Ethnobotanical Uses, Nutritional Composition, Phytochemicals, Biological Activities, and Propagation of the Genus Brachystelma (Apocynaceae)
Abstract
1. Introduction
2. Methods
3. Distribution and Botanical Description of Brachystelma Species
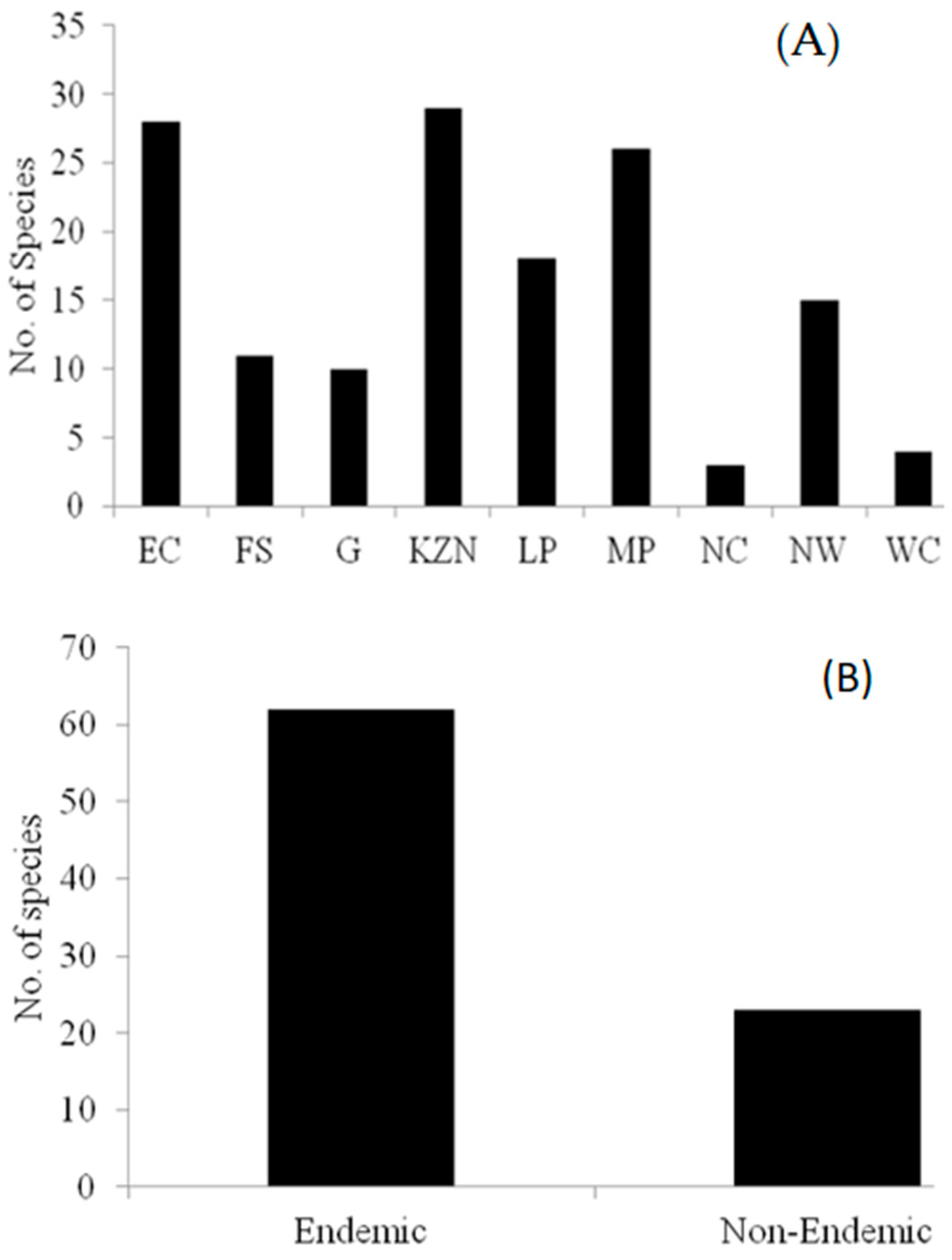
3.1. Distribution of Brachystelma Species
3.2. Overview of Botanical Aspects of Brachystelma Species
3.3. Taxonomy of Brachystelma Species
4. Ethnobotanical Applications of Brachystelma Species
4.1. Uses as Food and for Nutritional Needs
4.2. Applications as Herbal Medicine
| #Species | Country/Region | Local Name(s) | Plant Part(s) | Uses | References |
|---|---|---|---|---|---|
| Brachystelma arnottii Baker (Brachystelma arnotii) | southern Africa | ns | Tuber | Snack, vegetable | [12,32] |
| Brachystelma barberiae Harv. ex Hook.f. | Botswana | ns | Tuber | Eaten raw, sometimes roasted | [48] |
| Brachystelma barberiae Harv. ex Hook.f. (Brachystelma barberae) | southern Africa | ns | Tuber | Snack, vegetable | [12,49] |
| Brachystelma bingeri A. Chev. (Synonym: Raphionacme splendens subsp. bingeri (A.Chev.) Venter) | Burkina Faso | Sensenega, Daffio | Roots, tuber | Appetite suppressant and thirst quencher | [40,50] |
| Brachystelma buchananii N.E.Br.(Synonym: Brachystelma magicum) | Tanzania | ns | Tuber | Wound healing, magic power | [10] |
| Brachystelma burchellii (Decne.) Peckover (Synonym: Macropetalum burchellii Decne.) | southern Africa | ns | Tuber | Wild food | [31,34] |
| Brachystelma circinnatum E.Mey (Brachystelma circinatum) | Lesotho | Bohobe-ba-setsomi | Tuber | Food for hunters | [33,37] |
| Brachystelma circinnatum E.Mey. (= Brachystelma filiforme Harv.) | southern Africa | ns | Tuber | Snack, vegetable, sweet preserve, wild food | [31,32,33,34,35,36] |
| Brachystelma cupulatum R.A.Dyer | Namibia | ns | Tuber | Snack, vegetable | [32] |
| Brachystelma dinteri Schltr. | southern Africa | ns | Tuber | Snack, vegetable | [31,32,35] |
| Brachystelma discoideum R.A.Dyer | Namibia | ns | Tuber | Snack, vegetable | [32] |
| Brachystelma edule Collett & Hemsl. | China | ns | Tuber | Wild food, cough, and reducing phlegm | [45] |
| Brachystelma edulis Coll. and Helmls | India | Galya, Hanuman batata | Tuber | Famine food, vegetable, decoction used for bodily discomfort, cough and cold, stomachache, headache, dysentery, enhancing fertility, applied on skin inflammation | [45,46] |
| Brachystelma foetidum Schltr. | Lesotho | Seru | ns | For colds in children | [37,42] |
| Brachystelma foetidum Schltr. | southern Africa | ns | Tuber | Snack, vegetable, meal, yeast | [31,33,34,37] |
| Brachystelma gerrardii Harv. | Swaziland (Kingdom of Eswatini) | Sidzendza | Tuber | Wild food | [38,51] |
| Brachystelma glabriflorum (F.Muell) Schltr. | Australia | Badju, Djalwak | Tuber | Wild food | [39] |
| Brachystelma glabriflorum (F.Muell) Schltr. | Thailand | ns | ns | ns | [28] |
| Brachystelma glabrum Hook. f. | India | ns | Tuber | Wild food | [52] |
| Brachystelma gracile E.A.Bruce | East Africa | ns | Tuber | Food, thirst quencher | [10] |
| Brachystelma gymnopodum (Schltr.) Bruyns (Synonym: Ceropegia pygmaea Schinz) | southern Africa | ns | Tuber | Snack, vegetable, wild food | [31,35] |
| Brachystelma johnstonii N.E.Br. | East Africa | Akurukuri, Naporokenyen | Tuber | Food, thirst quencher, medicine for chest pain | [10] |
| Brachystelma kerrii Craib | Cambodia, Thailand | ns | Whole plant | Medicine: bodily discomfort | [29,30] |
| Brachystelma laevigatum Hook.f. | India | ns | Whole plant | Attractive foliage | [17] |
| Brachystelma mahajanii Kambale & S. R. Yadav | India | ns | ns | ns | [23] |
| Brachystelma nallamalayana sp. nov | India | ns | Tuber | Food | [24] |
| Brachystelma naorojii P.Tetali & al. | India | ns | Tuber | Food and medicine: stomach-ache, cough and colds | [45] |
| Brachystelma pauciflorum Duthie | India | ns | Tuber | Wild food | [17] |
| Brachystelma plocamoides Oliv. | East Africa | ns | Tuber | Food, thirst quencher | [10] |
| Brachystelma pullaiahi Rao et al. | India | Nakshtralamokka, Nemithigadda | Tuber | Wild food (eaten raw) | [53] |
| Brachystelma rubellum (E.Mey.) Peckover | East Africa | Mkumbe, Muthunga | Tuber | Food, thirst quencher | [10] |
