Axillary Bud Fate Shapes Plant Architecture in Horticultural Crops
Abstract
1. Introduction
2. Plant Architecture in Selected Crop Species
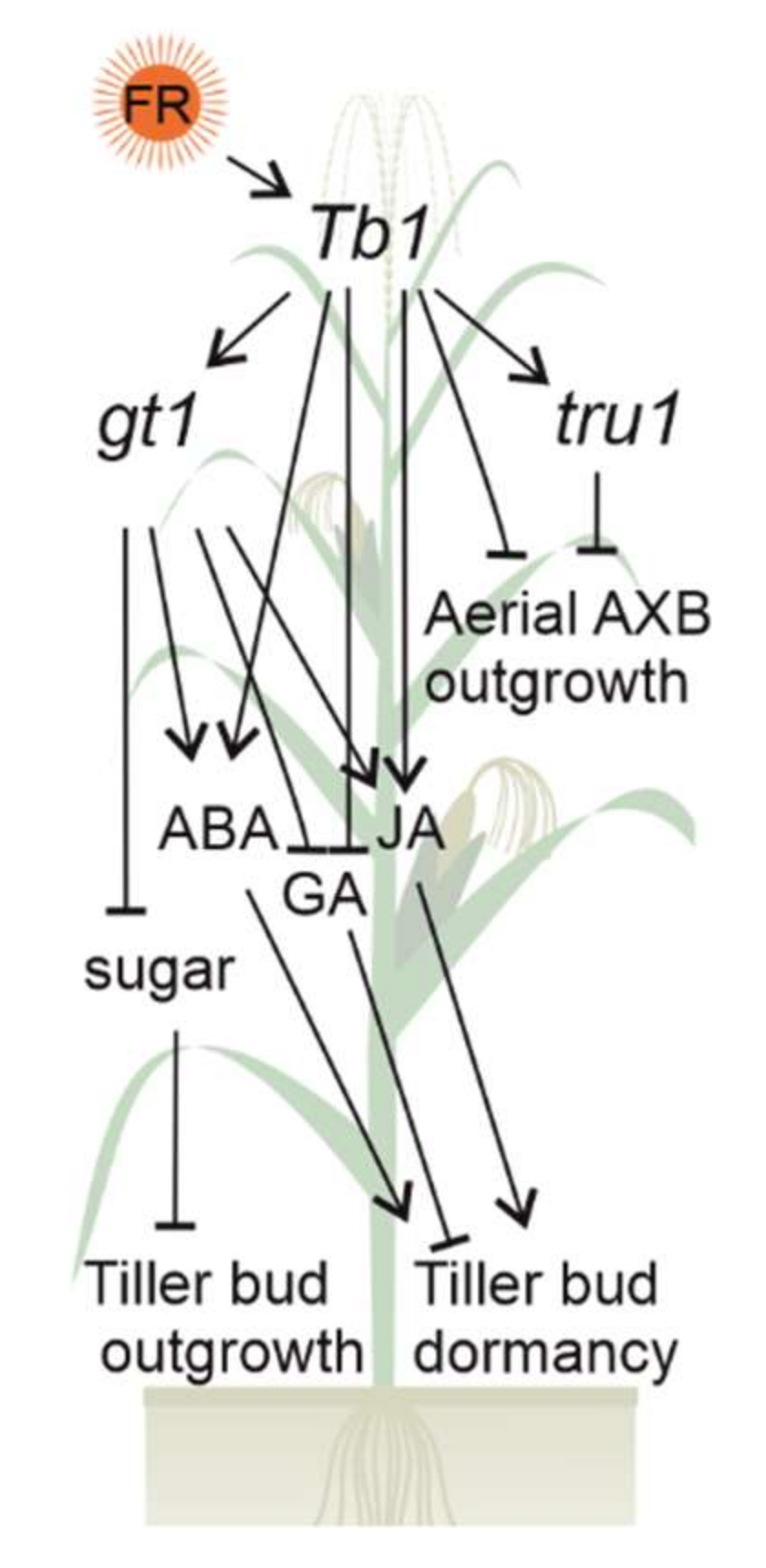
2.1. Maize
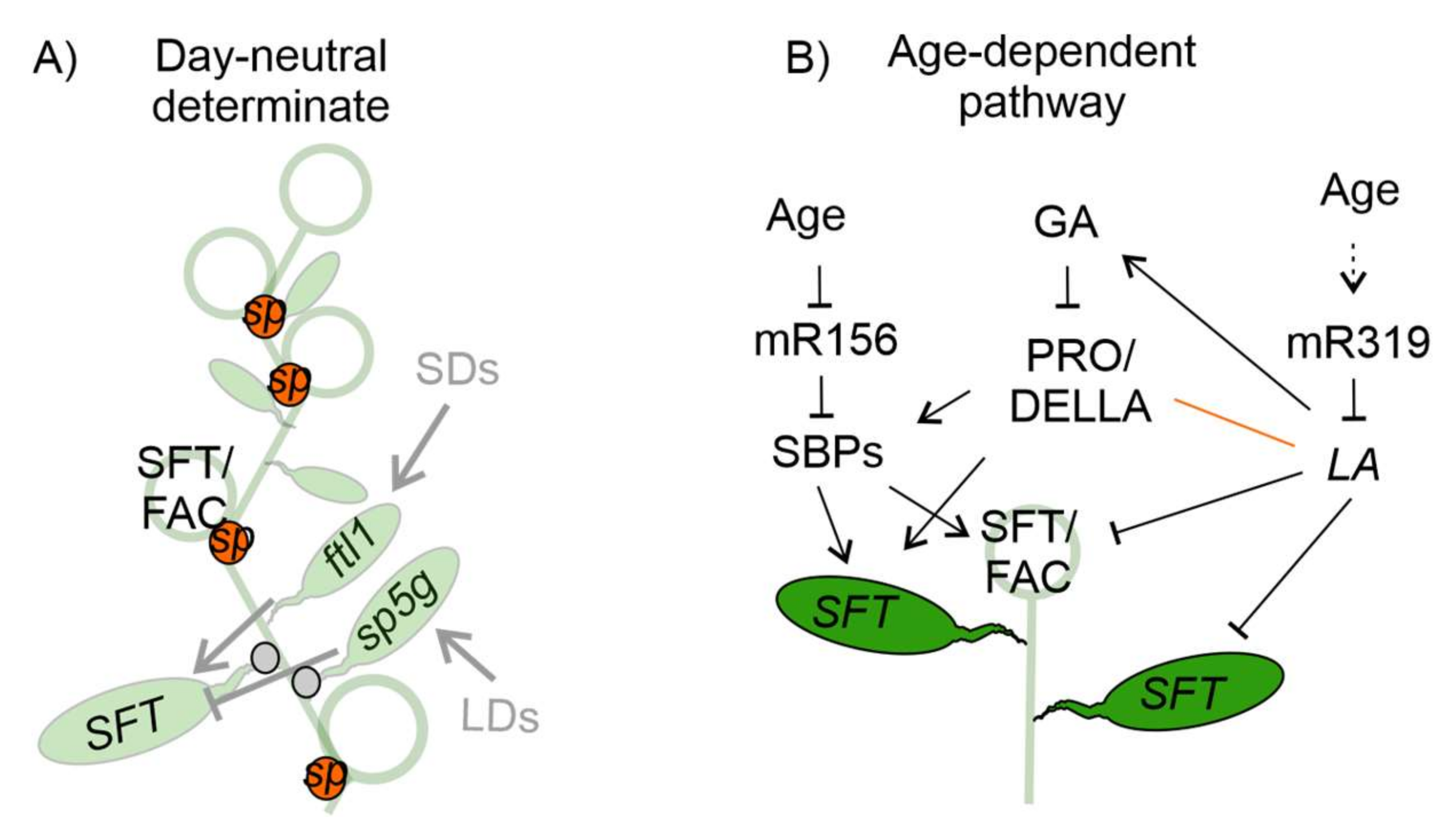
2.2. Tomato
2.3. Apple
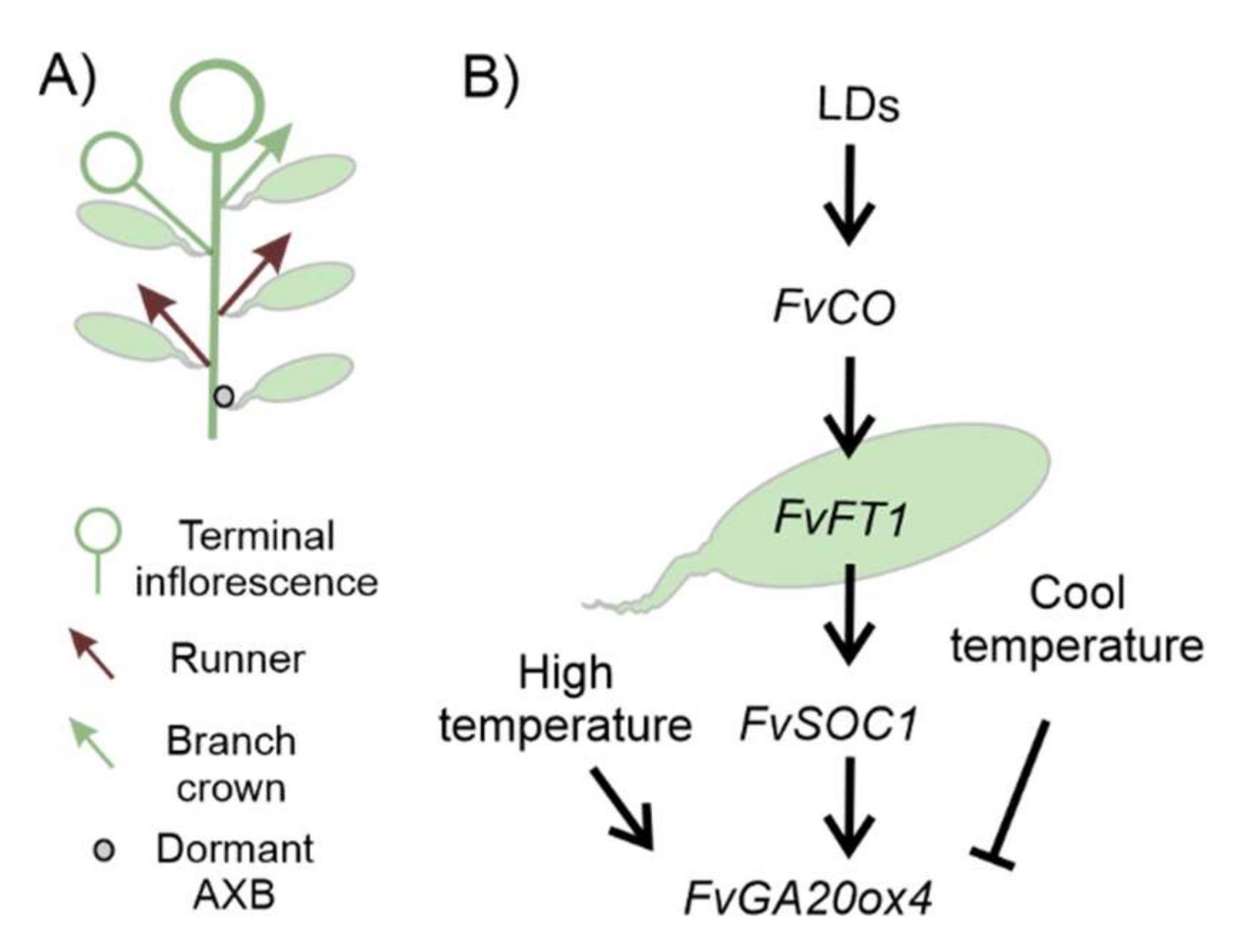
2.4. Strawberry
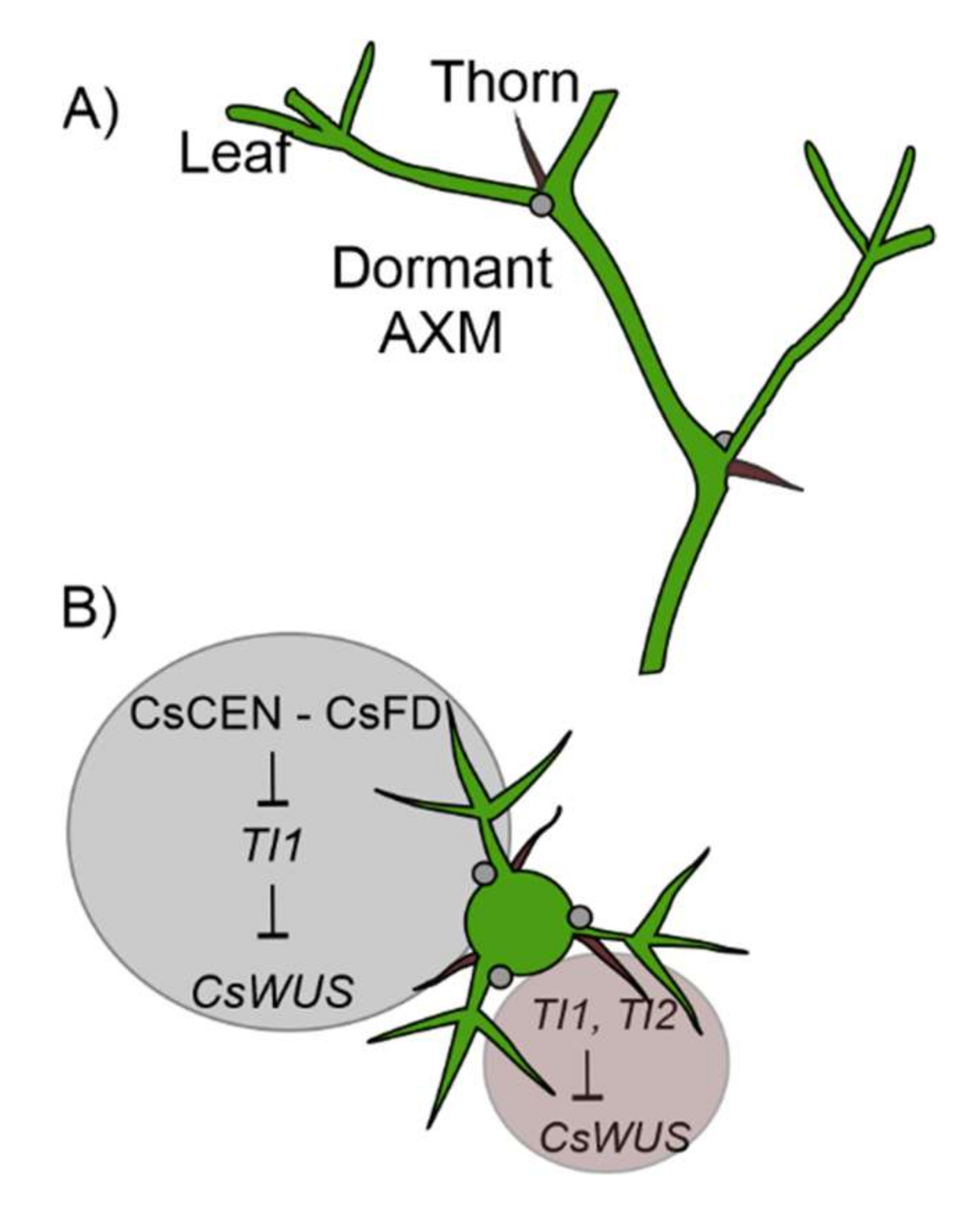
2.5. Citrus
3. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Khush, G.S. Green revolution: The way forward. Nat. Rev. 2001, 2, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Vernoux, T.; Besnard, F.; Godin, C. What shoots can teach about theories of plant form. Nat. Plants 2021, 7, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiao, Y. Axillary meristem initiation—A way to branch out. Curr. Opin. Plant Biol. 2018, 41, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Leyser, O. The control of shoot branching: An example of plant information processing. Plant Cell Environ. 2009, 32, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Iltis, H.H. From teosinte to maize: The catastrophic sexual transmutation. Science 1983, 222, 886–894. [Google Scholar] [CrossRef]
- Doebley, J.; Stec, A.; Hubbard, L. The evolution of apical dominance in maize. Nature 1997, 386, 485–488. [Google Scholar] [CrossRef]
- Doebley, J.; Stec, A. Genetic analysis of the morphological differences between maize and teosinte. Genetic 1991, 129, 285–295. [Google Scholar] [CrossRef]
- Doebley, J.S.; Stec, A.; Gustus, C. Teosinte branched1 and the origin of maize: Evidence for epistasis and the evolution of dominance. Genetics 1995, 141, 333–346. [Google Scholar] [CrossRef]
- Burnham, C. Teosinte branched. Maize Genet. Coop. News Lett. 1959, 33, 74. [Google Scholar]
- Hubbard, L.; McSteen, P.; Doebley, J.; Hake, S. Expression patterns and mutant phenotype of teosinte branched1 correlate with growth suppression in maize and teosinte. Genetics 2002, 162, 1927–1935. [Google Scholar] [CrossRef]
- Cubas, P.; Lauter, N.; Doebley, J.; Coen, E. The TCP domain: A motif found in proteins regulating plant growth and development. Plant J. 1999, 18, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.M.; Wagler, T.N.; Quijada, P.; Doebley, J. A distant upstream enhancer at the maize domestication gene tb1 has pleiotropic effects on plant and inflorescent architecture. Nat. Genet. 2006, 38, 594–597. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, J.; Yan, J.; Song, R. Two transposable element insertions are causative mutations for the major domestication gene teosinte branched 1 in modern maize. Cell Res. 2011, 21, 1267–1270. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, W.F. Maize developmental genetics: Genes of morphogenesis. Annu. Rev. Genet. 1988, 22, 353–385. [Google Scholar] [CrossRef] [PubMed]
- Shaver, D.L. Perennial Maize. J. Hered. 1967, 58, 271–273. [Google Scholar] [CrossRef]
- Whipple, C.J.; Kebrom, T.H.; Weber, A.L.; Yang, F.; Hall, D.; Meeley, R.; Schmidt, R.; Doebley, J.; Brutnell, T.P.; Jackson, D.P. grassy tillers1 promotes apical dominance in maize and responds to shade signals in the grasses. Proc. Natl. Acad. Sci. USA 2011, 108, E506–E512. [Google Scholar] [CrossRef]
