Tomato (Solanum lycopersicum L.) Genotypes Respond Differently to Long-Term Dry and Humid Heat Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Screening Conditions and Data Collection
2.3. Data Analysis
3. Results
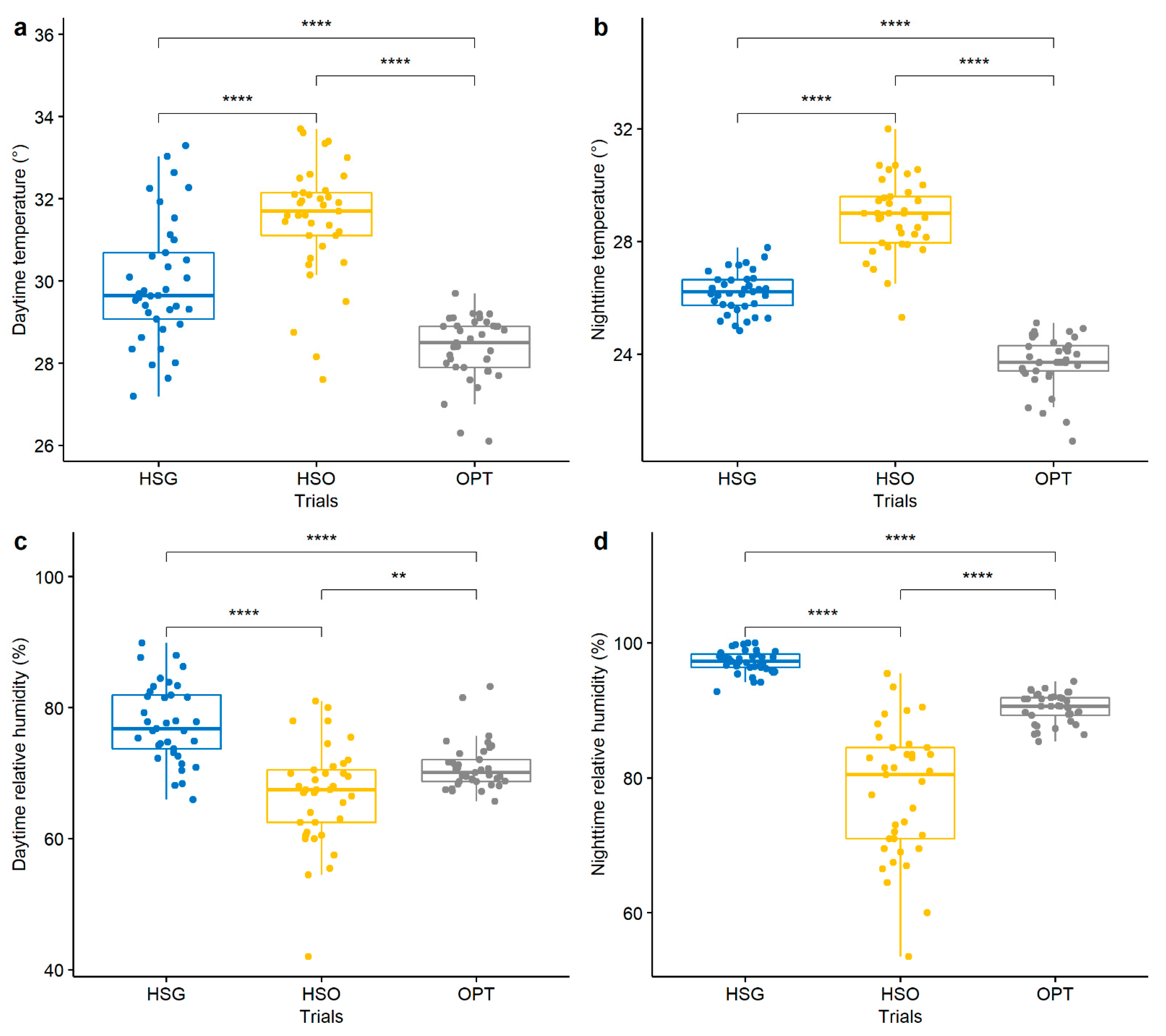
3.1. Temperatures and Relative Humidity in the Growing Environments
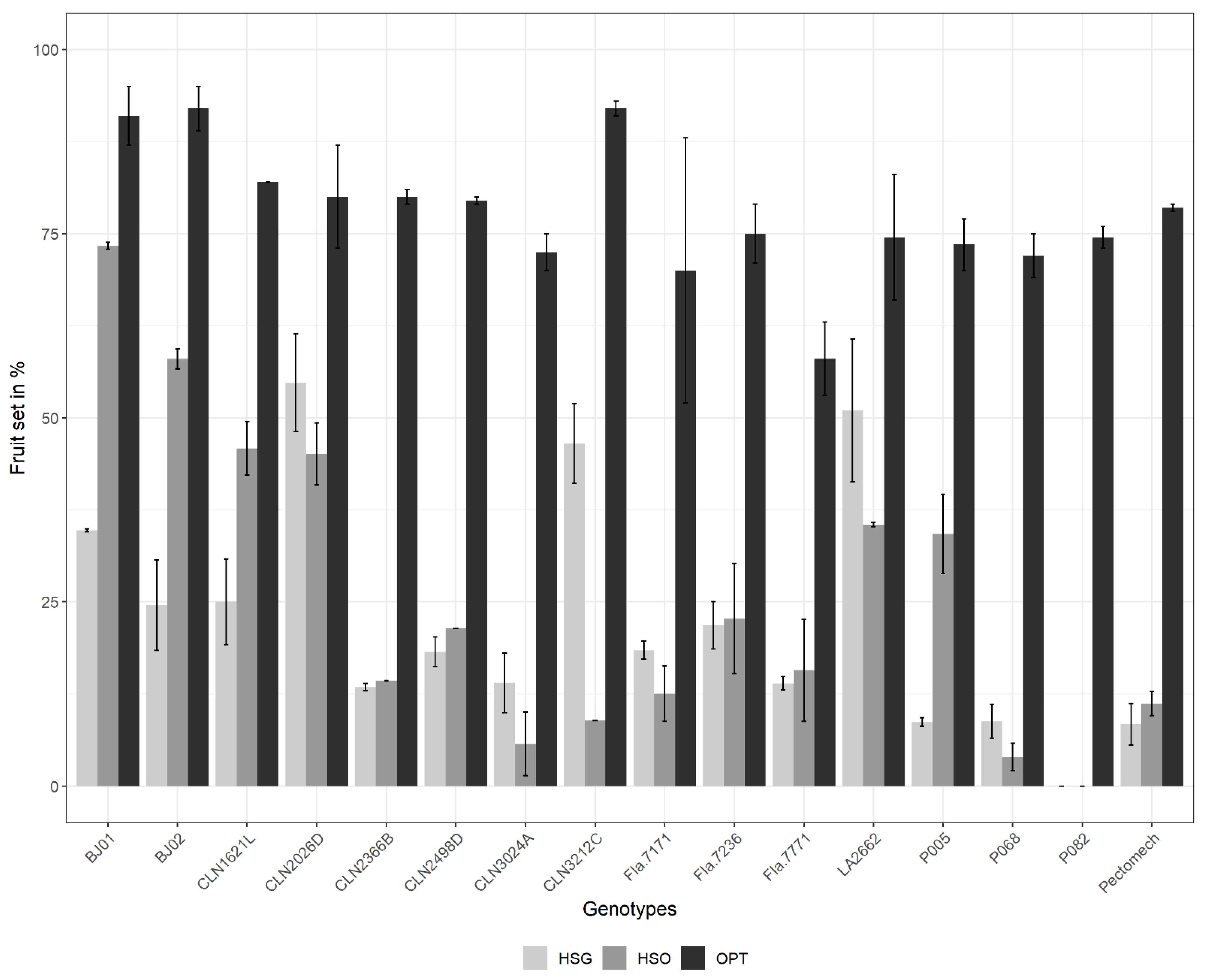
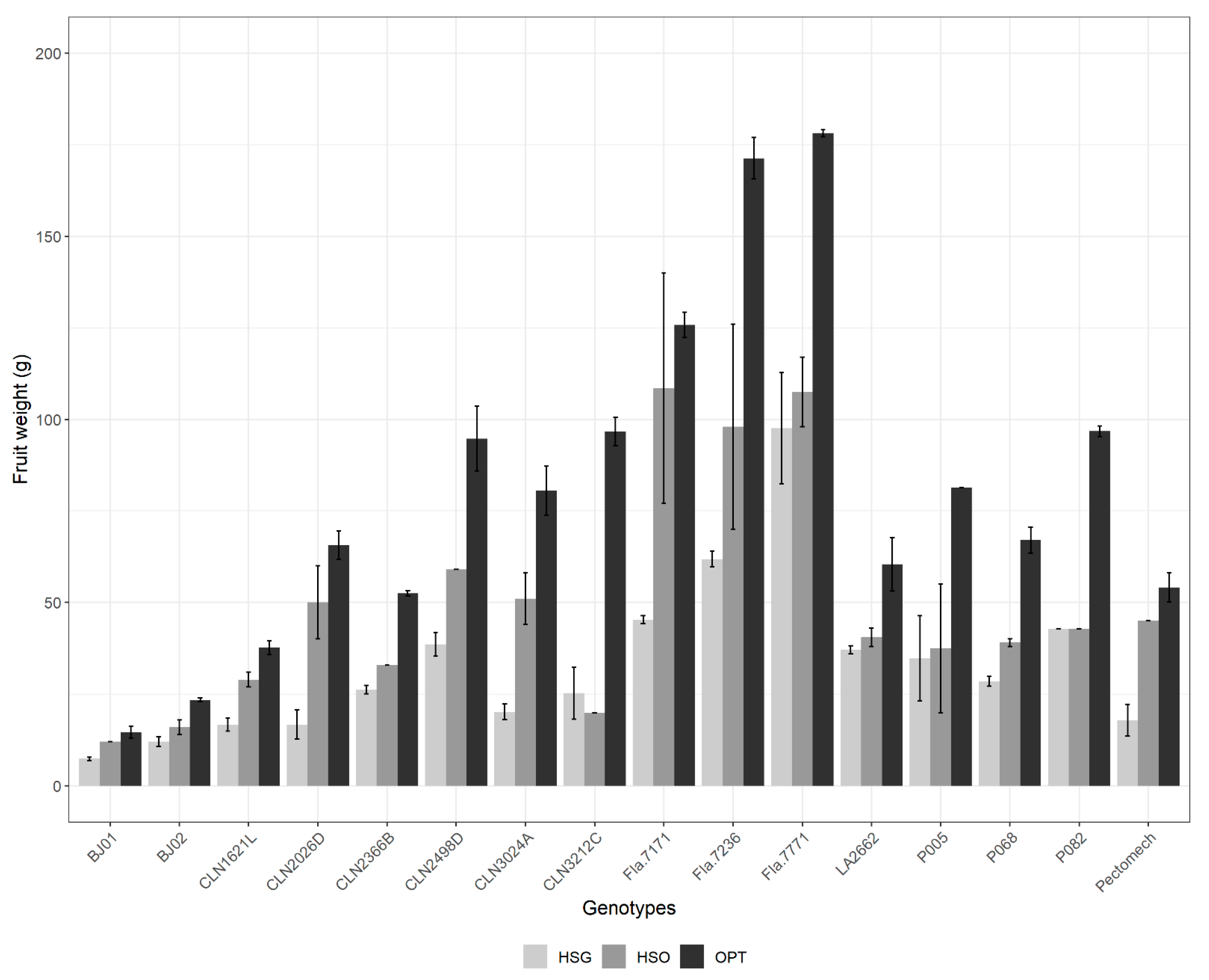
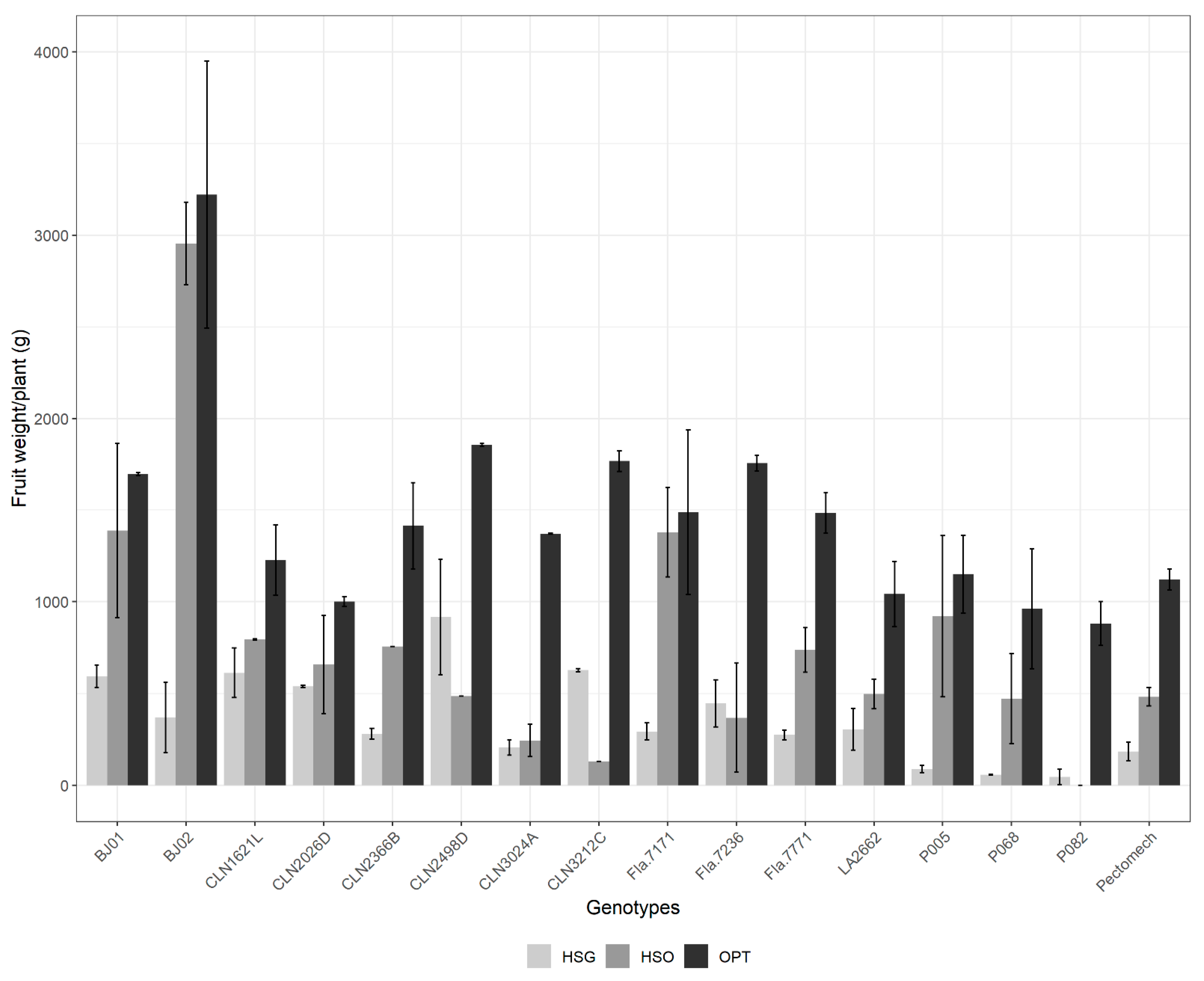
3.2. Performance of the Genotypes for Yield Components under Long Term Heat Stress Regimes and Optimal Season Trials
3.2.1. Fruit Set Percentage
3.2.2. Number of Fruits per Plant
3.2.3. Fruit Weight
3.2.4. Fruit Weight per Plant
3.3. Heat Stress Tolerance Indices