| Brachystelma schultzei (Schltr.) Bruyns (Synonym: Tenaris schultzei (Schltr.) E.Phillips) | southern Africa | ns | Tuber | Wild food | [31,36] |
| Brachystelma simplex Schltr. | East Africa | ns | Tuber | Food, thirst quencher | [10] |
| Brachystelma thunbergii N.E.Br (currently regarded as ambiguous) | South Africa | Kamb(a)roo | Tuber | Snack, vegetable, meal, yeast, sweet preserve | [34,41] |
| Brachystelma togoense Schltr. | Nigeria | ns | Whole plant | Medicine: dysentery, cough and cold, wounds, stomachache, typhoid, erectile dysfunction | [43,44] |
| Brachystelma tuberosum (Meerb.) R.Br. ex Sims | southern Africa | ns | Tuber | Wild food | [31] |
| Brachystelma vartakii Kambale & S. R. Yadav | India | ns | ns | ns | [23] |
| Brachystelma volubile Hook.f. | India | Telugu | Tuber | Wild food | [20,52] |
5. Nutritional and Phytochemical Aspects
5.1. Nutritional Composition of Brachystelma Species
| Component | Content |
|---|---|
| Proximate (Brachystelma edulis, BE and Brachystelma naorojii, BN) | |
| Ash (% of fresh weight) | BE leaves: 12 [45] |
| BE tuber: 11–11.5 [27,46] | |
| BN leaves: 7.8 [58] | |
| BN tuber: 6 [58] | |
| Crude fat (% DW) | 0.12 [46] |
| Crude fiber (% DW) | 8.0 [46] |
| Crude protein (g/100 g DW) | 3.93 [46] |
| Dry matter (% FW) | BE leaves: 8.61 [45] |
| BE tuber: 19.3 [46] | |
| BN leaves: 5.8 [58] | |
| BN tuber: 7.17 [58] | |
| Energy value (k cal/100 g DW) | 302.4 [46] |
| Moisture (% FW) | BE leaves: 91.4 [45] |
| BE tuber: 80.8–97.01 [27,46] | |
| BN leaves: 94 [58] | |
| BN tuber: 93 [58] | |
| Total carbohydrates (% DW) | 3.61 [46] |
| Mineral composition (mg/100 g DW)—Brachystelma edulis [46] | |
| Calcium | 464.8 |
| Copper | 0.94 |
| Iron | 40.3 |
| Magnesium | 186.7 |
| Manganese | 3.27 |
| Phosphorus | 143.4 |
| Potassium | 416.3 |
| Sodium | 9.5 |
| Zinc | 1.07 |
5.2. Phytochemical Profile of Brachystelma Species
6. Biological Activities of Brachystelma Species Extracts and Isolated Compounds
| Biological Activity | Species | Plant Part Extraction/Solvent | Method/Assay Positive Control | Key Results | Reference |
|---|---|---|---|---|---|
| Acetylcholine esterase (AChE) inhibition | Brachystelma bingeri | Tuber methanol | AChE inhibition effect Galanthamine (10 μg/mL, 100%) | Extract (tested at 100 μg/mL) had 50% inhibition of enzyme. | [62] |
| Acute toxicity | Brachystelma bingeri | Tuber methanol | In vivo testing using six (6) mice to determine the lethal dose (LD50) for 72 h | Up to 3000 mg/kg of body weight extracts had no mortality. An indication that the LD50 value is greater than 3000 mg/kg of body weight. | [62] |
| Antioxidant | Brachystelma bingeri | Tuber methanol | 2,2 diphenyl-1-picrylhydrazyl (DPPH) quercetin (82%) | Extract had low (6%) radical scavenging activity. | [62] |
| Antioxidant | Brachystelma bingeri | Tuber methanol | Ferric-reducing antioxidant power (FRAP) Quercetin (4.69 mmol EAA/g) | Low ferric-reducing power, 0.013 mmol equivalents ascorbic acid per gram of extract (mmol EAA/g extract). | [62] |
| Antioxidant | Brachystelma bingeri | Tuber methanol | 2,2′-azinobis—(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) Quercetin (70 mmET/g) | Moderate activity, 25 mmol Trolox equivalents (mmTE)/g of dry extract. | [62] |
| Antioxidant | Brachystelma edulis | Tuber and leaves | Enzyme (peroxidase) activity | A total of 8.43 and 4.07 (unit/min/mg protein) for tubers and leaves, respectively. | [72] |
| Antioxidant | Brachystelma edulis | Tuber and leaves | Enzyme (catalase) activity | A total of 0.3 and 0.39 (unit/min/mg protein) for tubers and leaves, respectively. | [72] |
| Antioxidant | Brachystelma edulis | Tuber and leaves | Enzyme (superoxide dismutase) activity | A total of 0.21 and 0.35 (unit/min/mg protein) for tubers and leaves, respectively. | [72] |
| Antioxidant | Brachystelma pulchellum | Whole plant methanol | Oxygen radical absorbance capacity (ORAC) | Cytokinin-treated in vitro-regenerants had approximately 35–72 μmol/g Trolox equivalents (TE). | [63] |
| Antioxidant | Brachystelma pygmaeum | Whole plant methanol | Oxygen radical absorbance capacity (ORAC) | Cytokinin-treated in vitro regenerants had approximately 45–76 μmol/g Trolox equivalents (TE). | [63] |