- Kebrom, T.H.; Burson, B.L.; Finlayson, S.A. Phytochrome B Represses Teosinte Branched1 Expression and Induces Sorghum Axillary Bud Outgrowth in Response to Light Signals. Plant Physiol. 2006, 140, 1109–1117. [Google Scholar] [CrossRef]
- Dong, Z.; Xiao, Y.; Govindarajulu, R.; Feil, R.; Siddoway, M.L.; Nielsen, T.; Lunn, J.E.; Hawkins, J.; Whipple, C.; Chuck, G. The regulatory landscape of a core maize domestication module controlling bud dormancy and growth repression. Nat. Commun. 2019, 10, 3810. [Google Scholar] [CrossRef]
- Dong, Z.; Li, W.; Unger-Wallace, E.; Yang, J.; Vollbrecht, E.; Chuck, G. Ideal crop plant architecture is mediated by tassels replace upper ears1, a BTB/POZ ankyrin repeat gene directly targeted by TEOSINTE BRANCHED1. Proc. Natl. Acad. Sci. USA 2017, 114, E8656–E8664. [Google Scholar] [CrossRef]
- Studer, A.J.; Wang, H.; Doebley, J.F. Selection During Maize Domestication Targeted a Gene Network Controlling Plant and Inflorescence Architecture. Genetic 2017, 207, 755–765. [Google Scholar] [CrossRef]
- Svitashev, S.; Schwartz, C.; Lenderts, B.; Young, J.K.; Cigan, A.M. Genome editing in maize directed by CRISPR–Cas9 ribonucleoprotein complexes. Nat. Commun. 2016, 7, 13274. [Google Scholar] [CrossRef]
- Lifschitz, E.; Eshed, Y. Universal florigenic signals triggered by FT homologues regulate growth and flowering cycles in perennial day-neutral tomato. J. Exp. Bot. 2006, 57, 3405–3414. [Google Scholar] [CrossRef]
- Sawhney, V.K.; Greyson, R.I. On the initiation of the inflorescence and floral organs in tomato (Lycopersicon esculentum). Can. J. Bot. 1972, 50, 1493–1495. [Google Scholar] [CrossRef]
- Yeager, A.F. Determinate growth in the tomato. J. Hered. 1927, 18, 263–265. [Google Scholar] [CrossRef]
- Silva, G.F.F.; Silva, E.M.; Correa, J.P.O.; Vicente, M.H.; Jiang, N.; Notini, M.M.; Junior, A.C.; De Jesus, F.A.; Castilho, P.; Carrera, E.; et al. Tomato floral induction and flower development are orchestrated by the interplay between gibberellin and two unrelated microRNA-controlled modules. New Phytol. 2019, 221, 1328–1344. [Google Scholar] [CrossRef] [PubMed]
- Pnueli, L.; Carmel-Goren, L.; Hareven, D.; Gutfinger, T.; Alvarez, J.; Ganal, M.; Zamir, D.; Lifschitz, E. The SELF-PRUNING gene of tomato regulates vegetative to reproductive switching of sympodial meristems and is the ortholog of CEN and TFL1. Development 1998, 125, 1979–1989. [Google Scholar] [CrossRef]
- Thouet, J.; Quinet, M.; Ormenese, S.; Kinet, J.M.; Périlleux, C. Revisiting the involvement of SELF-PRUNING in the sympodial growth of tomato. Plant Physiol. 2008, 148, 61–64. [Google Scholar] [CrossRef]
- Molinero-Rosales, N.; Latorre, A.; Jamilena, M.; Lozano, R. SINGLE FLOWER TRUSS regulates the transition and maintenance of flowering in tomato. Planta 2004, 218, 427–434. [Google Scholar] [CrossRef]
- Lifschitz, E.; Ayre, B.G.; Eshed, Y. Florigen and anti-florigen—A systemic mechanism for coordinating growth and termination in flowering plants. Front. Plant Sci. 2014, 5, 465. [Google Scholar] [CrossRef]
