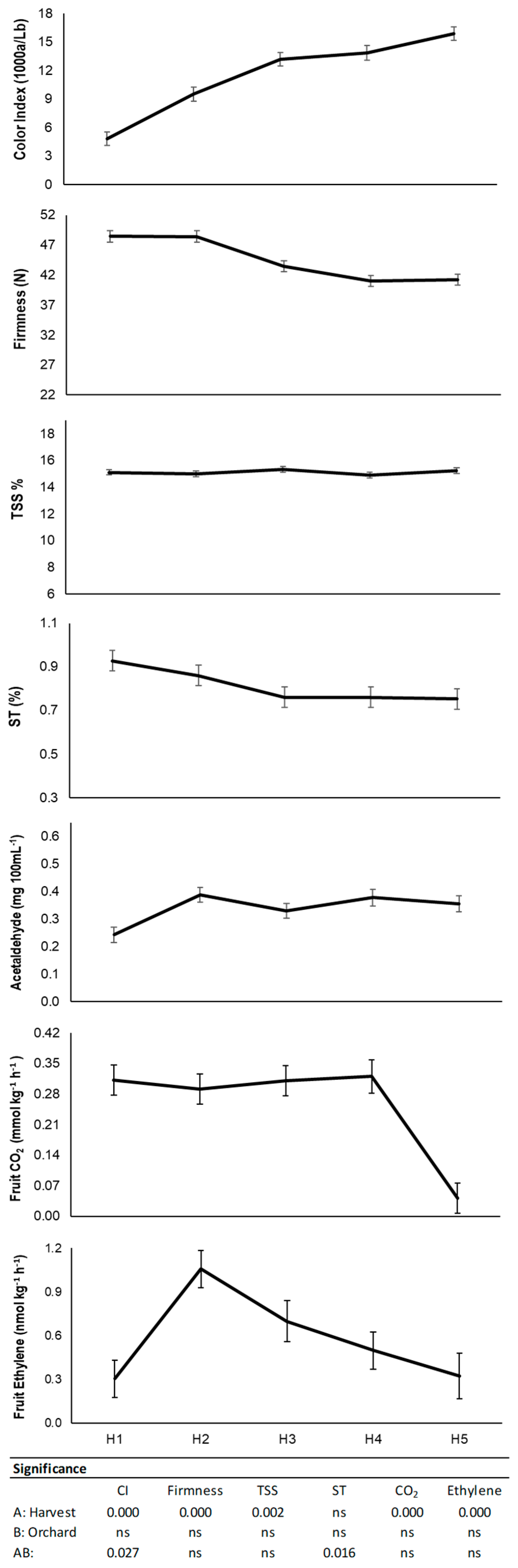
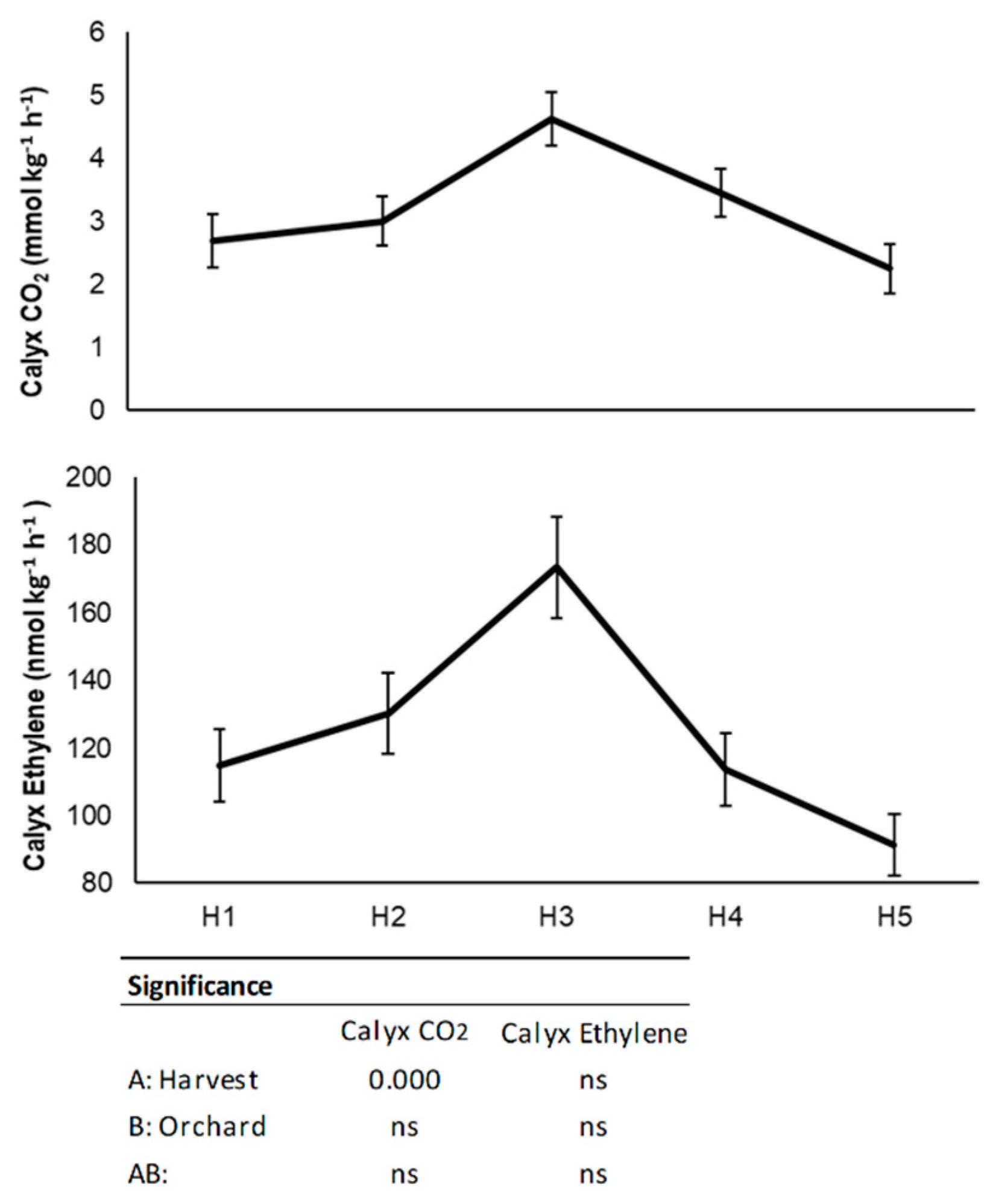
3. Results and Discussion
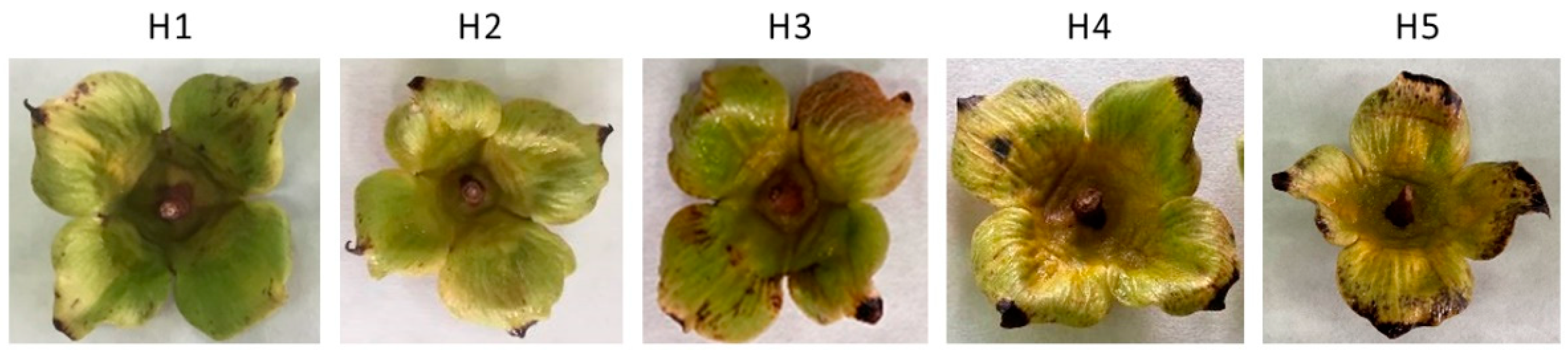
3.1. Physico-Chemical Characterization at Harvest
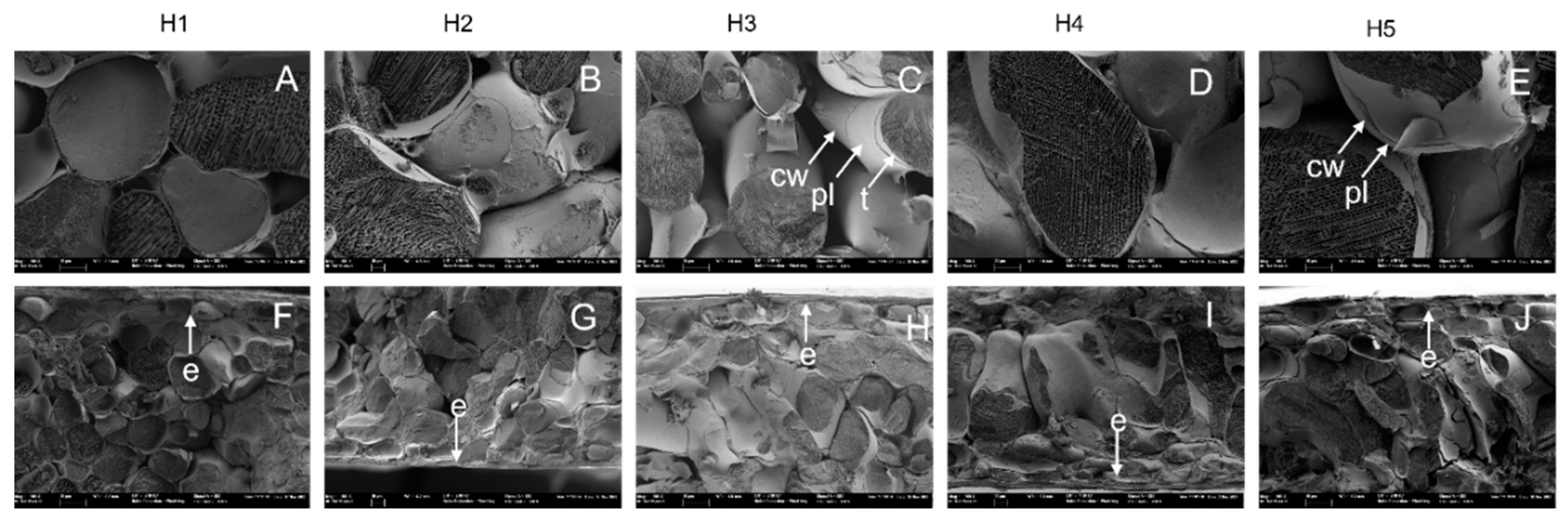
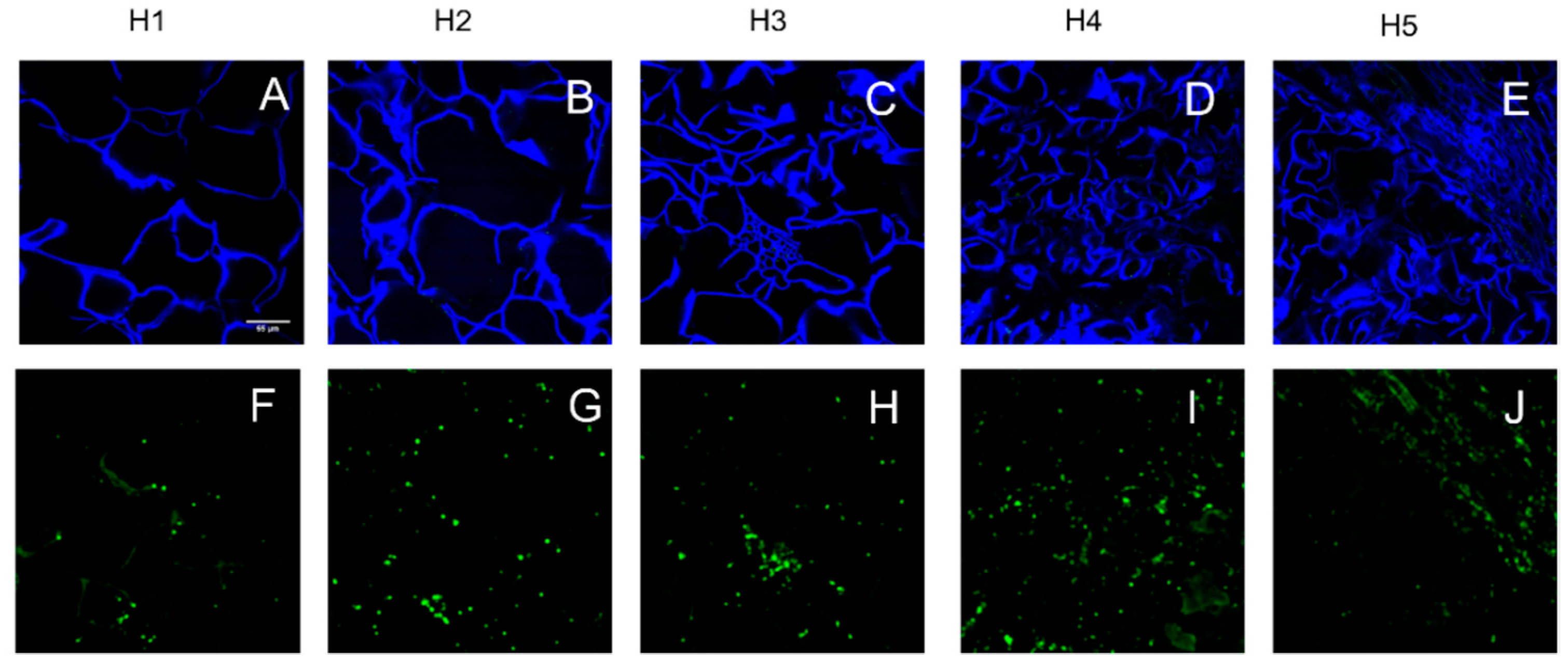
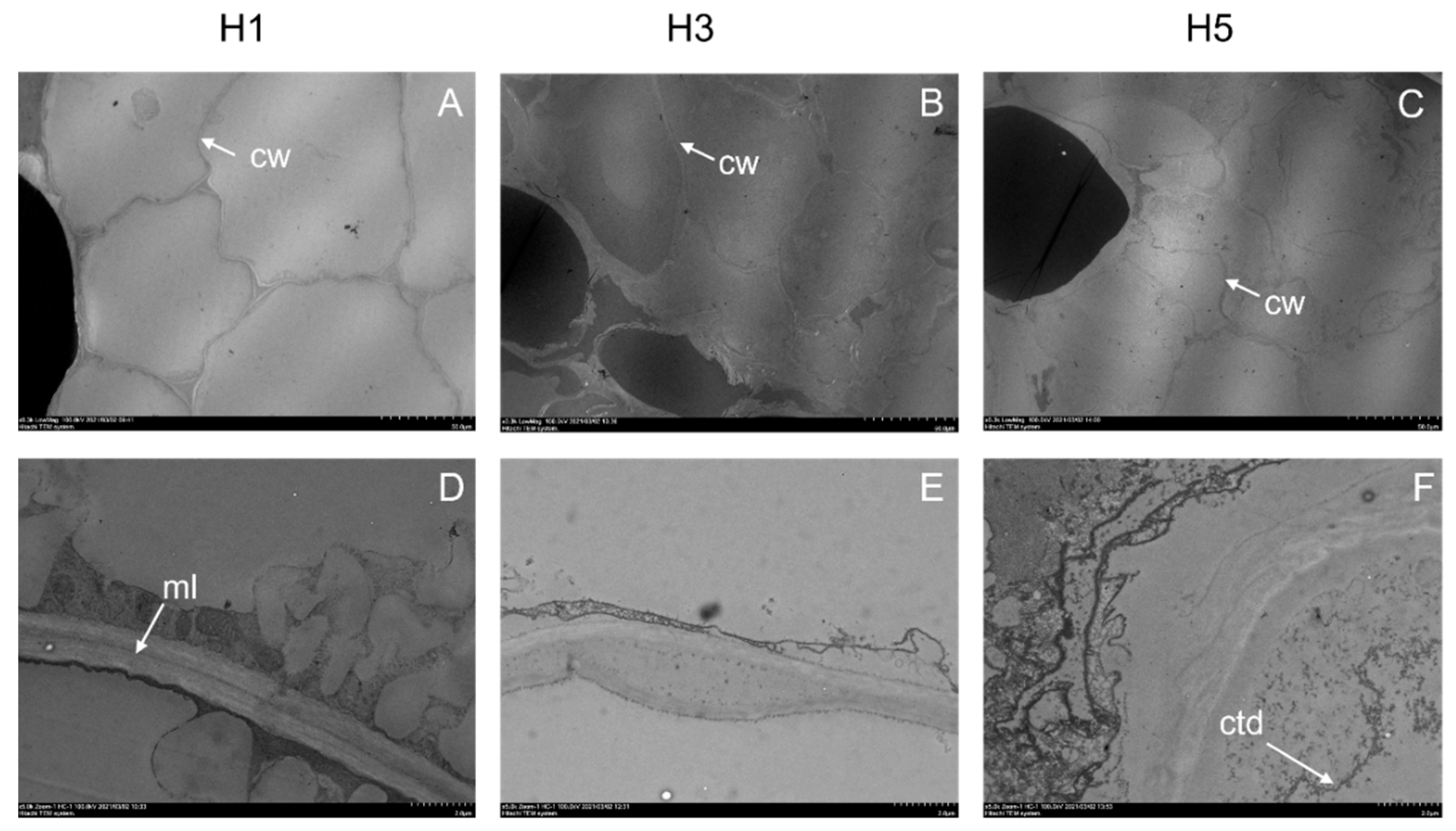
3.2. Parenchyma Structure at Harvest
3.3. Firmness Loss during Cold Storage Depending on Harvest Time
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giordani, E.; Picardi, E.; Radice, S. Morfología y fisiología. In El Cultivo del Caqui; Badenes, M.L., Intrigliolo, D.S., Salvador, A., Vicent, A., Eds.; Generalitat Valenciana: Valencia, Spain, 2015; pp. 17–33. [Google Scholar]
- IndexBox. Global Persimmon Market Overview. 2020. Available online: App.indexbox.io. (accessed on 27 October 2021).
- Novillo, P.; Gil, R.; Besada, C.; Salvador, A. Astringency removal of “Rojo brillante” persimmon by combining CO2 and ethanol application. Acta Hortic. 2015, 1079, 599–604. [Google Scholar] [CrossRef]
- Besada, C.; Arnal, L.; Salvador, A. Improving storability of persimmon cv. Rojo brillante by combined use of preharvest and postharvest treatments. Postharvest. Biol. Technol. 2008, 50, 169–175. [Google Scholar]