7. Conservation Status of Brachystelma Species and Sustainability
7.1. Conservation Status of Brachystelma Species
7.2. Propagation of Brachystelma Species
7.3. Micropropagation of Brachystelma Species
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meve, U.; Heiduk, A.; Liede-schumann, S. Origin and early evolution of Ceropegieae (Apocynaceae-Asclepiadoideae). Syst. Biodivers. 2017, 15, 143–155. [Google Scholar] [CrossRef]
- Murthy, K.S.R. Traditional uses, pharmacognostic, phytochemical, pharmacological, and in vitro propagation studies in Brachystelma species. In Monograph on Brachystelma and Ceropegia in India; Pullaiah, T., Karuppusamy, S., Murthy, K.S.R., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 81–95. [Google Scholar]
- Bruyns, P.V.; Klak, C.; Hanáček, P. Recent radiation of Brachystelma and Ceropegia (Apocynaceae) across the Old World against a background of climatic change. Mol. Phylogenet. Evol. 2015, 90, 49–66. [Google Scholar] [CrossRef] [PubMed]
- Bruyns, P. Ceropegia, Brachystelma and Tenaris in South West Africa. Dinteria 1984, 1984, 3–80. [Google Scholar]
- Bruyns, P. A new species of Brachystelma (Apocynaceae) from South Tropical Africa. Novon A J. Bot. Nomencl. 2006, 16, 452–453. [Google Scholar] [CrossRef]
- Bruyns, P.V. Three new species of genus Brachystelma (Apocynaceae, Asclepiadoideae, Ceropegieae) from South Tropical and Southern Africa. Novon A J. Bot. Nomencl. 2009, 19, 18–22. [Google Scholar] [CrossRef]
- Bruyns, P.V.; Klak, C.; Hanáček, P. A revised, phylogenetically-based concept of Ceropegia (Apocynaceae). S. Afr. J. Bot. 2017, 112, 399–436. [Google Scholar] [CrossRef]
- Hlophe, N.P. Micropropagation of Three Brachystelma Species and Investigations of their Phytochemical Content and Antioxidant Activity. Master’s Dissertation, University of KwaZulu-Natal, Pietermaritzburg, South Africa, 2017. [Google Scholar]
- Peckover, R. Taxonomic questions within the genus Brachystelma: A few examples. Aloe 1993, 30, 114. [Google Scholar]
- Masinde, P.S. A revision of Brachystelma Sims (Apocynaceae: Asclepiadoideae-Ceropegieae) in East Africa. Kew Bull. 2007, 62, 37–84. [Google Scholar]
- Van Wyk, B.E. A family-level floristic inventory and analysis of medicinal plants used in Traditional African Medicine. J. Ethnopharmacol. 2020, 249, 112351. [Google Scholar] [CrossRef]
- Welcome, A.K.; Van Wyk, B.E. An inventory and analysis of the food plants of southern Africa. S. Afr. J. Bot. 2019, 122, 136–179. [Google Scholar] [CrossRef]
- Wong, S.K.; Lim, Y.Y.; Abdullah, N.R.; Nordin, F.J. Assessment of antiproliferative and antiplasmodial activities of five selected Apocynaceae species. BMC Compl. Altern. Med. 2011, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Omino, E.A.; Kokwaro, J.O. Ethnobotany of Apocynaceae species in Kenya. J. Ethnopharmacol. 1993, 40, 167–180. [Google Scholar] [CrossRef]
- Dyer, R.A. Ceropegia, Brachystelma and Riocreuxia in Southern Africa; AA Balkema: Rotterdam, The Netherlands, 1983. [Google Scholar]
- Venu, P.; Prasad, K. The existential crisis in Indian Brachystelma (Apocynaceae). Curr. Sci. 2015, 109, 680–682. [Google Scholar]
- Singh, A.K. Probable agricultural biodiversity heritage sites in India: XI. The upper Gangetic Plains region. Asian Agri-Hist. 2012, 16, 21–44. [Google Scholar]
- Prasad, K.; Prasanna, P.V.; Meve, U.; Rao, M.S.; Thulasaiah, T. Brachystelma annamacharyae sp. nov. (Apocynaceae) from the Seshachalam hills of Andhra Pradesh (India). Nord. J. Bot. 2016, 34, 360–363. [Google Scholar] [CrossRef]
- Dzerefos, C.M.; Witkowski, E.T.F.; Kremer-Köhne, S. Aiming for the biodiversity target with the social welfare arrow: Medicinal and other useful plants from a critically endangered grassland ecosystem in Limpopo Province, South Africa. Int. J. Sustain. Dev. World Ecol. 2017, 24, 52–64. [Google Scholar] [CrossRef]
- Rajakullayiswamy, K.; Sandhyarani, S.; Karuppusamy, S.; Pullaiah, T. The rediscovery of Brachystelma volubile (Apocynaceae–Asclepiadoideae). Rheedea 2012, 22, 107–110. [Google Scholar]
- SANBI. South African National Biodiversity Institute (SANBI). List of SA Red Data Listed Species. Available online: http://www.sanbi.org/index.php?option=com_docman&task=documentdetails&id=43 (accessed on 18 January 2022).
