Boosting Polyamines to Enhance Shoot Regeneration in Potato (Solanum tuberosum L.) Using AgNO3
Abstract
:1. Introduction
2. Materials and Methods
2.1. Explant Preparation
2.2. Callus Induction and Plant Regeneration
2.3. Quantification of Total Chlorophyll
2.4. Polyamine Determinations
2.5. H2O2 Determination
2.6. Lipid Peroxidation
2.7. Comet Assay
2.8. Statistical Analysis
3. Results
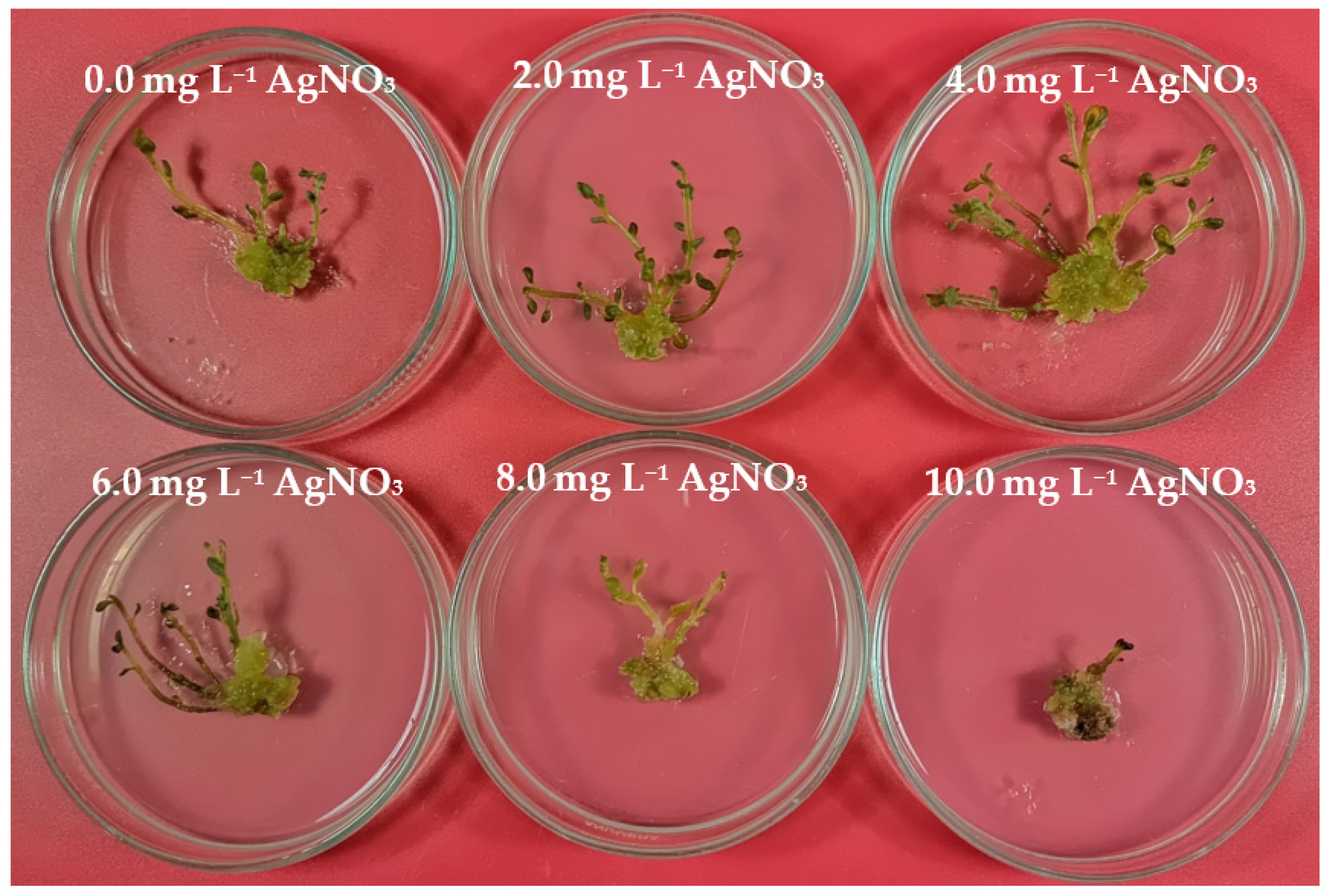
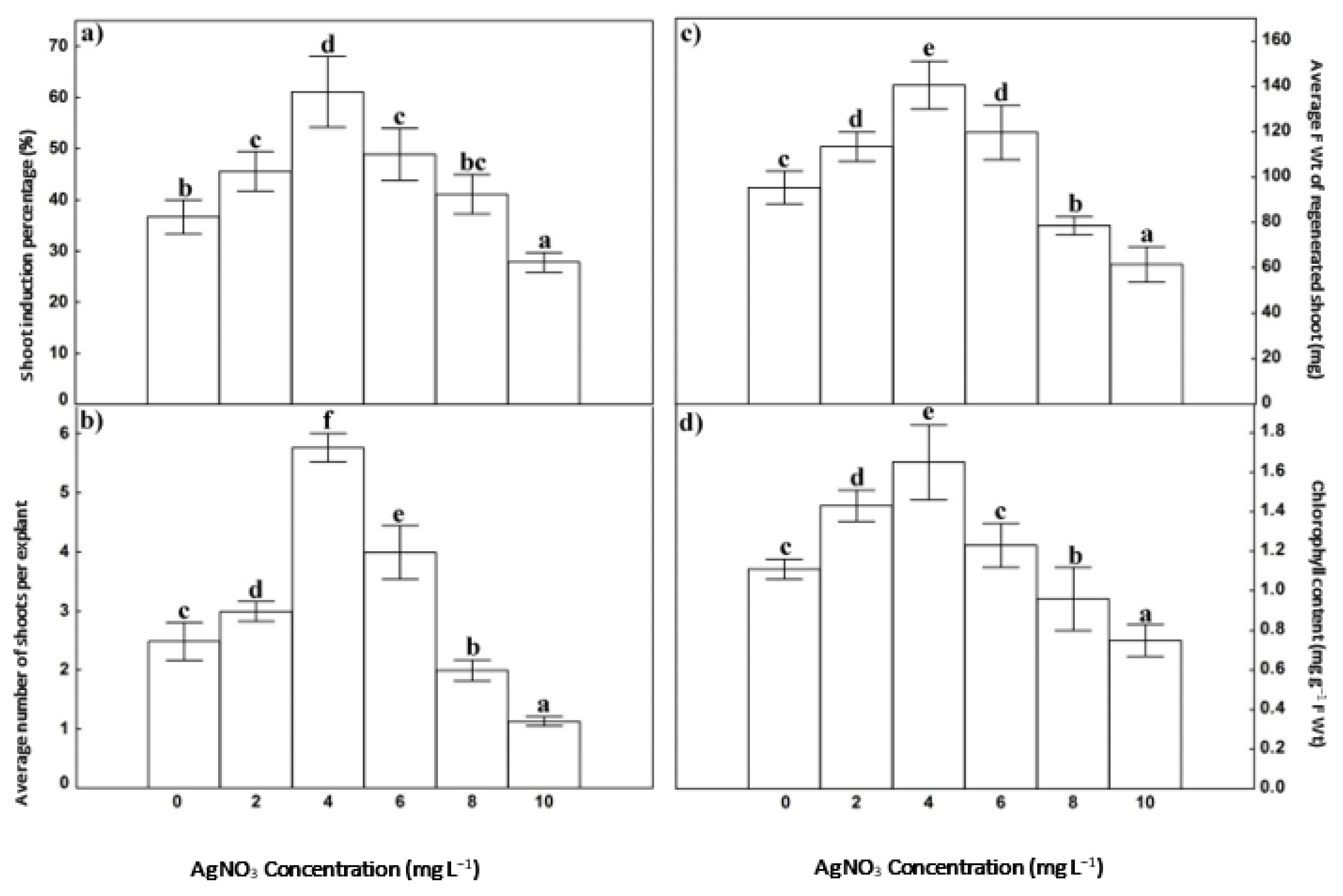
3.1. Callus Induction and Shoot Regeneration
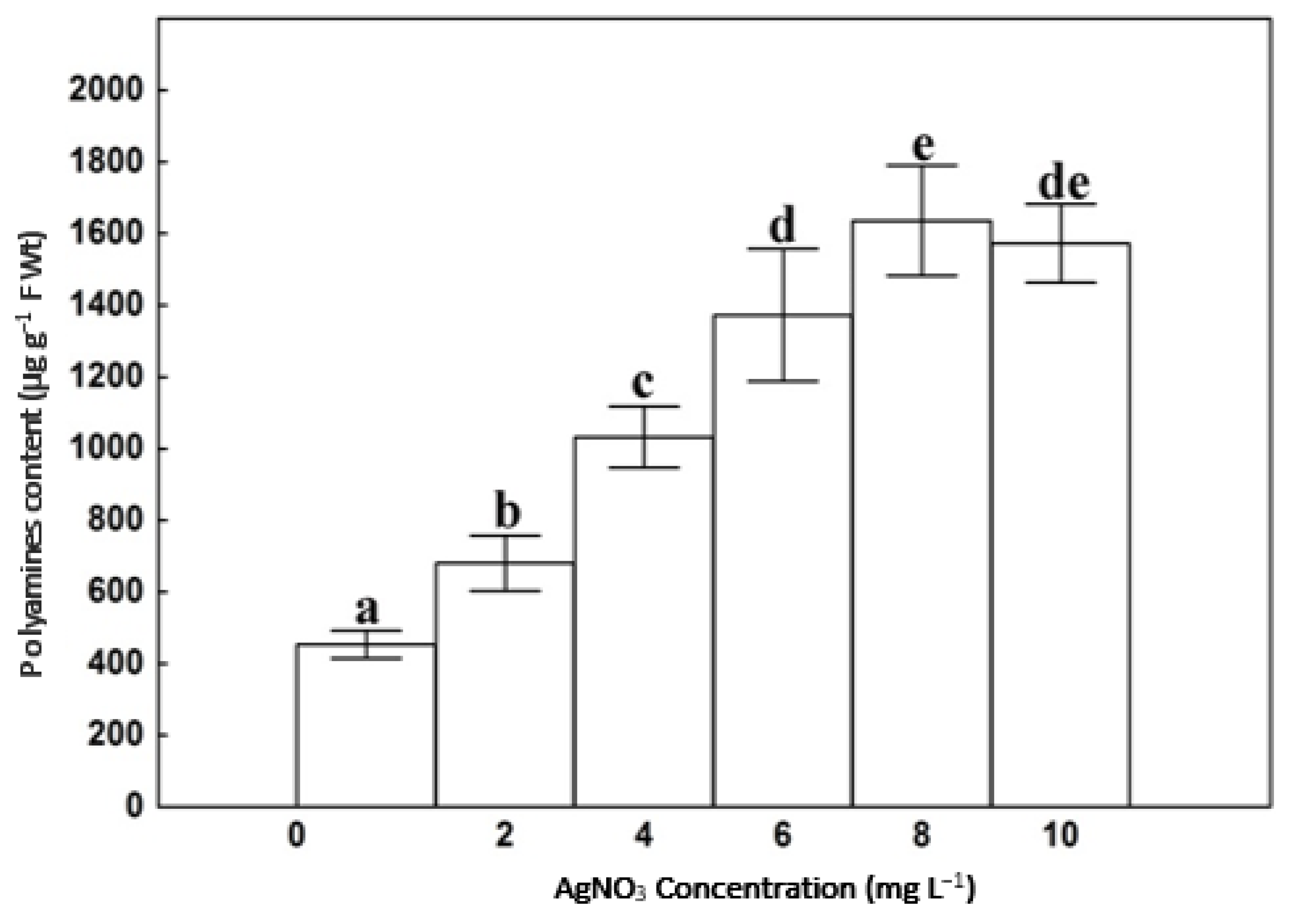
3.2. Polyamine Content
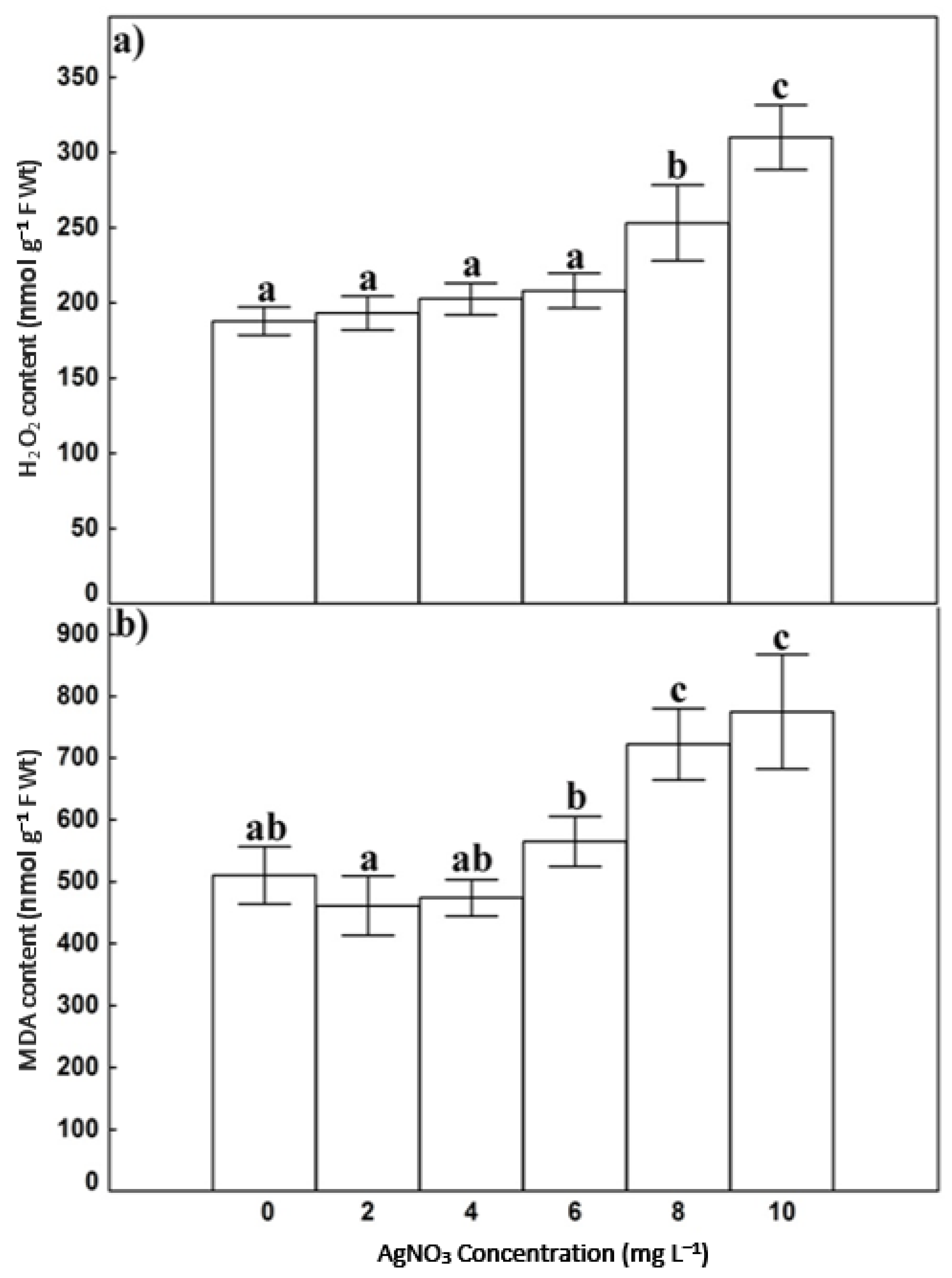
3.3. H2O2 and MDA
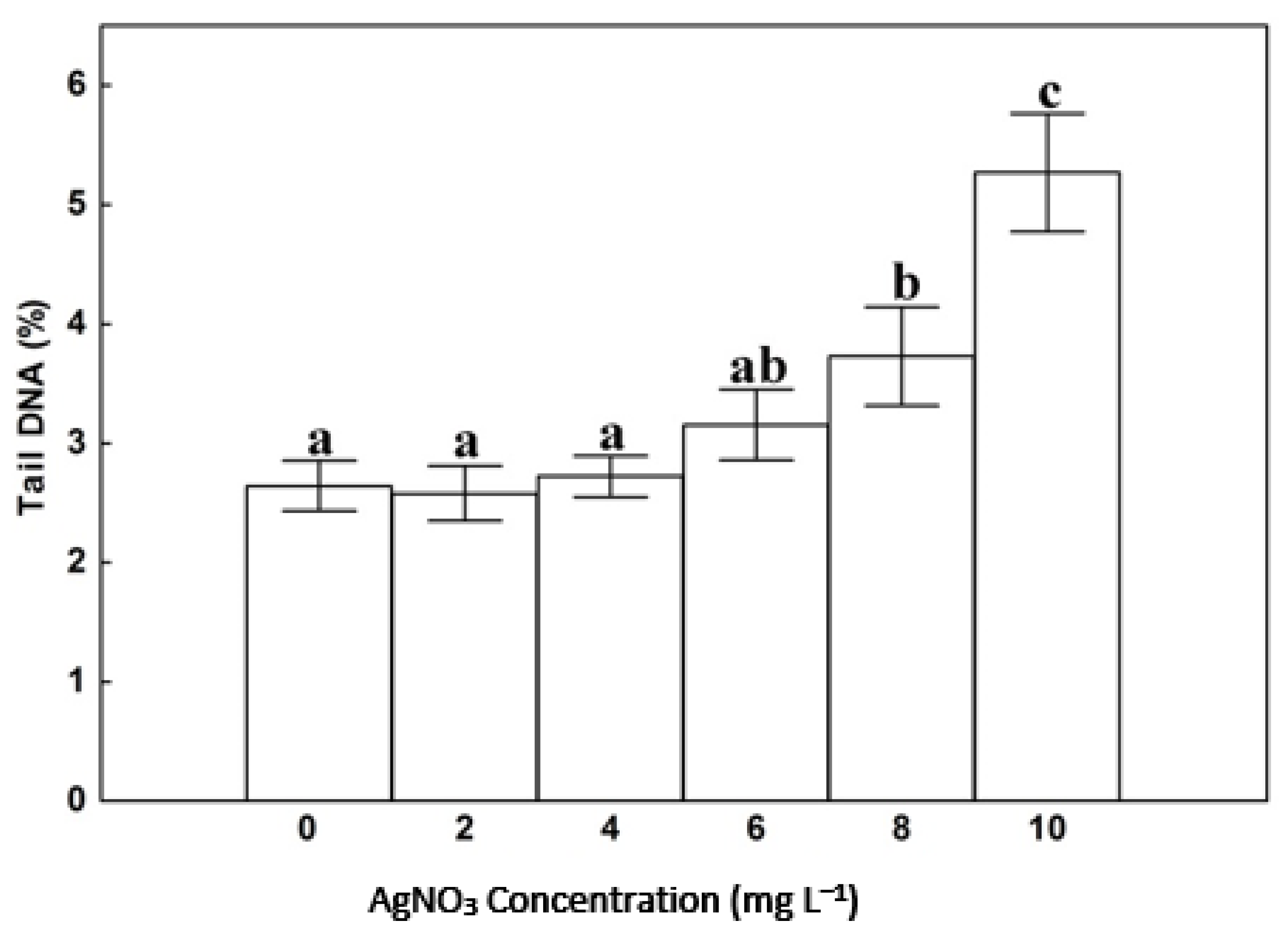
3.4. DNA Damage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Devaux, A.; Goffart, J.-P.; Kromann, P.; Andrade-Piedra, J.; Polar, V.; Hareau, G. The Potato of the Future: Opportunities and Challenges in Sustainable Agri-Food Systems. Potato Res. 2021, 64, 681–720. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, K.; Akhtar, M.H. Potato Production, Usage, and Nutrition—a Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Fen, X.; Yu, W.; Hu, H.; Dai, X. Progress of Potato Staple Food Research and Industry Development in China. J. Integr. Agric. 2017, 16, 2924–2932. [Google Scholar] [CrossRef]
- Maroufpour, B.; Rad, F.A.; Yazdanseta, S. Bioethanol Production as Biofuel from Potato Peel Using Saccharomyces Cerevisiae PTCC 5052 and Zymomonas Mobilis PTCC 1718. Bioagro 2019, 31, 177–184. [Google Scholar]
- Diambra, L.A. Genome Sequence and Analysis of the Tuber Crop Potato. Nature 2011, 475, 189–195. [Google Scholar]
- Hameed, A.; Zaidi, S.S.-A.; Shakir, S.; Mansoor, S. Applications of New Breeding Technologies for Potato Improvement. Front. Plant Sci. 2018, 9, 925. [Google Scholar] [CrossRef]
- Fouad, A.; Hafez, R.; Hosni, H. Authentication of Three Endemic Species of the Caryophyllaceae from Sinai Peninsula Using DNA Barcoding. Egypt. J. Bot. 2019, 59, 483–491. [Google Scholar] [CrossRef] [Green Version]
- Ashrafzadeh, S.; Leung, D.W. Novel Potato Plants with Enhanced Cadmium Resistance and Antioxidative Defence Generated after in Vitro Cell Line Selection. PLoS ONE 2017, 12, e0185621. [Google Scholar] [CrossRef] [Green Version]
- Ricroch, A.E.; Hénard-Damave, M.-C. Next Biotech Plants: New Traits, Crops, Developers and Technologies for Addressing Global Challenges. Crit. Rev. Biotechnol. 2016, 36, 675–690. [Google Scholar] [CrossRef]
- Ohnuma, M.; Teramura, H.; Shimada, H. A Simple Method to Establish an Efficient Medium Suitable for Potato Regeneration. Plant Biotechnol. J. 2020, 37, 25–30. [Google Scholar] [CrossRef]