- Pnueli, L.; Gutfinger, T.; Hareven, D.; Ben-Naim, O.; Ron, N.; Adir, N.; Lifschitz, E. Tomato SP-Interacting Proteins Define a Conserved Signaling System That Regulates Shoot Architecture and Flowering. Plant Cell 2001, 13, 2687. [Google Scholar] [CrossRef]
- Soyk, S.; Müller, N.A.; Park, S.J.; Schmalenbach, I.; Jiang, K.; Hayama, R.; Zhang, L.; van Eck, J.; Jiménez-Gómez, J.M.; Lippman, Z.B. Variation in the flowering gene SELF PRUNING 5G promotes day-neutrality and early yield in tomato. Nat. Genet. 2016, 49, 162–168. [Google Scholar] [CrossRef]
- Song, J.; Zhang, S.; Wang, X.; Sun, S.; Liu, Z.; Wang, K.; Wan, H.; Zhou, G.; Li, R.; Yu, H.; et al. Variations in Both FTL1 and SP5G, Two Tomato FT Paralogs, Control Day-Neutral Flowering. Mol. Plant 2020, 13, 939–942. [Google Scholar] [CrossRef] [PubMed]
- Parapunova, V.; Busscher, M.; Busscher-Lange, J.; Lammers, M.; Karlova, R.; Bovy, A.G.; Angenent, G.C.; de Maagd, R.A. Identification, cloning and characterization of the tomato TCP transcription factor family. BMC Plant Biol. 2014, 14, 157. [Google Scholar] [CrossRef] [PubMed]
- Yanai, O.; Shani, E.; Russ, D.; Ori, N. Gibberellin partly mediates LANCEOLATE activity in tomato. Plant J. 2011, 68, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Shalit, A.; Rozman, A.; Goldshmidt, A.; Alvarez, J.P.; Bowman, J.L.; Eshed, Y.; Lifschitz, E. The flowering hormone florigen functions as a general systemic regulator of growth and termination. Proc. Natl. Acad. Sci. USA 2009, 106, 8392–8397. [Google Scholar] [CrossRef]
- Krieger, U.; Lippman, Z.B.; Zamir, D. The flowering gene SINGLE FLOWER TRUSS drives heterosis for yield in tomato. Nat. Genet. 2010, 42, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Liberatore, K.; Park, S.J.; Alvarez, J.P.; Lippman, Z.B. Tomato Yield Heterosis Is Triggered by a Dosage Sensitivity of the Florigen Pathway That Fine-Tunes Shoot Architecture. PLoS Genet. 2013, 9, e1004043. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yang, X.; Yu, Y.; Si, X.; Zhai, X.; Zhang, H.; Dong, W.; Gao, C.; Xu, C. Domestication of wild tomato is accelerated by genome editing. Nat. Biotechnol. 2018, 36, 1160–1163. [Google Scholar] [CrossRef]
- Groot, S.P.; Keizer, L.C.; de Ruiter, W.; Dons, J.J. Seed and fruit set of the lateral suppressor mutant of tomato. Sci. Hortic. 1994, 59, 157–162. [Google Scholar] [CrossRef]
- Zsögön, A.; Cermak, T.; Voytas, D.; Peres, L.E.P. Genome editing as a tool to achieve the crop ideotype and de novo domestication of wild relatives: Case study in tomato. Plant Sci. 2017, 256, 120–130. [Google Scholar] [CrossRef]
- Potter, D.; Eriksson, T.; Evans, R.C.; Oh, S.; Smedmark, J.; Morgan, D.R.; Kerr, M.; Robertson, K.R.; Arsenault, M.; Dickinson, T.A.; et al. Phylogeny and classification of Rosaceae. Oesterreichische Bot. Z. 2007, 266, 5–43. [Google Scholar] [CrossRef]
- Costes, E.; Crespel, L.; Denoyes, B.; Morel, P.; Demene, M.-N.; Lauri, P.-É. Bud structure, position and fate generate various branching patterns along shoots of closely related Rosaceae species: A review. Front. Plant Sci. 2014, 5, 666. [Google Scholar] [CrossRef] [PubMed]