- Rivera, D.; Allkin, R.; Obón, C.; Alcaraz, F.; Verpoorte, R.; Heinrich, M. What is in a name? The need for accurate scientific nomenclature for plants. J. Ethnopharmacol. 2014, 152, 393–402. [Google Scholar] [CrossRef]
- Kambale, S.S.; Surveswaran, S.; Yadav, S.R. Two new species of Brachystelma Sims (Apocynaceae: Asclepiadoideae-Ceropegieae) from the Western Ghats of India. Kew Bull. 2014, 69, 9493. [Google Scholar] [CrossRef]
- Prasad, K.; Rao, B.R.P. Brachystelma nallamalayana sp. Nov. (Apocynaceae: Asclepiadoideae: Ceropegieae) from India. J. Threat. Taxa 2014, 5, 4904–4906. [Google Scholar] [CrossRef]
- Peckover, R.G. An additional distribution range for the rare Ceropegia macmasteri and Brachystelma cathcartense in the Eastern Cape, South Africa. CactusWorld 2017, 35, 171–175. [Google Scholar]
- Prasad, K.; Venu, P. Rediscovery and notes on Brachystelma maculatum (Apocynaceae). Rheedea 2018, 28, 73–77. [Google Scholar] [CrossRef]
- Rajaram, M.S.; Rathod, J.; Dilip, V. Pharmacognostical studies on the tuber of Brachystelma edulis Coll. and Helmsl.-an endemic to Peninsular, India. World J. Pharm. Pharm. Sci. 2014, 3, 1958–1965. [Google Scholar]
- Choopan, T. Vascular plants in Petrified Forest Park, Ban Tak District, Tak Province. Burapha Sci. J. 2019, 24, 170–189. [Google Scholar]
- Chuakul, W.; Boonpleng, A. Survey on medicinal plants in Ubon Ratchathani province (Thailand). Thai J. Phytopharm. 2004, 11, 33–54. [Google Scholar]
- Maxwell, J.F. Vegetation and vascular flora of the Mekong River, Kratie and Steung Treng Provinces, Cambodia. Maejo Int. J. Sci. Technol. 2009, 3, 143–211. [Google Scholar]
- Fox, F.W.; Young, M.E.N. Food from the Veld: Edible Wild Plants of Southern Africa Botanically Identified and Described; Delta Books: Johannesburg, South Africa, 1982. [Google Scholar]
- Leffers, A. Gemsbok Bean & Kalahari Truffle: Traditional Plant Use by Jul’hoansi in North-Eastern Namibia; Gamsberg Macmillan: Pretoria, South Africa, 2003. [Google Scholar]
- Moteetee, A.; van Wyk, B.-E. Sesotho names for exotic and indigenous edible plants in southern Africa. Bothalia 2006, 36, 8. [Google Scholar] [CrossRef]
- Smith, C.A. Common names of South African plants. In Memoirs of the Botanical Survey of South Africa 35; Department of Agricultural Technical Services: Pretoria, South Africa, 1966. [Google Scholar]
- Von Koenen, E. Medicinal, Poisonous, and Edible Plants in Namibia; Klaus Hess: Pretoria, South Africa, 2001. [Google Scholar]
- Wehmeyer, A. Edible wild plants of southern Africa: Data on the nutrient contents of over 300 species. In CSIR Report; CSIR, National Food Research Institute: Pretoria, South Africa, August 1986; p. 46. [Google Scholar]
- Guillarmod, A.J. Flora of Lesotho (Basutoland); Verlag von Cramer: Lehre, Germany, 1971. [Google Scholar]
- Ogle, B.M.; Grivetti, L.E. Legacy of the chameleon: Edible wild plants in the Kindom of Swaziland, Southern Africa. A cultural, ecological, nutritional study. Part II—Demographics, species availability and dietary use, analysis by ecological zone. Ecol. Food Nutr. 1985, 17, 1–30. [Google Scholar] [CrossRef]
- Smith, N.M. Ethnobotanical field notes from the northern territory, Australia. J. Adel. Bot. Gard. 1991, 14, 1–65. [Google Scholar]
- Pare, D.; Hilou, A.; Ouedraogo, N.; Guenne, S. Ethnobotanical study of medicinal plants used as anti-obesity remedies in the nomad and hunter communities of Burkina Faso. Medicines 2016, 3, 9. [Google Scholar] [CrossRef]
- Youngblood, D. Identification and quantification of edible plant foods in the Upper (Nama) Karoo, South Africa. Econ. Bot. 2004, 58, S43–S65. [Google Scholar] [CrossRef]
- Moteetee, A.; Van Wyk, B.E. The medical ethnobotany of Lesotho: A review. Bothalia 2011, 41, 209–228. [Google Scholar] [CrossRef]
- Ekalu, A.; Ayo, R.; Habila, J.D.; Hamisu, I. Phaeophytin and triterpenoids from Brachystelma togoense Schltr, a Nigerian medicinal herb. Asian J. Chem. Sci. 2019, 6, 1–5. [Google Scholar] [CrossRef]
- Ekalu, A.; Ayo, R.G.-O.; Habila, J.D.; Hamisu, I. A bioactive (2R, 3R)-dihydroflavonol-3-O-α-L-rhamnoside from Bracystelma togoense Schtlr. Nat. Appl. Sci. J. 2019, 11, 32–38. [Google Scholar]
- More, S.R.; Jadhav, V. Pharmacognostic and phytochemical analysis of leaves of Brachystelma edulis Coll. and Helmsl. World J. Pharm. Pharm. Sci. 2015, 5, 963–970. [Google Scholar]