- Salvador, A.; Cuquerella, J.; Martínez-Jávega, J.M.; Monterde, A.; Navarro, P. 1-MCP preserves the firmness of stored persimmon “Rojo brillante”. J. Food Sci. 2004, 69, 69–73. [Google Scholar] [CrossRef]
- Salvador, A.; Arnal, L.; Monterde, A.; Martínez-Jávega, J.M. Influence of ripening stage at harvest on chilling injury symptoms of persimmon cv. Rojo Brillante stored at different temperatures. Food Sci. Technol. Int. 2005, 11, 359–365. [Google Scholar] [CrossRef]
- Salvador, A.; Arnal, L.; Besada, C.; Larrea, V.; Quiles, A.; Pérez-Munuera, I. Physiological and structural changes during ripening and deastringency treatment of persimmon fruit cv. “Rojo brillante”. Postharvest. Biol. Technol. 2007, 46, 181–188. [Google Scholar] [CrossRef]
- Testoni, A. Post-harvest and processing of persimmon fruit Testoni. In CIHEAM—Options Méditerranéennes; CIHEAM: Zaragoza, Spain, 2002. [Google Scholar]
- Agustí, M.; Juan, M.; Martínez-Fuentes, A.; Mesejo, C.; Almela, V. Calcium nitrate delays climacteric of persimmon fruit. Ann. Appl. Biol. 2004, 144, 65–69. [Google Scholar] [CrossRef]
- Arnal, L.; Del Río, M.A. Effect of cold storage and removal astringency on quality of persimmon fruit (Diospyros kaki L.) cv. Rojo brillante. Food Sci. Technol. Int. 2004, 10, 179–185. [Google Scholar] [CrossRef]