3.3.1. Average Rank of the Genotypes
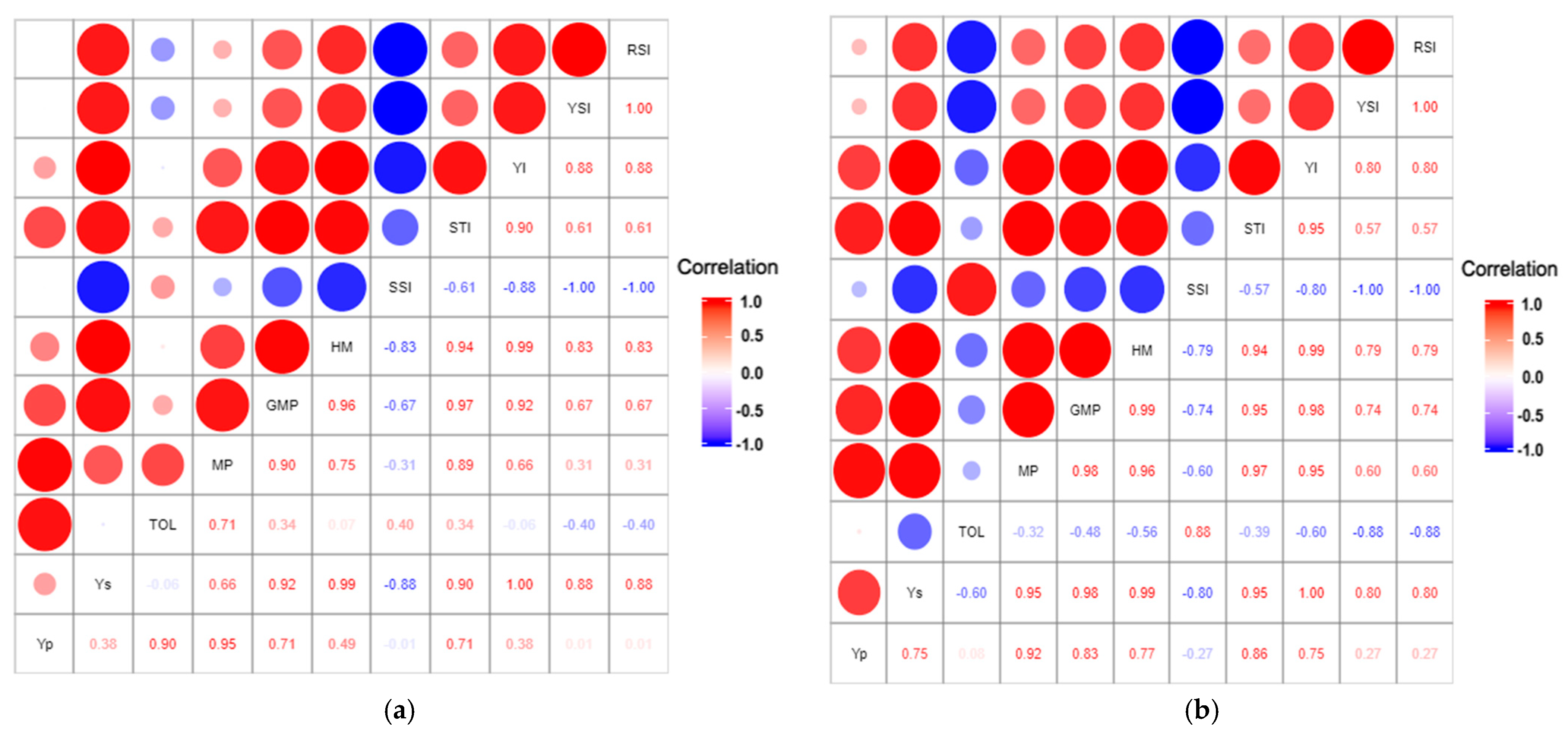
3.3.2. Association between Fruit Weight per Plant and Stress Tolerance Indices
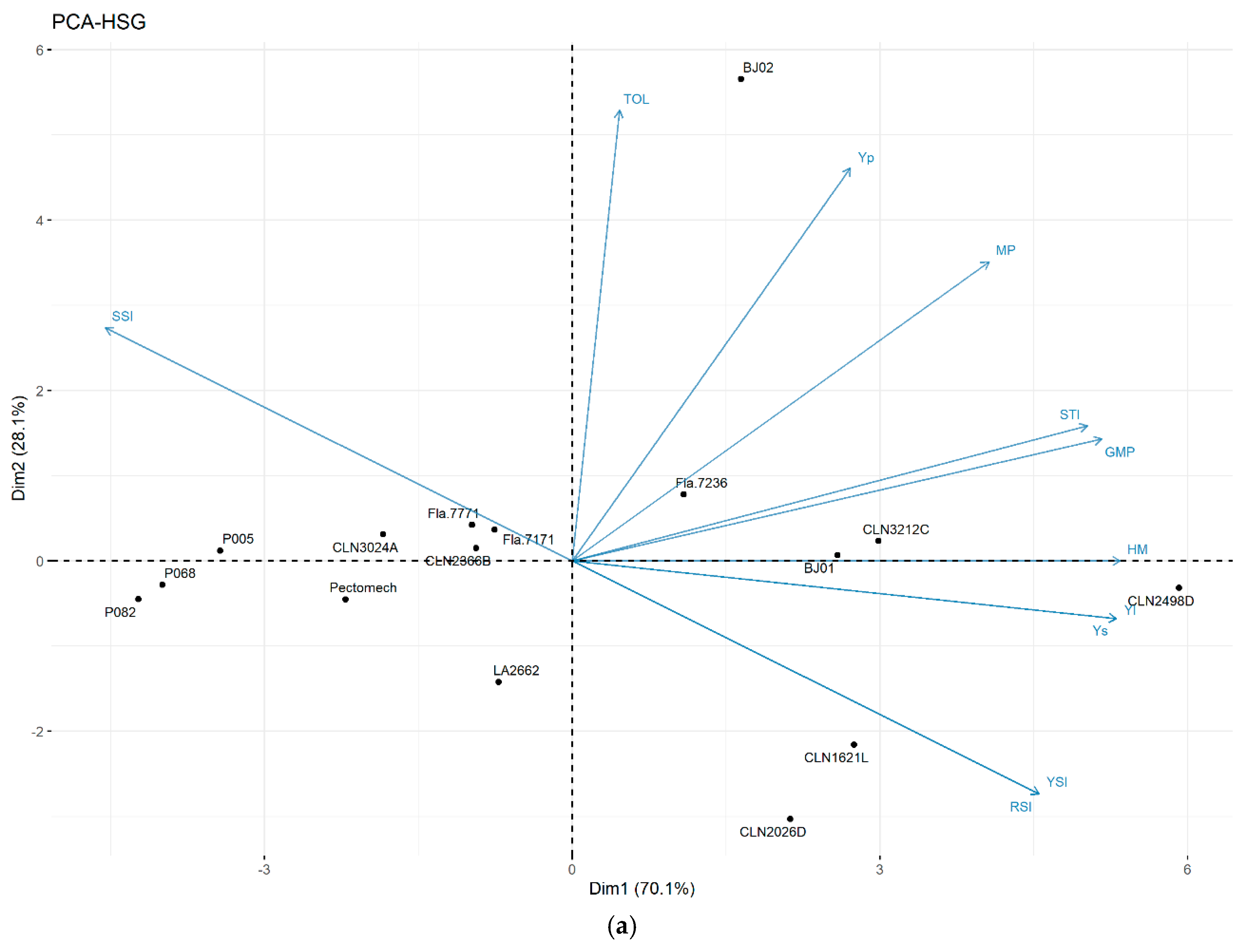
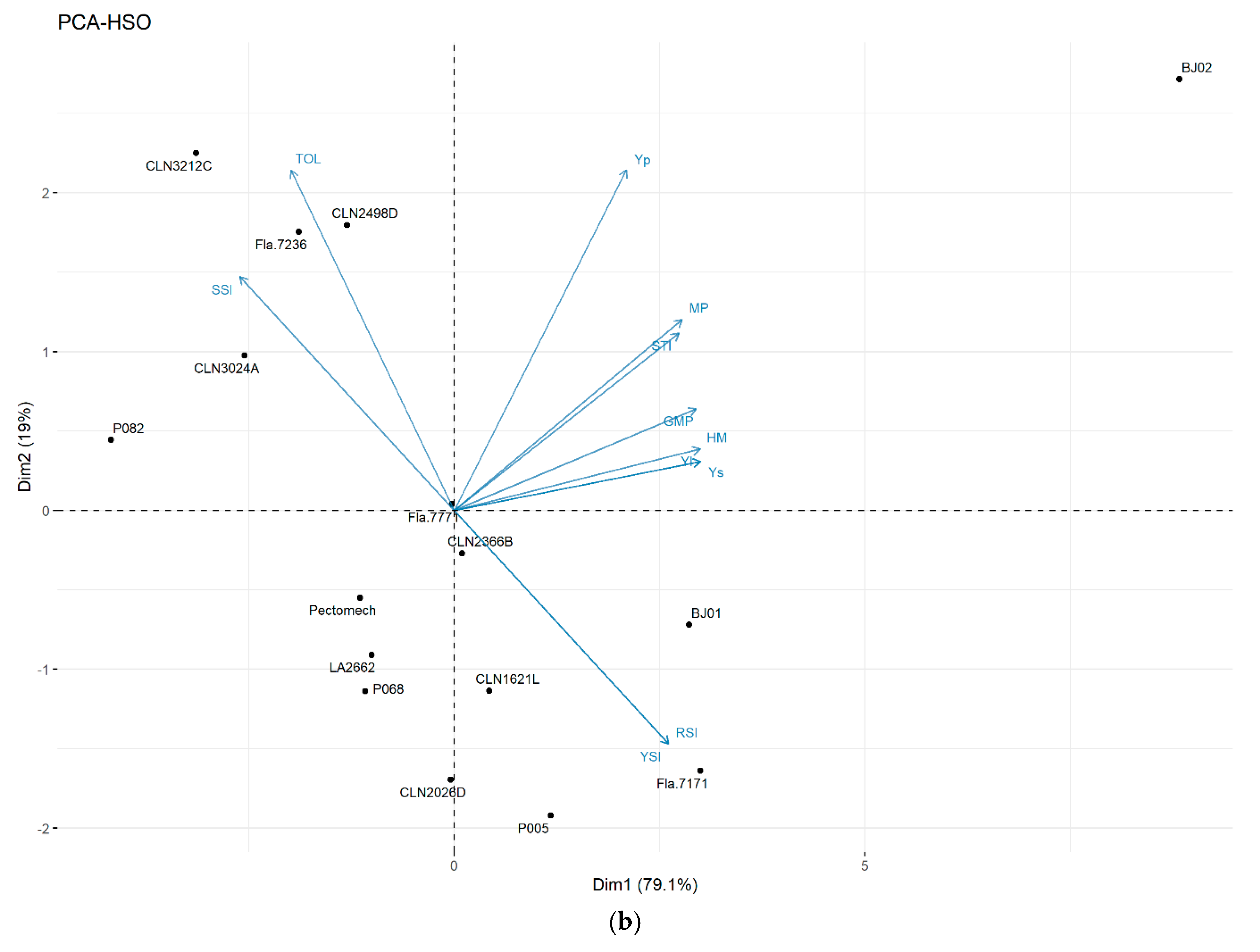
3.3.3. Principal Component Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Plant Husbandry
Appendix A.1. Greenhouse Trial (HSG)
Appendix A.2. Open Field Trial (HSO)
Appendix A.3. Optimal Season Trial (OPT)
References
- FAOSTAT FAOSATA. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 5 April 2021).
- Adimabuno, A.M. Marketing and Market Queens: A Study of Tomato Farmers in the Upper East Region of Ghana. Ph.D. Thesis, University of Bonn, Bonn, Germany, 2010; p. 175. [Google Scholar]