- Deshmukh, S.; Rathod, V. Nutritional evaluation of some wild edible tuberous plants. Asian J. Pharm. Clin. Res. 2013, 6, 58–60. [Google Scholar]
- Heinrich, M.; Ankli, A.; Frei, B.; Weimann, C.; Sticher, O. Medicinal plants in Mexico: Healers’ consensus and cultural importance. Soc. Sci. Med. 1998, 47, 1859–1871. [Google Scholar] [CrossRef]
- Campbell, A. The use of wild food plants, and drought in Botswana. J. Arid Environ. 1986, 11, 81–91. [Google Scholar] [CrossRef]
- Mogg, A.O.D. Important Plants of Sterkfontein: An Illustrated Guide; University of the Witwatersrand: Johannesburg, South Africa, 1975. [Google Scholar]
- Belem, M.; Nabaloum, M.; Yameogo, J. Strategy of conservation and protection of wild edible plants diversity in Burkina Faso. ANADOLU Ege Tarımsal Araştırma Enstitüsü Derg. 2017, 27, 82–90. [Google Scholar]
- Dlamini, B. Swaziland Flora: Their Local Names and Uses; Ministry of Agriculture and Cooperatives, Forestry Section: Mbabane, Swaziland, 1981. [Google Scholar]
- Sadasivaiah, B.; Rao, B.R.P. Tribe: Ceropegieae (Apocynaceae, Asclepidoideae) in Eastern Ghats of Andhra Pradesh, India. Multidiscip. Approaches Angiosperm Syst. Publ. Cell Univ. Kalayni West Bengal India 2012, 1, 86–94. [Google Scholar]
- Rao, B.R.P.; Prasad, K.; Sadasivaiah, B.; Basha, S.K.; Babu, M.S.; Prasanna, P. A new species of Brachystelma R. Br.(Apocynaceae: Asclepiadoideae-Ceropegieae) from India. Taiwania 2011, 56, 223–226. [Google Scholar]
- Chivandi, E.; Mukonowenzou, N.; Nyakudya, T.; Erlwanger, K.H. Potential of indigenous fruit-bearing trees to curb malnutrition, improve household food security, income and community health in Sub-Saharan Africa: A review. Food Res. Int. 2015, 76, 980–985. [Google Scholar] [CrossRef]
- Ogle, B.M.; Grivetti, L.E. Legacy of the chameleon: Edible wild plants in the kingdom of Swaziland, Southern Africa. A cultural, ecological, nutritional study. Part IV—Nutritional analysis and conclusions. Ecol. Food Nutr. 1985, 17, 41–64. [Google Scholar] [CrossRef]
- Titchenal, C.A.; Dobbs, J. Nutritional value of vegetables. In Handbook of Food Science, Technology, and Engineering; Hui, Y.H., Ed.; CRC Taylor and Francis: Boca Raton, FL, USA, 2006; Volume 1, pp. 23–38. [Google Scholar]
- Uusiku, N.P.; Oelofse, A.; Duodu, K.G.; Bester, M.J.; Faber, M. Nutritional value of leafy vegetables of sub-Saharan Africa and their potential contribution to human health: A review. J. Food Compos. Anal. 2010, 23, 499–509. [Google Scholar] [CrossRef]
- More, S.R.; Jadhav, V. Pharmacognostic and phytochemical investigations of Brachystelma naorojii. World J. Pharm. Pharm. Sci. 2017, 6, 924–934. [Google Scholar]
- Aremu, A.O.; Moyo, M. Health benefits and biological activities of spiny monkey orange (Strychnos spinosa Lam.): An African indigenous fruit tree. J. Ethnopharmacol. 2022, 283, 114704. [Google Scholar] [CrossRef]
- Van Wyk, B.E. The potential of South African plants in the development of new food and beverage products. S. Afr. J. Bot. 2011, 77, 857–868. [Google Scholar] [CrossRef]
- Wansi, J.D.; Devkota, K.P.; Tshikalange, E.; Kuete, V. Alkaloids from the medicinal plants of Africa. In Medicinal Plant Research in Africa; Kuete, V., Ed.; Elsevier: Oxford, UK, 2013; pp. 557–605. [Google Scholar] [CrossRef]
- Pare, D.; Hilou, A.; Yabre, S.; Sombie, N.E.; Guenne, S.; Traoré, A.; Nacoulma, O.G. Phytochemical study and evaluation of the biological activity of anorectic plants used in the Seno province (Burkina Faso). J. Sci. Res. Rep. 2019, 23, 1–13. [Google Scholar] [CrossRef]
- Hlophe, N.P.; Aremu, A.O.; Gruz, J.; Van Staden, J.; Finnie, J.F. Influence of different cytokinins on the phenolic acids and antioxidant activity of two Brachystelma species. Plant Cell Tiss. Organ. Cult. 2021, 145, 689–699. [Google Scholar] [CrossRef]
- Herrmann, K.; Nagel, C.W. Occurrence and content of hydroxycinnamic and hydroxybenzoic acid compounds in foods. Crit. Rev. Food Sci. Nutr. 1989, 28, 315–347. [Google Scholar] [CrossRef]
- Gülçin, İ. Antioxidant activity of food constituents: An overview. Arch. Toxicol. 2012, 86, 345–391. [Google Scholar] [CrossRef] [PubMed]
- Stintzing, F.C.; Kammerer, D.; Schieber, A.; Adama, H.; Nacoulma, O.G.; Carle, R. Betacyanins and phenolic compounds from Amaranthus spinosus L. and Boerhavia erecta L. Z. Für Nat. C 2004, 59, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hilou, A.; Millogo-Rasolodimby, J.; Nacoulma, O.G. Betacyanins are the most relevant antioxidant molecules of Amaranthus spinosus and Boerhavia erecta. J. Med. Plants Res. 2013, 7, 645–652. [Google Scholar]