- Costes, E.; Guedon, Y. Modelling branching patterns on 1-year-old trunks of six apple cultivars. Ann. Bot. 2002, 89, 513–524. [Google Scholar] [CrossRef]
- Kervella, J.; Pagès, L.; Génard, M. Growth Context and Fate of Axillary Meristems of Young Peach Trees. Influence of Parent Shoot Growth Characteristics and of Emergence Date. Ann. Bot. 1995, 76, 559–567. [Google Scholar] [CrossRef]
- Foster, T.; Johnston, R.; Seleznyova, A. A Morphological and Quantitative Characterization of Early Floral Development in Apple (Malus x domestica Borkh.). Ann. Bot. 2003, 92, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Wilkie, J.D.; Sedgley, M.; Olesen, T. Regulation of floral initiation in horticultural trees. J. Exp. Bot. 2008, 59, 3215–3228. [Google Scholar] [CrossRef]
- Barthélémy, D.; Caraglio, Y. Plant Architecture: A Dynamic, Multilevel and Comprehensive Approach to Plant Form, Structure and Ontogeny. Ann. Bot. 2007, 99, 375–407. [Google Scholar] [CrossRef]
- DeJong, T.; Negron, C.; Favreau, R.; Day, K.; Costes, E.; Lopez, G.; Guedon, Y. Using concepts of shoot growth and architecture to understand and predict responses of peach trees to pruning. Acta Hortic. 2012, 962, 225–232. [Google Scholar] [CrossRef]
- Costes, E.; Guédon, Y. Deciphering the ontogeny of a sympodial tree. Trees 2011, 26, 865–879. [Google Scholar] [CrossRef]
- Fortanier, E.J.; Jonkers, H. Juvenility and maturity of plants as influenced by their ontogenetical and physiological ageing. Acta Hortic. 1976, 56, 37–44. [Google Scholar] [CrossRef]
- Segura, V.; Cilas, C.; Costes, E. Dissecting apple tree architecture into genetic, ontogenetic and environmental effects mixed linear model. New Phytol. 2008, 178, 302–314. [Google Scholar] [CrossRef]
- Fyhrie, K.; Llinàs, M.T.P.; López, G.; DeJong, T. How does peach fruit set on sylleptic shoots borne on epicormics compare with fruit set on proleptic shoots? Eur. J. Hortic. Sci. 2018, 83, 3–11. [Google Scholar] [CrossRef]
- Prats-Llinàs, M.T.; López, G.; Fyhrie, K.; Pallas, B.; Guédon, Y.; Costes, E.; DeJong, T.M. Long proleptic and sylleptic shoots in peach (Prunus persica L. Batsch) trees have similar, predetermined, maximum numbers of nodes and bud fate patterns. Ann. Bot. 2019, 123, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Cornille, A.; Antolín, F.; Garcia, E.; Vernesi, C.; Fietta, A.; Brinkkemper, O.; Kirleis, W.; Schlumbaum, A.; Roldán-Ruiz, I. A Multifaceted Overview of Apple Tree Domestication. Trends Plant Sci. 2019, 24, 770–782. [Google Scholar] [CrossRef] [PubMed]
- Webster, A. Vigour mechanisms in dwarfing rootstocks for temperate FRUIT trees. Acta Hortic. 2004, 658, 29–41. [Google Scholar] [CrossRef]
- Zhou, Y.; Tian, X.; Yao, J.; Zhang, Z.; Wang, Y.; Zhang, X.; Li, W.; Wu, T.; Han, Z.; Xu, X.; et al. Morphological and photosynthetic responses differ among eight apple scion-rootstock combinations. Sci. Hortic. 2020, 261, 108981. [Google Scholar] [CrossRef]
