Abstract
The occurrence of tipburn is a severe problem in horticulture crop production. A previous study suggested that, in lisianthus, tipburn is caused by imbalanced calcium (Ca) distribution. However, there are few studies on the effects of other cations on tipburn incidence in lisianthus cultivars. In this study, to determine the effect of Magnesium (Mg) concentration in nutrient solution on tipburn incidence and Ca and Mg acquisition, three lisianthus cultivars (‘Celeb Wine’: CW, ‘Reina White’: RW, and ‘Voyage Peach’: VP) were cultivated under different Mg concentrations in nutrient solution (12 ppm, 34.7 ppm, and 75.4 ppm). Under high nutritional Mg concentrations, CW and RW had significantly decreased tipburn severity, while VP showed no significant differences in tipburn severity among the treatments. Total Ca concentrations in all cultivars significantly increased at higher nutritional Mg concentrations, which indicated that Mg application in lisianthus cultivars promoted Ca acquisition. Furthermore, it was suggested that CW and RW had the ability to distribute increased Mg to the tip of the upper leaves, thereby promoting Ca distribution. Thus, a decrease in tipburn severity in CW and RW at higher nutritional Mg concentrations appeared to occur.
1. Introduction
The occurrence of calcium (Ca) deficiency disorder is a severe problem in horticulture crop production. Ca (calcium) deficiency disorder occurs even when plants are grown under sufficient Ca conditions. It is affected by several biotic (e.g., plant growth rate [,,], Ca acquisition competence [], and Ca distribution [,,]) and abiotic factors (e.g., humidity [,], nutrient conditions [,], temperature [,], and light []). The mechanism of Ca deficiency disorder is complex and involves multiple factors []. Thus, to clarify the cause of the Ca deficiency disorder, we should carefully demonstrate the effects of each factor on the occurrence of Ca deficiency disorder.
Lisianthus (Eustoma grandiflorum (Raf.) Shinn.) is a species in the family Gentianaceae. In Japan, its cultivars are one of the most famous cut flowers, and their wholesale value ranked fifth among cut flowers in 2017. In addition, lisianthus cultivars produced worldwide are bred by Japanese seed companies. However, rosetting [], blasting (flower abortion) [], and tipburn are considerable problems in their production.
Tipburn is a Ca deficiency disorder at the tip of new leaves. For lisianthus cultivar production in Japan, tipburn predominantly occurs in summer and causes shipment delay, plant quality deterioration, and economic losses. Kuronuma et al. [,] demonstrated that there are varietal differences in tipburn incidence and severity among the cultivars, with little relevance to varietal differences in plant growth rate and Ca acquisition competence. In contrast, tipburn cultivars with high damage tended to accumulate excessive Ca in their roots, resulting in Ca deficiency in new leaves [,,]. Accordingly, it was suggested that tipburn in lisianthus cultivars was primarily caused by the imbalanced Ca distribution. Furthermore, the effects of humidity conditions [] and nutritional Ca concentration [,] on tipburn incidence in lisianthus cultivars were demonstrated. However, there are few studies on the effects of nutritional composition, particularly major cations, except Ca, on tipburn incidence in lisianthus cultivars.
Boron (B), potassium (K), and magnesium (Mg) are key cations that affect the occurrence of tipburn. In Chinese cabbage (Brassica rapa ssp. pekinensis Rupr.), lettuce (Lactuca sativa L.), and strawberry (Fragaria ananassa Duchesne), B deficiency in new leaves significantly affects tipburn incidence [,,]. In contrast, high K and Mg contents in nutrient solutions are considered to inhibit Ca absorption in plants [,,]. We conducted a preliminary test to demonstrate the effects of B, K, and Mg contents on tipburn incidence in lisianthus cultivars. Several lisianthus cultivars have been cultivated under different B, K, and Mg concentrations in nutrient solutions. Interestingly, only the effect of differences in Mg concentration in the nutrient solution on tipburn incidence was observed. Therefore, we focused on effect of Mg and built the following hypothesis. Mg concentrations in nutrient solutions influence tipburn incidence and Ca acquisition in lisianthus cultivars.