- Patil, K.S.; Bhalsing, S.R. Ethnomedicinal uses, phytochemistry and pharmacological properties of the genus Boerhavia. J. Ethnopharmacol. 2016, 182, 200–220. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, İ.; Elmastaş, M.; Aboul-Enein, H.Y. Determination of antioxidant and radical scavenging activity of Basil (Ocimum basilicum L. Family Lamiaceae) assayed by different methodologies. Phytother. Res. 2007, 21, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Van Vuuren, S.; Holl, D. Antimicrobial natural product research: A review from a South African perspective for the years 2009–2016. J. Ethnopharmacol. 2017, 208, 236–252. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, S.; Jadhav, V. Antioxidant activity of some wild edible tuberous plants. Int. J. Pharm. 2014, 4, 236–239. [Google Scholar]
- Ekalu, A.; Ayo, R.G.-O.; Habila, J.D.; Hamisu, I. Isolation and microbial screening of 1-methylcyclopentene from Bracystelma togoense Schtlr. Org. Med. Chem. Int. J. 2019, 8, 78–85. [Google Scholar] [CrossRef]
- Ekalu, A.; Ayo, R.G.-O.; Habila, J.; Hamisu, İ. Bioactivity of phaeophytin A, α-amyrin and lupeol from Brachystelma togoense Schltr. J. Turk. Chem. Soc. Sect. A Chem. 2019, 6, 411–418. [Google Scholar] [CrossRef]
- Chavan, J.J.; Gaikwad, N.B.; Dixit, G.B.; Yadav, S.R.; Bapat, V.A. Biotechnological interventions for propagation, conservation and improvement of ‘Lantern Flowers’ (Ceropegia spp.). S. Afr. J. Bot. 2018, 114, 192–216. [Google Scholar] [CrossRef]
- Adnan, M.; Jan, S.; Mussarat, S.; Tariq, A.; Begum, S.; Afroz, A.; Shinwari, Z.K. A review on ethnobotany, phytochemistry and pharmacology of plant genus Caralluma R. Br. J. Pharm. Pharmacol. 2014, 66, 1351–1368. [Google Scholar] [CrossRef] [PubMed]
- Muthukrishnan, S.; Franklin Benjamin, J.H.; Sathishkannan, G.; Senthil Kumar, T.; Rao, M.V. In vitro propagation of genus Ceropegia and retrosynthesis of cerpegin—A review. Int. J. Pharm. Sci. Rev. Res. 2013, 46, 315–330. [Google Scholar] [CrossRef]
- Wyatt, R.; Broyles, S.B.; Lipow, S.R. Pollen-ovule ratios in milkweeds (Asclepiadaceae): An exception that probes the rule. Syst. Bot. 2000, 25, 171–180. [Google Scholar] [CrossRef]
- Moyo, M.; Bairu, M.W.; Amoo, S.O.; Van Staden, J. Plant biotechnology in South Africa: Micropropagation research endeavours, prospects and challenges. S. Afr. J. Bot. 2011, 77, 996–1011. [Google Scholar] [CrossRef][Green Version]
- Pathak, M.R.; Abido, M.S. The role of biotechnology in the conservation of biodiversity. J. Exp. Biol. Agric. Sci. 2014, 2, 352–363. [Google Scholar]
- Aremu, A.O.; Bairu, M.W.; Doležal, K.; Finnie, J.F.; Van Staden, J. Topolins: A panacea to plant tissue culture challenges? Plant Cell Tissue Organ. Cult. 2012, 108, 1–16. [Google Scholar] [CrossRef]
- Cruz-Cruz, C.A.; González-Arnao, M.T.; Engelmann, F. Biotechnology and Conservation of Plant Biodiversity. Resources 2013, 2, 73–95. [Google Scholar] [CrossRef]
- Singh, A. Micropropagation of Plants. In Plant Biology and Biotechnology: Volume II: Plant Genomics and Biotechnology; Bahadur, B., Venkat Rajam, M., Sahijram, L., Krishnamurthy, K.V., Eds.; Springer: New Delhi, India, 2015; pp. 329–346. [Google Scholar] [CrossRef]
- Hlophe, N.P.; Aremu, A.O.; Doležal, K.; Van Staden, J.; Finnie, J.F. Cytokinin-facilitated plant regeneration of three Brachystelma species with different conservation status. Plants 2020, 9, 1657. [Google Scholar] [CrossRef]
- Revathi Lakshmi, S.; Parthibhan, S.; Ahamed Sherif, N.; Senthil Kumar, T.; Rao, M.V. Micropropagation, in vitro flowering, and tuberization in Brachystelma glabrum Hook.f., an endemic species. Vitr. Cell. Dev. Biol.-Plant 2017, 53, 64–72. [Google Scholar] [CrossRef]
- Aremu, A.O.; Fawole, O.A.; Makunga, N.P.; Masondo, N.A.; Moyo, M.; Buthelezi, N.M.D.; Amoo, S.O.; Spíchal, L.; Doležal, K. Applications of cytokinins in horticultural fruit crops: Trends and future prospects. Biomolecules 2020, 10, 1222. [Google Scholar] [CrossRef] [PubMed]
- Aremu, A.O.; Doležal, K.; Van Staden, J. New cytokinin-like compounds as a tool to improve rooting and establishment of micropropagated plantlets. In Proceedings of the 2017: International Society for Horticultural Science (ISHS), Leuven, Belgium, Bolzona, Italy, 27–30 June 2017; pp. 497–504. [Google Scholar]