- Pérez-Munuera, I.; Hernando, I.; Larrea, V.; Besada, C.; Arnal, L.; Salvador, A. Microstructural study of chilling injury alleviation by 1-methylcyclopropene in persimmon. HortScience 2009, 44, 742–745. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Cuesta, J.; Cuquerella, Y.J.M.J. Definition of a color index for citrus fruit degreening. Proc. Int. Soc. Citric. 1981, 2, 773–778. [Google Scholar]
- Taira, S.; Ono, M. Reduction of astringency in persimmon caused by adhesion of tannins to cell wall fragments. In I International Persimmon Symposium 436; ISHS: Chang Mai City, Thailand, 1996; pp. 235–242. [Google Scholar]
- Sugiura, A.; Kataoka, I.; Tomana, T. Use of refractometer to determine soluble solids of astringent fruits of Japanese persimmon (Diospyros kaki L.). J. Hortic. Sci. 1983, 58, 241–246. [Google Scholar] [CrossRef]
- Fathi-Najafabadi, A.; Besada, C.; Gil, R.; Calatayud, M.A.; Salvador, A. Chlorophyll fluorescence imaging as a tool to evaluate calyx senescence during the ripening of persimmon fruit treated with gibberellic acid. Postharvest. Biol. Technol. 2021, 179, 111582. [Google Scholar] [CrossRef]