Mg is an important nutrient for plant growth because it influences various metabolic processes and reactions (e.g., photophosphorylation, photosynthetic carbon dioxide fixation, protein synthesis, and chlorophyll formation) [,]. In general, Mg deficiency symptoms appear as yellowing leaves.
In this study, to elucidate the effect of Mg concentration in nutrient solution on tipburn incidence and Ca and Mg acquisition, three lisianthus cultivars (‘Celeb Wine’: CW, ‘Reina White’: RW, and ‘Voyage Peach’: VP) were cultivated under different Mg concentrations in nutrient solution. The incidence of tipburn and Ca and Mg concentrations in each organ of the cultivars were investigated.
2. Materials and Methods
2.1. Plant Material
CW (Celeb Wine), RW (Reina White), and VP (Voyage Peach) were used in this study. CW and VP are cultivars highly damaged by tipburn, and there have been no significant differences in tipburn incidence between low and high Ca concentrations in nutrient solution [,]. RW is a tipburn medium-damaged cultivar that has significantly mitigated tipburn incidence and severity under high Ca concentrations in nutrient solution []. The seedling method was the same as in the previous report [,,,,].
2.2. Treatments
We established three treatments with different Mg concentrations (control, Mg+, and Mg++). Nutrient salts used in the control treatment (Cont.) were shown in Table 1. They were dissolved by distilled water. In addition to these salts, MgCl2·6H2O was added at concentrations of 4.3 and 8.6 g/ 20 L in the Mg+ and Mg++ treatments, respectively. The theoretical Mg2+ and chlorine ion (Cl−) concentrations of the nutrient solution were as follows: Cont., 12 ppm (0.49 mM) Mg2+ and Cl− is not included; Mg+ treatment, 34.7 ppm (1.43 mM) Mg2+ and 75 ppm (2.11 mM) Cl−; and Mg++ treatment, 75.4 ppm (3.10 mM) Mg2+ and 150 ppm (4.22 mM) Cl−.

Table 1.
Nutrient salts used in the control treatment (Cont.).
The plants were maintained in a phytotron (Table 2). The irrigation system of each treatment supplied the nutrient solution for 30 min by bottom watering each morning. To adopt randomized block design, the plants in each treatment group were randomly divided into three plots.

Table 2.
Environmental conditions of the phytotron used in this study.
2.3. Sampling
At the beginning of this experiment (0 weeks), we randomly sampled ten plants of each cultivar. Subsequently, to collect data before the onset of tipburn, 10 plants were sampled in each treatment at 4 weeks. At 8 weeks, 12 plants were harvested for each treatment. We washed the harvested plants with distilled water. Then, they were immediately divided into roots and shoots. Moreover, the top (first) and lower leaves (4weeks: fourth and 8 weeks: seventh) on the main stem were picked up and analyzed.
2.4. Tipburn Severity and Incidence
We calculated the tipburn severity and incidence using the same method and formula as in our previous reports [,,,,].
2.5. Mesurments of Ca and Mg Concentrations
The harvested plants were separated by organ and dried at 70 °C for 72 h. Leaves picked up as top and lower leaves were distinguished between leaf tip and the remainder (leaf base). We used an atomic absorption spectrophotometer (Z-5300; Hitachi, Ltd., Tokyo, Japan) to measure Ca and Mg concentrations. The total (whole-plant) Ca and Mg concentrations were calculated by dividing the whole-plant Ca and Mg contents by the total dry weight.
2.6. Statistical Analysis
We used SPSS (version 22.0; IBM Corp. Japan, Tokyo, Japan) for data analyses. ANOVA (one-way analysis of variance) was conducted (p < 0.05). Homoscedasticity was assessed using Levene’s test. We conducted a correlation analysis to determine the effects of Mg concentration on Ca acquisition and occurrence of tipburn.