- Van Wesenbeeck, C.F.; Venus, V.; Keyzer, M.A.; Wesselman, B.; Asare Kye, D. Development of a Horticulture Production Chain in Western Africa: A Case Study of Tomatoes in Burkina Faso and Ghana; Centre for World Food Studies: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Beecher, G.R. Nutrient Content of Tomatoes and Tomato Products. Exp. Biol. Med. 1998, 218, 98–100. [Google Scholar] [CrossRef]
- Fufa, F.; Hanson, P.; Dagnoko, S.; Dhaliwal, M. AVRDC—The world vegetable center tomato breeding in Sub-Saharan Africa: Lessons from the past, present work, and future prospects. Acta Hortic. 2011, 911, 87–98. [Google Scholar] [CrossRef]
- Robinson, E.J.Z.; Kolavalli, S.L. The Case of Tomato in Ghana: Productivity; International Food Policy Research Institute: Accra, Ghana, 2010. [Google Scholar]
- Amikuzuno, J.; von Cramon-Taubadel, S. Seasonal variation in price transmission between tomato markets in Ghana. J. Afr. Econ. 2012, 21, 669–686. [Google Scholar] [CrossRef]
- Schreinemachers, P.; Wu, M.H.; Uddin, M.N.; Ahmad, S.; Hanson, P. Farmer training in off-season vegetables: Effects on income and pesticide use in Bangladesh. Food Policy 2016, 61, 132–140. [Google Scholar] [CrossRef] [Green Version]
- Asgedom, S.; Struik, P.C.; Heuvelink, E.; Araia, W. Opportunities and constraints of tomato production in Eritrea. Afr. J. Agric. Res. 2011, 6, 956–967. [Google Scholar]
- Adeoti, R.; Koffi-Tessio, E.; Coulibaly, O.; Manyong, V.; Oloukoï, L. Spatial integration of Vegetable Markets in West Africa: Case of Tomato (Lycopersicum esculentum) between Benin and Burkina Faso. In Proceedings of the 4th International Conference of the African Association of Agricultural Economists, Hammamet, Tunisia, 22–25 September 2013; pp. 1–25. [Google Scholar]
- Mensah, A.C.G.; Assogba Komlan, F.; Yarou, B.; Aisso, C.B.R.; Eké, H.; Amadji, L.G. Quel type d ’abri pour mieux réussir la production de tomate de contre saison au Sud-Bénin? Bull. Rech. Agron. Bénin 2019, 205–217. Available online: https://www.researchgate.net/publication/340570349_Quel_type_d'abri_pour_mieux_reussir_la_production_de_tomate_de_contre_saison_au_Sud-Benin (accessed on 14 August 2021).