- Besada, C.; Salvador, A. Postharvest biology and technology of persimmon. In Postharvest Biology and Technology of Temperate Fruits; Mir, S.A., Shah, M.A., Mir, M.M., Eds.; Springer: Cham, Switzerland, 2018; pp. 1–415. [Google Scholar]
- Besada, C.; Gil, R.; Fathi, A.; Navarro, P. Efecto de la temperatura de transporte en la calidad del caqui. Levante Agric. 2017, 3, 277–279. [Google Scholar]
- Salvador, A.; Arnal, L.; Carot, J.M.; Carvalho, C.P.; Jabaloyes, J.M. Influence of different factors on firmness and color evolution during the storage of persimmon cv. “Rojo brillante”. J. Food Sci. 2006, 71, S169–S175. [Google Scholar] [CrossRef]
- Tessmer, M.A.; Besada, C.; Hernando, I.; Appezzato-da-Glória, B.; Quiles, A.; Salvador, A. Microstructural changes while persimmon fruits mature and ripen. Comparison between astringent and non-astringent cultivars. Postharvest. Biol. Technol. 2016, 120, 52–60. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, J.; Mantri, N.; Jiang, L.; Ying, S.; Chen, S.; Feng, X.; Cao, Y.; Chen, Z.; Ren, L.; et al. An effective combination storage technology to prolong storability, preserve high nutrients and antioxidant ability of astringent persimmon. Sci. Hortic. 2018, 241, 304–312. [Google Scholar] [CrossRef]