3. Results and Discussion
3.1. Tipburn Severity and Incidence and Plant Growth
For CW (Celeb Wine), tipburn severity significantly decreased with increasing nutritional Mg concentrations; however, there were no differences in tipburn incidence among the treatment groups (Figure 1). RW (Reina White) exhibited a significant decrease in tipburn severity and incidence at higher Mg concentrations. In contrast, for VP (Voyage Peach), both tipburn severity and incidence did not differ significantly under different treatments.
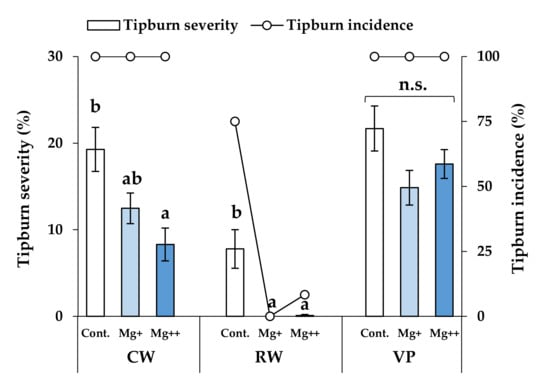
Figure 1.
Tipburn severity and incidence in each cultivar and each treatment at 8 weeks. Data are means ± SE. Significant differences among means are indicated by different letters. n.s. represents no significant differences among the treatments. Cultivars: Celeb Wine (CW), Reina White (RW), and Voyage Peach (VP).
At the start of the experiment, the total dry weight of RW (14.0 ± 1.0 mg) was significantly greater than that of VP (10.1 ± 1.0 mg). CW (13.0 ± 0.8 mg) was not significantly different from RW and VP. There were no significant differences in the total Mg (CW: 3.7 ± 0.4 mg/g, RW: 3.8 ± 0.2 mg/g, and VP: 4.0 ± 0.3 mg/g) and Ca concentrations (CW: 2.2 ± 0.2 mg/g, RW: 2.5 ± 0.1 mg/g, and VP: 2.5 ± 0.2 mg/g) among the cultivars.
At 4 weeks, all cultivars had no significant differences in total dry weight under different treatments (Table 3). Furthermore, at the end of the experiment (8 weeks), none of the cultivars showed a decreasing tendency in total dry weights depending on nutritional Mg concentrations (Table 3).

Table 3.
Total dry weight in each cultivar and treatment at 4 weeks and 8 weeks.
In general, high plant growth rates cause tipburn [,,]. However, our results showed that Mg application can mitigate tipburn severity and incidence in several lisianthus cultivars without decreasing plant growth rates. Chen et al. demonstrated that lisianthus grew well even under Mg concentrations of zero in nutrient solution []; however, Mg application might contribute to preventing tipburn, increasing and promoting plant quality in lisianthus production.
For CW, applying more Mg than the amount applied in the Mg++ treatment may mitigate the tipburn disorder because tipburn severity in the Mg++ treatment was the lowest among the treatments. In contrast, for RW and VP, it was suggested that Mg concentration in Mg+ treatment was suitable for tipburn mitigation and plant growth, because in the Mg+ treatment, they exhibited the lowest tipburn severity and highest plant dry weight among the treatments.
3.2. Mg and Ca Acquisition
At the end of the experiment (8 weeks), the total Mg concentration in CW in the Mg++ treatment group was significantly higher than that in the other treatment groups (Figure 2A). For RW, the total Mg concentrations in the Mg+ and Mg++ treatment groups were significantly higher than those in the control group. Mg concentration in the Mg+ treatment group in VP was significantly higher than that in the other treatment groups. Moreover, for CW and RW, increments in total Mg concentration were observed from 4 weeks to the end of the experiment (8 weeks; Figure 2A). These results indicated that CW and RW tended to acquire more Mg at higher nutritional Mg concentrations.