- Roberts, L.W. The initiation of Xylem differentiation. Bot. Rev. 1969, 35, 201–250. [Google Scholar] [CrossRef]
- Saeed, M.; Dodd, P.B.; Sohail, L. Anatomical studies of stems, roots and leaves of selected citrus rootstock varieties in relation to their varieties in relation to their vigour. J. Hortic. For. 2010, 2, 87–94. [Google Scholar]
- Song, C.; Zhang, D.; Zhang, J.; Zheng, L.; Zhao, C.; Ma, J.; An, N.; Han, M. Expression analysis of key auxin synthesis, transport, and metabolism genes in different young dwarfing apple trees. Acta Physiol. Plant. 2016, 38, 43. [Google Scholar] [CrossRef]
- Kamboj, J.S.; Browning, G.; Quinlan, J.D.; Blake, P.S.; Baker, D.A. Polar transport of [3H]-IAA in apical shoot segments of different apple rootstocks. J. Hortic. Sci. 1997, 72, 773–780. [Google Scholar] [CrossRef]
- Li, H.L.; Zhang, H.; Yu, C.; Ma, L.; Wang, Y.; Zhang, X.Z.; Han, Z.H. Possible roles of auxin and zeatin for initiating the dwarfing effect of M9 used as apple rootstock or interstock. Acta Physiol. Plant. 2011, 34, 235–244. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, X.; Wu, T.; Xu, X.; Han, Z.; Wang, Y. Methylation effect on IPT5b gene expression determines cytokinin biosynthesis in apple rootstock. Biochem. Biophys. Res. Commun. 2017, 482, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Kamboj, J.S.; Quinlan, J.D. The apple rootstock and its influence on endogenous hormones. Acta Hortic. 1998, 463, 143–152. [Google Scholar] [CrossRef]
- Tworkoski, T.; Fazio, G. Effects of Size-Controlling Apple Rootstocks on Growth, Abscisic Acid, and Hydraulic Conductivity of Scion of Different Vigor. Int. J. Fruit Sci. 2015, 15, 369–381. [Google Scholar] [CrossRef]
- McKim, S.M. Moving on up—Controlling internode growth. New Phytol. 2020, 226, 672–678. [Google Scholar] [CrossRef]
- Bulley, S.M.; Wilson, F.M.; Hedden, P.; Phillips, A.L.; Croker, S.J.; James, D.J. Modification of gibberellin biosynthesis in the grafted apple scion allows control of tree height independent of the rootstock. Plant Biotechnol. J. 2005, 3, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Van Hooijdonk, B.; Woolley, D.; Warrington, I.; Tustin, S. Rootstocks Modify Scion Architecture, Endogenous Hormones, and Root Growth of Newly Grafted ‘Royal Gala’ Apple. J. Am. Soc. Hortic. Sci. 2011, 136, 93–102. [Google Scholar] [CrossRef]
- Dong, Y.; Ye, X.; Xiong, A.; Zhu, N.; Jiang, L.; Qu, S. The regulatory role of gibberellin related genes DKGA2ox1 and MIR171f_3 in persimmon dwarfism. Plant Sci. 2021, 310, 110958. [Google Scholar] [CrossRef]
- El-Sharkawy, I.; El Kayal, W.; Prasath, D.; Fernández, H.; Bouzayen, M.; Svircev, A.M.; Jayasankar, S. Identification and genetic characterization of a gibberellin 2-oxidase gene that controls tree stature and reproductive growth in plum. J. Exp. Bot. 2011, 63, 1225–1239. [Google Scholar] [CrossRef]
- Harrison, N.; Harrison, R.J.; Barber-Perez, N.; Cascant-Lopez, E.; Cobo-Medina, M.; Lipska, M.; Conde-Ruíz, R.; Brain, P.; Gregory, P.J.; Fernandez-Fernandez, F. A new three-locus model for rootstock-induced dwarfing in apple revealed by genetic mapping of root bark percentage. J. Exp. Bot. 2016, 67, 1871–1881. [Google Scholar] [CrossRef]