- Masondo, N.A.; Aremu, A.O.; Finnie, J.F.; Van Staden, J. Growth and phytochemical levels in micropropagated Eucomis autumnalis subspecies autumnalis using different gelling agents, explant source, and plant growth regulators. Vitr. Cell. Dev. Biol.-Plant 2015, 51, 102–110. [Google Scholar] [CrossRef]
- Carmo, L.P.; Wallace do Nascimento Moura, C.; Lima-Brito, A. Effects of seaweed extracts on the in vitro multiplication of plants. In Biostimulants for Crops from Seed Germination to Plant Development; Gupta, S., Van Staden, J., Eds.; Academic Press: London, UK, 2021; pp. 211–230. [Google Scholar] [CrossRef]
- Méndez-Hernández, H.A.; Ledezma-Rodríguez, M.; Avilez-Montalvo, R.N.; Juárez-Gómez, Y.L.; Skeete, A.; Avilez-Montalvo, J.; De-la-Peña, C.; Loyola-Vargas, V.M. Signaling overview of plant somatic embryogenesis. Front. Plant Sci. 2019, 10, 77. [Google Scholar] [CrossRef] [PubMed]
- Sahijram, L.; Bahadur, B. Somatic embryogenesis. In Plant Biology and Biotechnology: Volume II: Plant Genomics and Biotechnology; Bahadur, B., Venkat Rajam, M., Sahijram, L., Krishnamurthy, K.V., Eds.; Springer: New Delhi, India, 2015; pp. 315–327. [Google Scholar] [CrossRef]


| Brachystelma Species | Content | Reference | |
|---|---|---|---|
| Qualitative Tests | |||
| Alkaloid | Brachystelma edulis | + | [27,45] |
| Brachystelma naorojii | + | [58] | |
| Coumarins | Brachystelma bingeri | + | [62] |
| Brachystelma naorojii | + | [58] | |
| Flavones | Brachystelma edulis | + | [45] |
| Glycosides | Brachystelma edulis | + | [27,45] |
| Brachystelma naorojii | + | [58] | |
| Phenol | Brachystelma edulis | + | [27] |
| Brachystelma naorojii | + | [58] | |
| Reducing sugars | Brachystelma edulis | + | [45] |
| Brachystelma naorojii | + | [58] | |
| Saponosides | Brachystelma bingeri | + | [62] |
| Saponin | Brachystelma edulis | + | [27] |
| Steroids and triterpenes | Brachystelma bingeri | + | [62] |
| Tannins | Brachystelma edulis | + | [27,45] |
| Brachystelma naorojii | + | [58] | |
| Spectrophotometric Technique | |||
| Total peholic | Brachystelma bingeri | 1.7 mg EGA/g | [62] |
| Ultra-High-Performance Liquid Chromatography–Tandem Mass Spectrometry (UHPLC-MS/MS) Analysis | |||
| Caffeic acid | Brachystelma pulchellum | 0.331–1.476 μg/g | [63] |
| Caffeic acid | Brachystelma pygmaeum | 0.445–1.282 μg/g | [63] |
| Ferulic acid | Brachystelma pulchellum | 10–55 μg/g | [63] |
| Ferulic acid | Brachystelma pygmaeum | 9–43 μg/g | [63] |
| Gallic acid | Brachystelma pulchellum | 0.119–0.40 μg/g | [63] |
| Gallic acid | Brachystelma pygmaeum | 0.263–0.565 μg/g | [63] |
| m-Hydroxybenzoic acid | Brachystelma pulchellum | 0.206–1.167 μg/g | [63] |
| m-Hydroxybenzoic acid | Brachystelma pygmaeum | 0.167–1.595 μg/g | [63] |
| p-Coumaric acid | Brachystelma pulchellum | 2–7 μg/g | [63] |
| p-Coumaric acid | Brachystelma pygmaeum | 1.352–2.783 μg/g | [63] |
| p-Hydroxybenzoic acid | Brachystelma pulchellum | 0.496–1.374 μg/g | [63] |
| p-Hydroxybenzoic acid | Brachystelma pygmaeum | 0.385–1.060 μg/g | [63] |
| Protocatechuic acid | Brachystelma pulchellum | 0.450–1.488 μg/g | [63] |
| Protocatechuic acid | Brachystelma pygmaeum | 0.223–0.955 μg/g | [63] |
| Salicylic acid | Brachystelma pulchellum | 0.839–2.464 μg/g | [63] |
| Salicylic acid | Brachystelma pygmaeum | 0.307–1.086 μg/g | [63] |
| Sinapic acid | Brachystelma pulchellum | 14–58 μg/g | [63] |
| Sinapic acid | Brachystelma pygmaeum | 30–98 μg/g | [63] |
| Syringic acid | Brachystelma pulchellum | 0.672–3.107 μg/g | [63] |
| Syringic acid | Brachystelma pygmaeum | 0.167–0.910 μg/g | [63] |
| Vanillic acid | Brachystelma pulchellum | 0.506–1.804 μg/g | [63] |
| Vanillic acid | Brachystelma pygmaeum | 0.663–2.834 μg/g | [63] |
| Compound | Species | Plant Part | Bioactivity Tested | Reference |
|---|---|---|---|---|
| (2R, 3R)-dihydroflavonol-3-O-a-L-rhamnoside | Brachystelma togoense | Aerial parts | Antimicrobial (significant antimicrobial effects) against Escherichia coli, Streptococcus typhi, and Candida albicans | [44] |
| 1-Methylcyclopentene | Brachystelma togoense | Aerial parts | Antimicrobial (noteworthy MIC) against Escherichia coli (0.1875 mg/mL) and Candida albicans (0.1875 g/mL) | [73] |
| Lupeol | Brachystelma togoense | Aerial parts | Antimicrobial (MIC was 0.18–0.37 mg/mL) against Escherichia coli, Staphylococcus aureus, Staphylococcus pneumoniae, Streptococcus typhi, and Candida albicans | [43,74] |