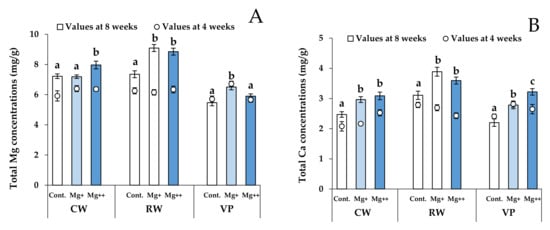
Figure 2.
Total Mg (A) and Ca concentrations (B) in each cultivar and each treatment at 4 (scatter plots) and 8 weeks (bar graph). Data are means ± SE. Significant differences among means are indicated by different letters. Cultivars: Celeb Wine (CW), Reina White (RW), and Voyage Peach (VP).
The total Ca concentrations in all cultivars are shown in Figure 2B. For CW and VP, the total Ca concentrations increased with increasing nutritional Mg concentrations (Figure 2B). Likewise, for RW, the total Ca concentrations in the Mg+ and Mg++ treatment groups were significantly higher than those in the control group. Total Ca concentrations in Mg+ and Mg++ treatment groups for all cultivars clearly increased from 4 weeks to the end of the experiment (8 weeks), except for the value of Mg+ treatment for VP (Figure 2B).
Antagonism between Mg and Ca is well documented, and high nutritional Mg concentrations generally disturb the acquisition of Ca in horticulture crops [,,]. However, a significant positive correlation between mean values of total Mg and Ca concentrations for all cultivars was observed (r = 0.776, p < 0.05). Our findings suggest that Mg application in lisianthus cultivars promotes Ca acquisition, leading to mitigation of tipburn incidence and severity. Certain researchers have demonstrated that an increase in nutritional Mg concentrations within a reasonable range increases chlorophyll content [] and promotes photosynthetic rates []. In addition, appropriate Mg application conditions increased yield and Ca acquirement in tomatoes (Solanum lycopersicum L.) []. Therefore, it is likely that the Mg applied in this study was within the range suitable for lisianthus growth.
In contrast, although VP acquired more Ca with increasing nutritional Mg concentrations, tipburn incidence and severity did not significantly decrease. This inconsistency should be discussed once the results of Mg and Ca distributions are revealed.
3.3. Mg and Ca Distribution
Mg and Ca concentrations in each organ (leaf, stem, and root) of all cultivars are shown in Figure 3 and Figure 4. For all cultivars, Mg concentrations in leaves and Ca concentrations in roots were the highest in all organs.
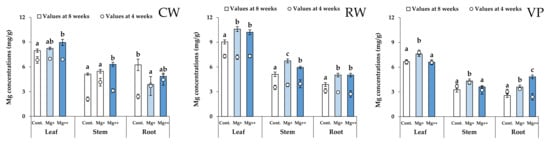
Figure 3.
Mg concentrations of leaf, stem, and root in each treatment group and each cultivar at 4 (scatter plots) and 8 weeks (bar graph). Data are means ± SE. Significant differences among means are indicated by different letters. Cultivars: Celeb Wine (CW), Reina White (RW), and Voyage Peach (VP).
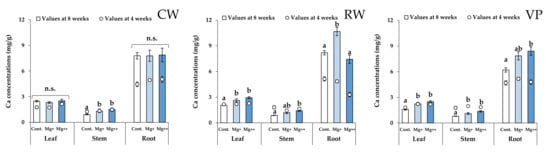
Figure 4.
Ca concentrations of leaf, stem, and root in each treatment group and each cultivar at 4 (scatter plots) and 8 weeks (bar graph). Data are means ± SE. Significant differences among means are indicated by different letters. n.s. represents no significant differences among the treatment groups. Cultivars: Celeb Wine (CW), Reina White (RW), and Voyage Peach (VP).