- Pilcher, R.R.; Celton, J.M.; Gardiner, S.E.; Tustin, D.S. Genetic Markers Linked to the Dwarfing Trait of Apple Rootstock ‘Malling 9′. J. Am. Soc. Hortic. Sci. 2008, 133, 100–106. [Google Scholar] [CrossRef]
- Kelsey, D.F.; Brown, S.K. ‘McIntosh Wijcik’: A columnar mutation of ‘McIntosh’ Apple proving useful in physiology and breeding research. Fruit Var. J. 1992, 46, 83–87. [Google Scholar]
- Otto, D.; Petersen, R.; Brauksiepe, B.; Braun, P.; Schmidt, E.R. The columnar mutation (“Co gene”) of apple (Malus × domestica) is associated with an integration of a Gypsy-like retrotransposon. Mol. Breed. 2013, 33, 863–880. [Google Scholar] [CrossRef]
- Okada, K.; Wada, M.; Moriya, S.; Katayose, Y.; Fujisawa, H.; Wu, J.; Kanamori, H.; Kurita, K.; Sasaki, H.; Fujii, H.; et al. Expression of a putative dioxygenase gene adjacent to an insertion mutation is involved in the short internodes of columnar apples (Malus x domestica). J. Plant Res. 2016, 129, 1109–1126. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.; Djozgic, H.; Rieger, B.; Rapp, S.; Schmidt, E.R. Columnar apple primary roots share some features of the columnar-specific gene expression profile of aerial plant parts as evidenced by RNA-Seq analysis. BMC Plant Biol. 2015, 15, 34. [Google Scholar] [CrossRef][Green Version]
- Wolters, P.J.; Schouten, H.J.; Velasco, R.; Si-Ammour, A.; Baldi, P. Evidence for regulation of columnar habit in apple by a putative 2OG-Fe(II) oxygenase. New Phytol. 2013, 200, 993–999. [Google Scholar] [CrossRef]
- Wada, M.; Iwanami, H.; Moriya, S.; Hanada, T.; Moriya-Tanaka, Y.; Honda, C.; Shimizu, T.; Abe, K.; Okada, K. A root-localized gene in normal apples is ectopically expressed in aerial parts of columnar apples. Plant Growth Regul. 2018, 85, 389–398. [Google Scholar] [CrossRef]
- Okada, K.; Wada, M.; Takebayashi, Y.; Kojima, M.; Sakakibara, H.; Nakayasu, M.; Mizutani, M.; Nakajima, M.; Moriya, S.; Shimizu, T.; et al. Columnar growth phenotype in apple results from gibberellin deficiency by ectopic expression of a dioxygenase gene. Tree Physiol. 2020, 40, 1205–1216. [Google Scholar] [CrossRef]
- Watanabe, D.; Takahashi, I.; Jaroensanti-Tanaka, N.; Miyazaki, S.; Jiang, K.; Nakayasu, M.; Wada, M.; Asami, T.; Mizutani, M.; Okada, K.; et al. The apple gene responsible for columnar tree shape reduces the abundance of biologically active gibberellin. Plant J. 2021, 105, 1026–1034. [Google Scholar] [CrossRef]
- Darrow, G.M. The Strawberry; Holt, Rinehart and Winston: New York, NY, USA, 1966. [Google Scholar]
- Battey, N.H.; le Miere, P.; Tehranifar, A.; Cecik, C.; Taylor, S.; Shrives, K.; Hadley, P.; Greenland, A.; Darby, J.; Wilkinson, M. Genetic and environmental control of flowering in strawberry. In Genetic and Environmental Manipulation of Horticultural Crops; Cockshull, K.E., Gray, D., Seymour, G.B., Thomas, B., Eds.; CABI: Wallingford, UK, 1998; pp. 111–131. [Google Scholar]