- Perez, K.; Froikin-Gordon, J.S.; Abdourhamane, I.K.; Levasseur, V.; Alfari, A.A.; Mensah, A.; Bonsu, O.; Habsatou, B.; Assogba-Komlan, F.; Mbaye, A.A.; et al. Connecting smallholder tomato producers to improved seed in West Africa. Agric. Food Secur. 2017, 6, 42. [Google Scholar] [CrossRef]
- Kugblenu, Y.O.; Danso, E.O.; Ofori, K.; Andersen, M.N.; Abenney-Mickson, S.; Sabi, E.B.; Plauborg, F.L.; Abekoe, M.K.; Jørgensen, S.T.; Jensen, C.R.; et al. Heat tolerance in field grown tomatoes (Lycopersicon esculentum Mill.) under semi-arid conditions of west Africa. Acta Hortic. 2013, 971, 99–106. [Google Scholar] [CrossRef]
- Kugblenu, Y.O.; Oppong-Danso, E.; Ofori, K.; Andersen, M.N.; Abenney-Mickson, S.; Sabi, E.B. Screening tomato genotypes for adaptation to high temperature in West Africa. Acta Agric. Scand. Sect. B Soil Plant. Sci. 2013, 63, 516–522. [Google Scholar] [CrossRef]
- Ayenan, M.A.T.; Danquah, A.; Hanson, P.; Ampomah-dwamena, C.; Sodedji, A.F.K.; Asante, I.K.; Danquah, E.Y. Accelerating Breeding for Heat Tolerance in Tomato (Solanum lycopersicum L.): An Integrated Approach. Agronomy 2019, 9, 720. [Google Scholar] [CrossRef] [Green Version]
- Driedonks, N.; Rieu, I.; Vriezen, W.H. Breeding for plant heat tolerance at vegetative and reproductive stages. Plant Reprod. 2016, 29, 67–79. [Google Scholar] [CrossRef] [Green Version]
- IPCC. Summary for Policymakers. An IPCC Special Report on the impacts of global warming of 1.5 °C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to 2018. In Global Warming of 1.5 °C; Masson-Delmotte, V., Zhai, P., Pörtner, H.-O., Roberts, D., Skea, J., Shukla, P.R., Pirani, A., Moufouma-Okia, W., Péan, C., Pidcock, R., et al., Eds.; World Meteorological Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Perkins-Kirkpatrick, S.E.; Lewis, S.C. Increasing trends in regional heatwaves. Nat. Commun. 2020, 11, 1–8. [Google Scholar] [CrossRef]
- Ro, S.; Chea, L.; Ngoun, S.; Stewart, Z.P.; Roeurn, S.; Theam, P.; Lim, S.; Sor, R.; Kosal, M.; Roeun, M.; et al. Response of tomato genotypes under different high temperatures in field and greenhouse conditions. Plants 2021, 10, 449. [Google Scholar] [CrossRef]
- Vijayakumar, A.; Shaji, S.; Beena, R.; Sarada, S.; Sajitha Rani, T.; Stephen, R.; Manju, R.V.; Viji, M.M. High temperature induced changes in quality and yield parameters of tomato (Solanum lycopersicum L.) and similarity coefficients among genotypes using SSR markers. Heliyon 2021, 7, e05988. [Google Scholar] [CrossRef]
- Sherzod, R.; Yang, E.Y.; Cho, M.C.; Chae, S.Y.; Chae, W.B. Physiological traits associated with high temperature tolerance differ by fruit types and sizes in tomato (Solanum lycopersicum L.). Hortic. Environ. Biotechnol. 2020, 61, 837–847. [Google Scholar] [CrossRef]
- Peet, M.; Sato, S.; Clémente, C.; Pressman, E. Heat stress increases sensitivity of pollen, fruit and seed production in tomatoes (Lycopersicon esculentum mill.) to non-optimal vapor pressure deficits. Acta Hortic. 2003, 618, 209–215. [Google Scholar] [CrossRef]
- Ayenan, M.A.T.; Danquah, A.; Hanson, P.; Asante, I.K.; Danquah, E.Y. Identification of new sources of heat tolerance in cultivated and wild tomatoes. Euphytica 2021, 217, 33. [Google Scholar] [CrossRef]
- Ayenan, M.A.T.; Danquah, A.; Agre, P.A.; Hanson, P.; Asante, I.K.; Danquah, E.Y. Genomic and phenotypic diversity of cultivated and wild tomatoes with varying levels of heat tolerance. Genes 2021, 12, 503. [Google Scholar] [CrossRef]
- Kassambara, A. “ggplot2” Based Publication Ready Plots. R Package Version 0.2.5. Available online: https://CRAN.R-project.org/package=ggpubr (accessed on 24 March 2021).
- Wobbrock, J.O.; Findlater, L.; Gergle, D.; Higgins, J.J. The Aligned Rank Transform for Nonparametric Factorial Analyses Using Only ANOVA Procedures. In Proceedings of the ACM Conference on Human Factors in Computing Systems (CHI ’11), Vancouver, Canada, 7–12 May 2011; pp. 143–146. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Pour-Aboughadareh, A.; Yousefian, M.; Moradkhani, H.; Moghaddam Vahed, M.; Poczai, P.; Siddique, K.H.M. iPASTIC: An online toolkit to estimate plant abiotic stress indices. Appl. Plant. Sci. 2019, 7, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Kassambara, A.; Mundt, F. factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R Package Version 1.0.6. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 24 March 2021).
- R Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 24 March 2021).