Mg and Ca concentrations in leaves (tip and base of lower and upper leaves) in all cultivars are shown in Figure 5 and Figure 6. These results were arranged for each cultivar, and the effects of Mg application on Ca distribution and occurrence of tipburn are discussed below.

Figure 5.
Mg concentrations in the base and tip of the lower and upper leaves in each treatment group and each cultivar at 4 (scatter plots) and 8 weeks (bar graph). Data are means ± SE. Significant differences among means are indicated by different letters. n.s. represents no significant differences among the treatment groups. Cultivars: Celeb Wine (CW), Reina White (RW), and Voyage Peach (VP).
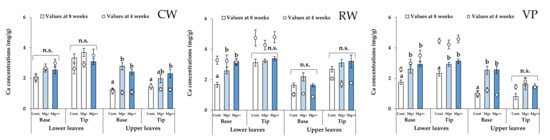
Figure 6.
Ca concentrations in the base and tip of the lower and upper leaves in each treatment group and each cultivar at 4 (scatter plots) and 8 weeks (bar graph). Data are means ± SE. Significant differences among means are indicated by different letters. n.s. represents no significant differences among the treatment groups. Cultivars: Celeb Wine (CW), Reina White (RW), and Voyage Peach (VP).
3.3.1. Celeb Wine (CW)
Mg concentrations in leaves and stems of CW increased with increasing nutritional Mg concentrations (Figure 3). In contrast, there were no significant differences in Ca concentrations in leaves among the treatment groups (Figure 4). These results indicate that increasing the Mg concentration in whole leaves has little effect on the increment of Ca concentration in whole leaves for CW.
Mg concentrations in the tip of the lower leaves and base of the upper leaves significantly increased at higher nutritional Mg concentrations for CW (Figure 5). Although Mg concentrations in the tips of upper leaves did not differ significantly among the treatment groups, their increasing tendency depended on the nutritional Mg concentrations. In addition, Mg concentrations in the upper leaves of the Mg+ and Mg++ treatment groups clearly increased from 4 weeks to the end of the experiment (8 weeks). Ca (calcium) concentrations in the upper leaves of CW significantly increased with increasing nutritional Mg concentrations (Figure 6), while there were no significant differences in Ca concentrations in the lower leaves among the treatment groups.
These results suggest that CW distributes more Mg for upper leaves under high Mg application conditions, promoting Ca distribution in the upper leaves. Therefore, a decrease in tipburn severity in CW at higher nutritional Mg concentrations appeared to occur. CW is a cultivar highly damaged by tipburn even under high nutritional Ca concentrations []. Mg application in CW can be an effective approach to mitigate the occurrence of tipburn.
3.3.2. Reina White (RW)
For the RW, Mg concentrations of each organ (leaf, stem, and root) in the Mg+ and Mg++ treatment groups were significantly higher than those in the control group (Figure 3). Likewise, Ca concentrations in the leaves and stems significantly increased with increasing nutritional Mg concentrations (Figure 4). In the lower leaves, Mg and Ca concentrations in the base significantly increased with increasing nutritional Mg concentrations (Figure 5 and Figure 6). In the upper leaves, although there were no significant differences in Mg and Ca concentrations among the treatment groups, these values in the Mg+ and Mg++ treatment groups clearly increased from 4 weeks to the end of the experiment (8 weeks), as well as in CW. In addition, Ca concentrations in the tips of upper leaves in RW were higher than those in the other cultivars.
Our results indicated that Mg application in RW increased both the whole leaf Mg and Ca concentrations. In addition, as with CW, RW likely distributes more Mg for upper leaves under high Mg application conditions, promoting Ca distribution in the upper leaves and mitigating tipburn severity and incidence.
3.3.3. Voyage Peach (VP)
For VP, only Mg concentrations in the roots significantly increased with increasing nutritional Mg concentrations (Figure 3). In contrast, Ca concentrations in each organ (leaf, stem, and root) significantly increased at higher nutritional Mg concentrations (Figure 4).