| Phaeophytin A | Brachystelma togoense | Aerial parts | Antimicrobial (MIC was 0.09–0.18 mg/mL) against Escherichia coli, Staphylococcus aureus, Staphylococcus pneumoniae, Streptococcus typhi, and Candida albicans | [43,74] |
| α-Amyrin | Brachystelma togoense | Aerial parts | Antimicrobial (MIC was 0.37 mg/mL) against Escherichia coli, Staphylococcus aureus, Staphylococcus pneumoniae, Streptococcus typhi, and Candida albicans | [43,74] |
| Species | Parameter Tested | Explant | Most Optimal Media Composition | Response | Reference |
|---|---|---|---|---|---|
| Brachystelma glabrum | Effect cytokinins (TDZ, BA) and auxins (IBA, NAA) on in vitro tuberisation | Shoot tip and nodal | TDZ + IBA | 60% response in the production of aerial tubers | [85] |
| Brachystelma glabrum | Effect of auxins (IAA, IB, NAA) on in vitro rooting | Shoot tip and nodal | NAA (0.5 mg/L) | 80% root induction and an average of 5.3 roots/micro-shoot | [85] |
| Brachystelma glabrum | Effect of BA with different auxins (NAA, IBA) on in vitro flowering | Shoot tip and nodal | BA + NAA (2 mg/L) | 60% of culture produced flower buds | [85] |
| Brachystelma glabrum | Effect of different cytokinins (BA, KIN, TDZ) on shoot induction | Shoot tip | TDZ (1 mg/L) | 90% shoot induction and an average of 4.7 shoots/shoot-tip explant | [85] |
| Brachystelma glabrum | Effect of different cytokinins (BA, KIN, TDZ) on shoot induction | Nodal | TDZ (1 mg/L) | 100% shoot induction and an average of 5.5 shoots/nodal explants | [85] |
| Brachystelma glabrum | Effect of TDZ (1 mg/L) with different auxins (IAA, IBA, NAA) on shoot proliferation | Shoot tip and nodal | TDZ + NAA (0.5 mg/L) | 85% shoot induction and an average of 10.6 shoots/shoot-tip explant 90% shoot induction and an average of 11.4 shoots/nodal explants | [85] |
| Brachystelma ngomense | Effect of auxin (IBA) on ex vitro rooting and acclimatisation | Nodal | 100 mg/L IBA pulsing for 3 min | Survival rate: 42% (4 weeks) and 5% (10 weeks) | [84] |
| Brachystelma ngomense | Effect of cytokinins (BA, iP, mTR) on shoot proliferation | Nodal | mTR (25 μM) | Average of 4.4 shoots/nodal explant | [84] |
| Brachystelma pulchellum | Effect of auxin (IBA) on ex vitro rooting and acclimatisation | Nodal | 100 mg/L IBA pulsing for 3 min | Survival rate: 35% (4 weeks) and 0% (10 weeks) | [84] |
| Brachystelma pulchellum | Effect of cytokinins (BA, iP, mTR) on shoot proliferation | Nodal | iP (25 μM) | Average of 2.04 shoots/nodal explant | [84] |
| Brachystelma pygmaeum | Effect of auxin (IBA) on ex vitro rooting and acclimatisation | Nodal | 100 mg/L IBA pulsing for 3 min | Survival rate: 30% (4 weeks) and 3% (10 weeks) | [84] |
| Brachystelma pygmaeum | Effect of cytokinins (BA, iP, mTR) on shoot proliferation | Nodal | BA (25 μM) | Average of 2.57 shoots/nodal ex-plant | [84] |
| Brachystelma pygmaeum | Effect of pulse dipping of IBA (100 mg/L) for different time intervals (3, 12 and 21 min) on ex vitro rooting and acclimatisation | Nodal | Similar response was observed across all treatments. | Survival rate: 5% for the three-time internal, whereas control had no survival | [84] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aremu, A.O.; Hlophe, N.P.; Van Staden, J.; Finnie, J.F. Ethnobotanical Uses, Nutritional Composition, Phytochemicals, Biological Activities, and Propagation of the Genus Brachystelma (Apocynaceae). Horticulturae 2022, 8, 122. https://doi.org/10.3390/horticulturae8020122
Aremu AO, Hlophe NP, Van Staden J, Finnie JF. Ethnobotanical Uses, Nutritional Composition, Phytochemicals, Biological Activities, and Propagation of the Genus Brachystelma (Apocynaceae). Horticulturae. 2022; 8(2):122. https://doi.org/10.3390/horticulturae8020122
Chicago/Turabian StyleAremu, Adeyemi Oladapo, Nqobile P. Hlophe, Johannes Van Staden, and Jeffrey F. Finnie. 2022. "Ethnobotanical Uses, Nutritional Composition, Phytochemicals, Biological Activities, and Propagation of the Genus Brachystelma (Apocynaceae)" Horticulturae 8, no. 2: 122. https://doi.org/10.3390/horticulturae8020122
APA StyleAremu, A. O., Hlophe, N. P., Van Staden, J., & Finnie, J. F. (2022). Ethnobotanical Uses, Nutritional Composition, Phytochemicals, Biological Activities, and Propagation of the Genus Brachystelma (Apocynaceae). Horticulturae, 8(2), 122. https://doi.org/10.3390/horticulturae8020122