- Bhattarai, S.; Harvey, J.; Djidonou, D.; Leskovar, D. Exploring Morpho-Physiological Variation for Heat Stress Tolerance in Tomato. Plants 2021, 10, 347. [Google Scholar] [CrossRef]
- Tashiro, T.; Wardlaw, I. The Response to High Temperature Shock and Humidity Changes Prior to and During the Early Stages of Grain Development in Wheat. Funct. Plant. Biol. 1990, 17, 551. [Google Scholar] [CrossRef]
- Harel, D.; Fadida, H.; Slepoy, A.; Gantz, S.; Shilo, K. The Effect of Mean Daily Temperature and Relative Humidity on Pollen, Fruit Set and Yield of Tomato Grown in Commercial Protected Cultivation. Agronomy 2014, 4, 167–177. [Google Scholar] [CrossRef]
- Barker, J.C. Effects of day and night humidity on yield and fruit quality of glasshouse tomatoes (Lycopersicon esculentum Mill.). J. Hortic. Sci. 1990, 65, 323–331. [Google Scholar] [CrossRef]
- Ruiz-Nieves, J.M.; Ayala-Garay, O.J.; Serra, V.; Dumont, D.; Vercambre, G.; Génard, M.; Gautier, H. The effects of diurnal temperature rise on tomato fruit quality. Can the management of the greenhouse climate mitigate such effects? Sci. Hortic. 2021, 278, 109836. [Google Scholar] [CrossRef]
- Dane, F.; Hunter, A.G.; Chambliss, O.L. Fruit Set, Pollen Fertility, and Combining Ability of Selected Tomato Genotypes under High- temperature Field Conditions. J. Am. Soc. Hort. Sci. 1991, 116, 906–910. [Google Scholar] [CrossRef]
- Ayenan, M.A. DLB Product Profile—High-yielding Hot-Set Tomato Hybrid (Benin). Available online: https://www.demandledbreeding.org/sites/g/files/zhg1501/f/2020/08/16/product_profile_handout_tomato_benin_a4.pdf (accessed on 12 August 2021).
- Scott, J.W.; Volin, R.B.; Bryan, H.H.; Olson, S.M. Use of Hybrids to Develop Heat Tolerant Tomato Cultivars. Proc. Fla. State Hortic. Soc. 1986, 99, 311–314. [Google Scholar]
- AVRDC (World Vegetable Center). Fresh Market Tomato, Lines Developed at the World Vegetable Center. AVTO0301 (CLN2498D). Available online: https://avrdc.org/download/seed/cat-fresh-mark-tomato/AVTO0301.pdf (accessed on 15 August 2021).
- AVRDC (World Vegetable Center). Fresh Market Tomato, Lines Developed at the World Vegetable Center. AVTO1314 (CLN3212C). Available online: https://avrdc.org/download/seed/AVTO_1314.pdf (accessed on 15 August 2021).
- University of Florida Released Germplasm. Available online: https://tombreeding.ifas.ufl.edu/relsgerm.htm#Heat-Tolerant (accessed on 16 October 2021).
- Osei, M.K.; Danquah, E.; Danquah, A.; Blay, E.; Adu-Dapaah, H. Hybridity testing of tomato F1 progenies derived from parents with varying fruit quality and shelf life using single nucleotide polymorphism (SNPs). Sci. Afr. 2020, 8, e00267. [Google Scholar] [CrossRef]
- AVRDC (World Vegetable Center). Fresh Market Tomato, Lines Developed at the World Vegetable Center. AVTO9801 (CLN1621L). Available online: https://avrdc.org/download/seed/cat-process-tomato/AVTO9801.pdf (accessed on 15 August 2021).
- Basavaraj, P.S.; Gireesh, C.; Bharamappanavara, M.; Manoj, C.A.; Ishwarya Lakshmi, V.G.; Honnappa; Ajitha, V.; Senguttuvel, P.; Sundaram, R.M.; Anantha, M.S. Stress tolerance indices for the identification of low phosphorus tolerant introgression lines derived from Oryza rufipogon Griff. Plant. Genet. Resour. Characterisation Util. 2021, 19, 328–338. [Google Scholar] [CrossRef]
- Mohammadi, R. Efficiency of yield-based drought tolerance indices to identify tolerant genotypes in durum wheat. Euphytica 2016, 211, 71–89. [Google Scholar] [CrossRef]
- WRB. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2015. [Google Scholar]

| Genotypes | Fruit Size Category a | Heat Tolerance Status |
|---|---|---|
| BJ01 | Small | Highly tolerant |
| BJ02 | Small | Highly tolerant |
| CLN1621L | Small | Moderately tolerant |
| CLN3024A | Medium | Highly sensitive |
| CLN2366B | Medium | Moderately tolerant |
| CLN2026D | Medium | Moderately tolerant |
| Pectomech | Medium | Highly sensitive |
| LA2662 | Medium | Moderately tolerant |
| P068 | Medium | Highly sensitive |
| P082 | Large | Highly sensitive |
| P005 | Large | Highly sensitive |
| CLN3212C | Large | Moderately sensitive |
| CLN2498D | Large | Moderately sensitive |
| Fla.7236 | Extra large | Highly sensitive |
| Fla.7771 | Extra large | Highly sensitive |