In the lower leaves, there were no significant differences in Mg concentrations at the base and tip among the treatment groups (Figure 5). Although Ca concentrations in the base and tip of the lower leaves increased with increasing nutritional Mg concentrations at 8 weeks, these Ca concentrations were clearly lower than those at 4 weeks (Figure 6).
In the upper leaves, Mg and Ca concentrations in the base increased depending on the nutritional Mg concentrations (Figure 5). However, Mg and Ca concentrations in the tip of upper leaves in VP were not significantly different among the treatment groups and were lower than those of the other cultivars. Moreover, these values at 8 weeks showed little difference from the values at 4 weeks.
These results indicated that Mg application for VP did not increase the Mg concentrations in whole leaves and tips of upper leaves, while Mg concentration in roots increased. Thus, no increment of Ca concentrations in the tip of the upper leaves and no decrease in tipburn severity and incidence were observed. In a previous study, excessive Ca accumulation in the roots was observed in VP under high nutritional Ca concentrations. Similarly, VP accumulated excessive Mg in the roots. It was suggested that the imbalanced Mg and Ca distribution caused the occurrence of tipburn in VP.
3.4. Relevance of Mg and Ca Concentrations in Same Organ
For mean values in all cultivars and treatment groups, we conducted a correlation analysis to elucidate the relevance of Mg and Ca concentrations in the same organ. From this analysis, significant positive correlations were observed in the tip and base of upper leaves (Mg × Ca concentration at the tip of upper leaves: r = 0.941, P < 0.001; Mg × Ca concentrations in base of upper leaves: r = 0.910, P < 0.01). No significant correlation was observed in the other organs (Mg × Ca concentrations in whole leaves: r = 0.628, P > 0.05; in stem: r = 0.472, P > 0.05; in root: r = 0.446, P > 0.05; in tip of lower leaves: r = 0.100, P > 0.05; and base of lower leaves: r = 0.580, P > 0.05). In addition, Mg and Ca concentrations in the tips of upper leaves were significantly negatively correlated with tipburn severity (tipburn severity × Mg concentration in tip of upper leaves: r = −0.930, P < 0.001; tipburn severity × Ca concentration in tip of upper leaves: r = −0.985, P < 0.001) and tipburn incidence (tipburn incidence × Mg concentration in tip of upper leaves: r = −0.738, P < 0.05; tipburn incidence × Ca concentration in tip of upper leaves: r = −0.821, P < 0.01).
These findings indicated that the ability of the plant to distribute Mg to the tips of upper leaves under sufficient Mg conditions was considerably important for distributing Ca to the tips of upper leaves and mitigating the occurrence of tipburn in lisianthus cultivars.
4. Conclusions
Although antagonism between Mg and Ca has been well documented in horticulture crops, this study indicated that Mg application in lisianthus cultivars promotes Ca acquisition. Moreover, it was suggested that the cultivars had the ability to distribute increased Mg to the tip of the upper leaf cloud distributed Ca for the same organ, leading to mitigation of tipburn incidence and severity. In this study, tipburn severity significantly decreased with increasing nutritional Mg concentrations in two (CW and RW) of the three cultivars. Thus, Mg application in lisianthus cultivars is an effective method for tipburn mitigation.