- Andrés, J.; Caruana, J.; Liang, J.; Samad, S.; Monfort, A.; Liu, Z.; Hytönen, T.; Koskela, E.A. Woodland strawberry axillary bud fate is dictated by a crosstalk of environmental and endogenous factors. Plant Physiol. 2021, 187, 1221–1234. [Google Scholar] [CrossRef]
- Heide, O.M. Photoperiod and Temperature Interactions in Growth and Flowering of Strawberry. Physiol. Plant. 1977, 40, 21–26. [Google Scholar] [CrossRef]
- Sønsteby, A. Short-day period and temperature interactions on growth and flowering of strawberry. Acta Hortic. 1997, 439, 609–616. [Google Scholar] [CrossRef]
- Heide, O.M.; Sønsteby, A. Interactions of temperature and photoperiod in the control of flowering of latitudinal and altitudinal populations of wild strawberry (Fragaria vesca). Physiol. Plant. 2007, 130, 280–289. [Google Scholar] [CrossRef]
- Sønsteby, A.; Heide, O.M. Long-day control of flowering in everbearing strawberries. J. Hortic. Sci. Biotechnol. 2007, 82, 875–884. [Google Scholar] [CrossRef]
- Sønsteby, A.; Heide, O.M. Long-day rather than autonomous control of flowering in the diploid everbearing strawberry Fragaria vesca ssp. semperflorens. J. Hortic. Sci. Biotechnol. 2008, 83, 360–366. [Google Scholar] [CrossRef]
- Tenreira, T.; Lange, M.J.P.; Lange, T.; Bres, C.; Labadie, M.; Monfort, A.; Hernould, M.; Rothan, C.; Denoyes, B. A Specific Gibberellin 20-Oxidase Dictates the Flowering-Runnering Decision in Diploid Strawberry. Plant Cell 2017, 29, 2168–2182. [Google Scholar] [CrossRef]
- Caruana, J.C.; Sittmann, J.W.; Wang, W.; Liu, Z. Suppressor of Runnerless Encodes a DELLA Protein that Controls Runner Formation for Asexual Reproduction in Strawberry. Mol. Plant 2018, 11, 230–233. [Google Scholar] [CrossRef]
- Sun, T.-P. The Molecular Mechanism and Evolution of the GA–GID1–DELLA Signaling Module in Plants. Curr. Biol. 2011, 21, R338–R345. [Google Scholar] [CrossRef]
- Kurokura, T.; Samad, S.; Koskela, E.; Mouhu, K.; Hytönen, T. Fragaria vesca CONSTANS controls photoperiodic flowering and vegetative development. J. Exp. Bot. 2017, 68, 4839–4850. [Google Scholar] [CrossRef]
- Koskela, E.A.; Mouhu, K.; Albani, M.C.; Kurokura, T.; Rantanen, M.; Sargent, D.J.; Battey, N.H.; Coupland, G.; Elomaa, P.; Hytönen, T. Mutation inTERMINAL FLOWER1Reverses the Photoperiodic Requirement for Flowering in the Wild Strawberry Fragaria vesca. Plant Physiol. 2012, 159, 1043–1054. [Google Scholar] [CrossRef]
- Rantanen, M.; Kurokura, T.; Jiang, P.; Mouhu, K.; Hytönen, T. Strawberry homologue of TERMINAL FLOWER1 integrates photoperiod and temperature signals to inhibit flowering. Plant J. 2015, 82, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Mouhu, K.; Kurokura, T.; Koskela, E.; Albert, V.A.; Elomaa, P.; Hytönen, T. The Fragaria vesca Homolog of SUPPRESSOR OF OVEREXPRESSION OF CONSTANS1 Represses Flowering and Promotes Vegetative Growth. Plant Cell 2013, 25, 3296–3310. [Google Scholar] [CrossRef] [PubMed]