- Martínez-Las Heras, R.; Amigo-Sánchez, J.C.; Heredia, A.; Castelló, M.L.; Andrés, A. Influence of preharvest treatments to reduce the seasonality of persimmon production on color, texture and antioxidant properties during storage. CYTA-J. Food. 2016, 14, 333–339. [Google Scholar] [CrossRef]
- Novillo, P.; Salvador, A.; Crisosto, C.; Besada, C. Influence of persimmon astringency type on physico-chemical changes from the green stage to commercial harvest. Sci. Hortic. 2016, 206, 7–14. [Google Scholar] [CrossRef]
- Conesa, C.; Laguarda-Miró, N.; Fito, P.; Seguí, L. Evaluation of Persimmon (Diospyros kaki Thunb. cv. Rojo brillante) industrial residue as a source for value added products. Waste Biomass Valorization 2020, 11, 3749–3760. [Google Scholar] [CrossRef]
- Del Bubba, M.; Giordani, E.; Pippucci, L.; Cincinelli, A.; Checchini, L.; Galvan, P. Changes in tannins, ascorbic acid and sugar content in astringent persimmons during on-tree growth and ripening and in response to different postharvest treatments. J. Food Compos. Anal. 2009, 22, 668–677. [Google Scholar] [CrossRef]
- Woolf, A.B.; Ben-Arie, R. Persimmon (Diospyros kaki L.). In Postharvest Biology and Technology of Tropical and Subtropical Fruits; Yahia, E.M., Ed.; Woodhead Publishing Limited: Sawston, Cambridge, UK, 2011; Volume 4, pp. 166–194. [Google Scholar]