Author Contributions
Conceptualization, T.K. and H.W.; methodology, T.K. and K.I.; validation, T.K., H.W. and K.I.; formal analysis, T.K. and K.I.; investigation, K.I.; resources, T.K. and H.W.; writing—original draft preparation, T.K.; writing—review and editing, H.W. and K.I.; visualization, T.K.; supervision, H.W.; project administration, T.K.; funding acquisition, T.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by JSPS KAKENHI, Grant Number JP19K15830.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cox, E.F.; McKee, J.M.T.; Dearman, A.S. The effect of growth rate on tipburn occurrence in lettuce. J. Hortic. Sci. 1976, 51, 297–309. [Google Scholar] [CrossRef]
- Collier, G.F.; Huntington, V.C. The relationship between leaf growth, calcium accumulation and distribution, and tipburn development in field-grown butterhead lettuce. Sci. Hortic. 1983, 21, 123–128. [Google Scholar] [CrossRef]
- Saure, M.C. Causes of the tipburn disorder in leaves of vegetables. Sci. Hortic. 1998, 76, 131–147. [Google Scholar] [CrossRef]
- Kuronuma, T.; Watanabe, Y.; Ando, M.; Watanabe, H. Relevance of tipburn incidence to the competence for Ca acquirement and Ca distributivity in lisianthus [Eustoma grandiflorum (Raf.) Shinn.] cultivars. Sci. Hortic. 2019, 246, 805–811. [Google Scholar] [CrossRef]
- Wiebe, H.J.; Schätzler, H.P.; Kühn, W. On the movement and distribution of calcium in white cabbage in dependence of the water status. Plant Soil 1977, 48, 409–416. [Google Scholar] [CrossRef]
- Barta, D.J.; Tibbitts, T.W. Calcium localization and tipburn development in lettuce leaves during early enlargement. J. Am. Soc. Hortic. Sci. 2020, 125, 294–298. [Google Scholar] [CrossRef]
- Olle, M.; Bender, I. Causes and control of calcium deficiency disorders in vegetables: A review. J. Hortic. Sci. Biotech. 2009, 84, 577–584. [Google Scholar] [CrossRef]
- Mason, G.F.; Guttridge, C.G. The influence of relative humidity and nutrition on leaf tipburn of strawberry. Sci. Hortic. 1975, 3, 339–349. [Google Scholar] [CrossRef]
- Kuronuma, T.; Watanabe, Y.; Ando, M.; Watanabe, H. Tipburn severity and calcium distribution in lisianthus (Eustoma grandiflorum (Raf.) Shinn.) cultivars under different relative air humidity conditions. Agronomy 2018, 8, 218. [Google Scholar] [CrossRef]
- Bautista, A.S.; López-Galarza, S.; Martínez, A.; Pascual, B.; Maroto, J.V. Influence of cation proportions of the nutrient solution on tipburn incidence in strawberry plants. J. Plant Nutr. 2009, 32, 1527–1539. [Google Scholar] [CrossRef]
- Kuronuma, T.; Ando, M.; Watanabe, H. Tipburn Incidence and Ca acquisition and distribution in lisianthus (Eustoma grandiflorum (Raf.) Shinn.) cultivars under different Ca concentrations in nutrient solution. Agronomy 2020, 10, 216. [Google Scholar] [CrossRef]
- Lee, J.G.; Choi, C.S.; Jang, Y.A.; Jang, S.W.; Lee, S.G.; Um, Y.C. Effects of air temperature and air flow rate control on the tipburn occurrence of leaf lettuce in a closed-type plant factory system. Hortic. Environ. Biotech. 2013, 54, 303–310. [Google Scholar] [CrossRef]
- Holmes, S.C.; Wells, D.E.; Pickens, J.M.; Kemble, J.M. Selection of heat tolerant lettuce (Lactuca sativa L.) cultivars grown in deep water culture and their marketability. Horticulturae 2019, 5, 50. [Google Scholar] [CrossRef]
- Sago, Y. Effects of light intensity and growth rate on tipburn development and leaf calcium concentration in butterhead lettuce. HortScience 2016, 51, 1087–1091. [Google Scholar] [CrossRef]