- Koskela, E.A.; Kurokura, T.; Toivainen, T.; Sønsteby, A.; Heide, O.M.; Sargent, D.J.; Isobe, S.; Jaakola, L.; Hilmarsson, H.; Elomaa, P.; et al. Altered regulation of TERMINAL FLOWER 1 causes the unique vernalisation response in an arctic woodland strawberry accession. New Phytol. 2017, 216, 841–853. [Google Scholar] [CrossRef]
- Wilson, F.M.; Harrison, K.; Armitage, A.D.; Simkin, A.J.; Harrison, R.J. CRISPR/Cas9-mediated mutagenesis of phytoene desaturase in diploid and octoploid strawberry. Plant Methods 2019, 15, 45. [Google Scholar] [CrossRef] [PubMed]
- Krueger, R.R.; Navarro, L. Citrus Germplasm Resources. In Citrus Genetics, Breeding and Biotechnology; Khan, I.A., Ed.; CAB International: Wallingford, Oxfordshire, UK, 2007; pp. 45–140. [Google Scholar]
- Gonzalez-Ibeas, D.; Ibanez, V.; Perez-Roman, E.; Borredá, C.; Terol, J.; Talon, M. Shaping the biology of citrus: I. Genomic determinants of evolution. Plant Genome 2021, e20104. [Google Scholar] [CrossRef]
- Wheaton, T.A.; Castle, W.S.; Whitney, J.D.; Tucker, D.P.H.; Muraro, R.P. A High density citrus planting. Proc. Fla. State Hortic. Soc. 1990, 103, 55–59. [Google Scholar]
- Ladaniya, M.S.; Marathe, R.A.; Das, A.K.; Rao, C.N.; Huchche, A.D.; Shirgure, P.S.; Murkute, A.A. High density planting studies in acid lime (Citrus aurantifolia Swingle). Sci. Hortic. 2020, 261, 108935. [Google Scholar] [CrossRef]
- Khan, I.A.; Kender, W.J. Citrus Breeding: Introduction and Objectives. In Citrus Genetics, Breeding and Biotechnology; Khan, I.A., Ed.; CAB International: Wallingford, UK, 2007. [Google Scholar]
- Jia, H.; Wang, N. Targeted genome editing of sweet orange using Cas9/sgRNA. PLoS ONE 2014, 9, e93806. [Google Scholar] [CrossRef]
- Zhang, F.; Leblanc, C.; Irish, V.F.; Jacob, Y. Rapid and efficient CRISPR/Cas9 gene editing in Citrus using the YAO promoter. Plant Cell Rep. 2017, 36, 1883–1887. [Google Scholar] [CrossRef]
- Jia, H.; Orbovic, V.; Wang, N. CRISPR-LbCas12a-mediated modification of citrus. Plant Biotechnol. J. 2019, 17, 1928–1937. [Google Scholar] [CrossRef]
- Zhang, F.; Rossignol, P.; Huang, T.; Wang, Y.; May, A.; Dupont, C.; Orbovic, V.; Irish, V.F. Reprogramming of Stem Cell Activity to Convert Thorns into Branches. Curr. Biol. 2020, 30, 2951–2961.e5. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, Y.; Irish, V.F. CENTRORADIALIS maintains shoot meristem indeterminacy by antagonizing THORN IDENTITY1 in Citrus. Curr. Biol. 2021, 31, 2237–2242.e4. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrés, J.; Koskela, E. Axillary Bud Fate Shapes Plant Architecture in Horticultural Crops. Horticulturae 2022, 8, 130. https://doi.org/10.3390/horticulturae8020130
Andrés J, Koskela E. Axillary Bud Fate Shapes Plant Architecture in Horticultural Crops. Horticulturae. 2022; 8(2):130. https://doi.org/10.3390/horticulturae8020130
Chicago/Turabian StyleAndrés, Javier, and Elli Koskela. 2022. "Axillary Bud Fate Shapes Plant Architecture in Horticultural Crops" Horticulturae 8, no. 2: 130. https://doi.org/10.3390/horticulturae8020130
APA StyleAndrés, J., & Koskela, E. (2022). Axillary Bud Fate Shapes Plant Architecture in Horticultural Crops. Horticulturae, 8(2), 130. https://doi.org/10.3390/horticulturae8020130