- Kitagawa, H.; Glucina, P. Persimmon culture in New Zealand. In DSIR Information Series 159; Science Information Publishing Centre: Wellington, New Zealand, 1984; p. 74. [Google Scholar]
- Besada, C.; Gil, R.; Bonet, L.; Quiñones, A.; Intrigliolo, D.; Salvador, A. Chloride stress triggers maturation and negatively affects the postharvest quality of persimmon fruit. Involvement of calyx ethylene production. Plant Physiol. Biochem. 2016, 100, 105–112. [Google Scholar] [CrossRef]
- Hernández-Carrión, M.; Vázquez-Gutiérrez, J.L.; Hernando, I.; Quiles, A. Impact of high hydrostatic pressure and pasteurization on the structure and the extractability of bioactive compounds of persimmon “Rojo brillante”. J. Food Sci. 2014, 79, C32–C38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vázquez-Gutiérrez, J.L.; Quiles, A.; Hernando, I.; Pérez-Munuera, I. Changes in the microstructure and location of some bioactive compounds in persimmons treated by high hydrostatic pressure. Postharvest Biol. Technol. 2011, 61, 137–144. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilhena, N.Q.; Quiles, A.; Gil, R.; Llorca, E.; Fernández-Serrano, P.; Vendrell, M.; Salvador, A. Slight Changes in Fruit Firmness at Harvest Determine the Storage Potential of the ‘Rojo Brillante’ Persimmon Treated with Gibberellic Acid. Horticulturae 2022, 8, 140. https://doi.org/10.3390/horticulturae8020140
Vilhena NQ, Quiles A, Gil R, Llorca E, Fernández-Serrano P, Vendrell M, Salvador A. Slight Changes in Fruit Firmness at Harvest Determine the Storage Potential of the ‘Rojo Brillante’ Persimmon Treated with Gibberellic Acid. Horticulturae. 2022; 8(2):140. https://doi.org/10.3390/horticulturae8020140
Chicago/Turabian StyleVilhena, Nariane Q., Amparo Quiles, Rebeca Gil, Empar Llorca, Paula Fernández-Serrano, Mario Vendrell, and Alejandra Salvador. 2022. "Slight Changes in Fruit Firmness at Harvest Determine the Storage Potential of the ‘Rojo Brillante’ Persimmon Treated with Gibberellic Acid" Horticulturae 8, no. 2: 140. https://doi.org/10.3390/horticulturae8020140
APA StyleVilhena, N. Q., Quiles, A., Gil, R., Llorca, E., Fernández-Serrano, P., Vendrell, M., & Salvador, A. (2022). Slight Changes in Fruit Firmness at Harvest Determine the Storage Potential of the ‘Rojo Brillante’ Persimmon Treated with Gibberellic Acid. Horticulturae, 8(2), 140. https://doi.org/10.3390/horticulturae8020140