- Collier, G.F.; Tibbitts, T.W. Tipburn of Lettuce. Hortic. Rev. 1982, 4, 49–67. [Google Scholar] [CrossRef]
- Ohkawa, K.; Kano, A.; Kanematsu, K.; Korenaga, M. Effects of air temperature and time on rosette formation in seedlings of Eustoma grandiflorum (Raf.) Shinn. Sci. Hortic. 1991, 48, 171–176. [Google Scholar] [CrossRef]
- Kawakatsu, K.; Ushio, A.; Fukuta, N. Anatomical Characterization of Flower-bud Blasting and Suppression Following Hormone Application in Eustoma grandiflorum (Raf.) Shinn. J. Jap. Soc. Hortic. Sci. 2012, 81, 101–108. [Google Scholar] [CrossRef][Green Version]
- Kuronuma, T.; Kinoshita, N.; Ando, M.; Watanabe, H. Difference of Ca distribution before and after the onset of tipburn in lisianthus [Eustoma grandiflorum (Raf.) Shinn.] cultivars. Sci. Hortic. 2020, 261, 108911. [Google Scholar] [CrossRef]
- Kuronuma, T.; Saotome, M.; Ando, M.; Watanabe, H. Excessive Calcium Accumulation in the Roots Is a Key Factor in Tipburn Incidence under High Ca Supply in Lisianthus (Eustoma grandiflorum) Cultivars. Agronomy 2020, 10, 1123. [Google Scholar] [CrossRef]
- Kuronuma, T.; Watanabe, H. Search for Candidate Genes Causing the Excessive Ca Accumulation in Roots of Tipburn-Damaged Lisianthus (Eustoma grandiflorum) Cultivars. Agriculture 2021, 11, 254. [Google Scholar] [CrossRef]
- Kuo, C.G.; Tsay, J.S.; Tsai, C.L.; Chen, R.J. Tipburn of Chinese cabbage in relation to calcium nutrition and distribution. Sci. Hortic. 1981, 14, 131–138. [Google Scholar] [CrossRef]
- Crisp, P.; Collier, G.F.; Thomas, T.H. The effect of boron on tipburn and auxin activity in lettuce. Sci. Hortic. 1976, 5, 215–226. [Google Scholar] [CrossRef]
- Mason, G.F.; Guttridge, C.G. The role of calcium, boron and some divalent ions in leaf tipburn of strawberry. Sci. Hortic. 1974, 2, 299–308. [Google Scholar] [CrossRef]
- Fageria, V.D. Nutrient interactions in crop plants. J. Plant Nutr. 2001, 24, 1269–1290. [Google Scholar] [CrossRef]
- Huber, D.M.; Jones, J.B. The role of magnesium in plant disease. Plant Soil 2013, 368, 73–85. [Google Scholar] [CrossRef]
- Cakmak, I.; Yazici, A.M. Magnesium: A forgotten element in crop production. Better Crops 2010, 94, 23–25. [Google Scholar]
- Guo, W.; Nazim, H.; Liang, Z.; Yang, D. Magnesium deficiency in plants: An urgent problem. Crop J. 2016, 4, 83–91. [Google Scholar] [CrossRef]
- Chen, C.T.; Lee, C.L.; Yeh, D.M. Effects of nitrogen, phosphorus, potassium, calcium, or magnesium deficiency on growth and photosynthesis of Eustoma. HortScience 2018, 53, 795–798. [Google Scholar] [CrossRef]
- Xue, X.; Wu, X.; Luo, X.; Wang, W.; Wang, D.; Zhang, Y.; Zhao, C. Effects of Potassium and Magnesium Deficiency on Leaf Physiological Characteristics and Chloroplast Ultrastructure of Anther Culture Seedling of Rubber Tree (Hevea brasiliensis). Chine. J. Trop. Crops 2019, 40, 1507. [Google Scholar] [CrossRef]
- Tatagiba, S.D.; DaMatta, F.M.; Rodrigues, F.A. Magnesium decreases leaf scald symptoms on rice leaves and preserves their photosynthetic performance. Plant Physiol. Biochem. 2016, 108, 49–56. [Google Scholar] [CrossRef]
- Yan, B.; Sun, Y.Y.; Wei, Y. Potassium–calcium antagonistic interaction under tomato magnesium deficiency and magnesium fertiliser regulation in solar greenhouse. Qual. Assur. Saf. Crops Foods 2020, 12, 76–86. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).