| Fla.7171 | Extra large | Highly sensitive |
| Trial | Day Temperature (°C) | Night Temperatures (°C) | Day Relative Humidity (%) | Night Relative Humidity (%) |
|---|---|---|---|---|
| Greenhouse (HSG) | 30.0 ± 1.5 | 26.2 ± 0.7 | 77.6 ± 5.8 | 97.2 ± 1.7 |
| Open field (HSO) | 31.5 ± 1.4 | 28.9 ± 1.3 | 66.7 ± 7.6 | 77.8 ± 9.6 |
| Optimal season (OPT) | 28.4 ± 0.8 | 23.7 ± 0.9 | 71.0 ± 3.6 | 90.4 ± 2.2 |
| Genotypes | Yp | Ys | TOL | MP | GMP | HM | SSI | STI | YI | YSI | RSI | AR | SD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BJ01 | 5 | 4 | 9 | 4 | 4 | 3 | 5 | 4 | 4 | 5 | 5 | 4.7 | 1.6 |
| BJ02 | 1 | 7 | 16 | 1 | 2 | 7 | 13 | 2 | 7 | 13 | 13 | 7.5 | 5.6 |
| Fla.7236 | 4 | 6 | 15 | 5 | 5 | 5 | 7 | 5 | 6 | 7 | 7 | 6.5 | 3.0 |
| Fla.7771 | 7 | 11 | 14 | 8 | 9 | 11 | 10 | 9 | 11 | 10 | 10 | 10.0 | 1.8 |
| Fla.7171 | 6 | 9 | 13 | 7 | 8 | 8 | 9 | 8 | 9 | 9 | 9 | 8.6 | 1.7 |
| P082 | 16 | 16 | 4 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 14.9 | 3.6 |
| P005 | 11 | 14 | 8 | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 13.2 | 1.9 |
| P068 | 15 | 15 | 5 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 14.1 | 3.0 |
| CLN3212C | 3 | 2 | 11 | 3 | 3 | 2 | 4 | 3 | 2 | 4 | 4 | 3.7 | 2.5 |
| CLN1621L | 10 | 3 | 2 | 6 | 6 | 4 | 2 | 6 | 3 | 2 | 2 | 4.2 | 2.6 |
| CLN3024A | 9 | 12 | 12 | 10 | 12 | 12 | 12 | 12 | 12 | 12 | 12 | 11.5 | 1.0 |
| CLN2366B | 8 | 10 | 10 | 9 | 10 | 10 | 8 | 10 | 10 | 8 | 8 | 9.2 | 1.0 |
| CLN2498D | 2 | 1 | 7 | 2 | 1 | 1 | 3 | 1 | 1 | 3 | 3 | 2.3 | 1.8 |
| CLN2026D | 14 | 5 | 1 | 11 | 7 | 6 | 1 | 7 | 5 | 1 | 1 | 5.4 | 4.3 |
| Pectomech | 12 | 13 | 6 | 13 | 13 | 13 | 11 | 13 | 13 | 11 | 11 | 11.7 | 2.1 |
| LA2662 | 13 | 8 | 3 | 12 | 11 | 9 | 6 | 11 | 8 | 6 | 6 | 8.5 | 3.1 |
| Genotypes | Yp | Ys | TOL | MP | GMP | HM | SSI | STI | YI | YSI | RSI | AR | SD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BJ01 | 5 | 2 | 4 | 2 | 2 | 2 | 3 | 2 | 2 | 3 | 3 | 2.7 | 1.0 |
| BJ02 | 1 | 1 | 3 | 1 | 1 | 1 | 2 | 1 | 1 | 2 | 2 | 1.5 | 0.7 |
| Fla.7236 | 4 | 13 | 15 | 7 | 10 | 13 | 13 | 10 | 13 | 13 | 13 | 11.3 | 3.3 |
| Fla.7771 | 7 | 7 | 11 | 5 | 4 | 5 | 8 | 4 | 7 | 8 | 8 | 6.7 | 2.1 |
| Fla.7171 | 6 | 3 | 1 | 3 | 3 | 3 | 1 | 3 | 3 | 1 | 1 | 2.5 | 1.5 |
| P082 | 16 | 16 | 12 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 15.6 | 1.2 |
| P005 | 11 | 4 | 2 | 8 | 6 | 4 | 4 | 6 | 4 | 4 | 4 | 5.2 | 2.5 |
| P068 | 15 | 12 | 7 | 15 | 13 | 12 | 9 | 13 | 12 | 9 | 9 | 11.5 | 2.6 |
| CLN3212C | 3 | 15 | 16 | 10 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 13.5 | 3.8 |
| CLN1621L | 10 | 5 | 6 | 9 | 7 | 7 | 6 | 7 | 5 | 6 | 6 | 6.7 | 1.6 |
| CLN3024A | 9 | 14 | 13 | 12 | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 13.3 | 1.6 |
| CLN2366B | 8 | 6 | 10 | 6 | 5 | 6 | 7 | 5 | 6 | 7 | 7 | 6.6 | 1.4 |
| CLN2498D | 2 | 10 | 14 | 4 | 8 | 9 | 12 | 8 | 10 | 12 | 12 | 9.2 | 3.6 |
| CLN2026D | 14 | 8 | 5 | 11 | 9 | 8 | 5 | 9 | 8 | 5 | 5 | 7.9 | 2.9 |
| Pectomech | 12 | 11 | 9 | 13 | 11 | 10 | 11 | 11 | 11 | 11 | 11 | 11.0 | 1.0 |
| LA2662 | 13 | 9 | 8 | 14 | 12 | 11 | 10 | 12 | 9 | 10 | 10 | 10.7 | 1.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayenan, M.A.T.; Danquah, A.; Hanson, P.; Asante, I.K.; Danquah, E.Y. Tomato (Solanum lycopersicum L.) Genotypes Respond Differently to Long-Term Dry and Humid Heat Stress. Horticulturae 2022, 8, 118. https://doi.org/10.3390/horticulturae8020118
Ayenan MAT, Danquah A, Hanson P, Asante IK, Danquah EY. Tomato (Solanum lycopersicum L.) Genotypes Respond Differently to Long-Term Dry and Humid Heat Stress. Horticulturae. 2022; 8(2):118. https://doi.org/10.3390/horticulturae8020118
Chicago/Turabian StyleAyenan, Mathieu Anatole Tele, Agyemang Danquah, Peter Hanson, Isaac Kwadwo Asante, and Eric Yirenkyi Danquah. 2022. "Tomato (Solanum lycopersicum L.) Genotypes Respond Differently to Long-Term Dry and Humid Heat Stress" Horticulturae 8, no. 2: 118. https://doi.org/10.3390/horticulturae8020118
APA StyleAyenan, M. A. T., Danquah, A., Hanson, P., Asante, I. K., & Danquah, E. Y. (2022). Tomato (Solanum lycopersicum L.) Genotypes Respond Differently to Long-Term Dry and Humid Heat Stress. Horticulturae, 8(2), 118. https://doi.org/10.3390/horticulturae8020118