- Muktadir, M.A.; Habib, M.A.; Mian, M.A.K.; Akhond, M.A.Y. Regeneration Efficiency Based on Genotype, Culture Condition and Growth Regulators of Eggplant (Solanum melongena L.). Agric. Nat. Resour. 2016, 50, 38–42. [Google Scholar] [CrossRef] [Green Version]
- Bordallo, P.N.; Silva, D.H.; Maria, J.; Cruz, C.D.; Fontes, E.P. Somaclonal Variation on in Vitro Callus Culture Potato Cultivars. Hortic. Bras. 2004, 22, 300–304. [Google Scholar] [CrossRef] [Green Version]
- Kumlay, A.M.; Ercisli, S. Callus Induction, Shoot Proliferation and Root Regeneration of Potato (Solanum tuberosum L.) Stem Node and Leaf Explants under Long-Day Conditions. Biotechnol. Biotechnol. Equip. 2015, 29, 1075–1084. [Google Scholar] [CrossRef] [Green Version]
- Skoog, F.; Miller, C. Chemical Regulation of Growth and Organ Formation in Plant Tissues Cultured In Vitro. Symp. Soc. Exp. Biol. 1957, 11, 118–130. [Google Scholar] [PubMed]
- Hasanuzzaman, M.; Alhaithloul, H.A.S.; Parvin, K.; Bhuyan, M.; Tanveer, M.; Mohsin, S.M.; Nahar, K.; Soliman, M.H.; Mahmud, J.A.; Fujita, M. Polyamine Action under Metal/Metalloid Stress: Regulation of Biosynthesis, Metabolism, and Molecular Interactions. Int. J. Mol. Sci. 2019, 20, 3215. [Google Scholar] [CrossRef] [Green Version]
- Rakesh, B.; Sudheer, W.; Nagella, P. Role of Polyamines in Plant Tissue Culture: An Overview. Plant Cell Tissue Organ. Cult. 2021, 145, 487–506. [Google Scholar] [CrossRef]
- Evans, P.T.; Malmberg, R.L. Do Polyamines Have Roles in Plant Development? Annu. Rev. Plant Biol. 1989, 40, 235–269. [Google Scholar] [CrossRef]
- Bais, H.P.; Ravishankar, G. Role of Polyamines in the Ontogeny of Plants and Their Biotechnological Applications. Plant Cell Tissue Organ. Cult. 2002, 69, 1–34. [Google Scholar] [CrossRef]
- Apelbaum, A.; Goldlust, A.; Icekson, I. Control by Ethylene of Arginine Decarboxylase Activity in Pea Seedlings and Its Implication for Hormonal Regulation of Plant Growth. Plant Physiol. 1985, 79, 635–640. [Google Scholar] [CrossRef] [Green Version]
- Smith, T. Plant Polyamines Metabolism And Function Polyamine Synthesis. In Polyamines And Ethylene: Biochemistry, Physiology And Interaction; American Society of Plant Physiologists: Rockville, MD, USA, 1985; pp. 1–23. [Google Scholar]
- Zhao, X.-C.; Qu, X.; Mathews, D.E.; Schaller, G.E. Effect of Ethylene Pathway Mutations upon Expression of the Ethylene Receptor ETR1 from Arabidopsis. Plant Physiol. 2002, 130, 1983–1991. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Parvatam, G.; Ravishankar, G.A. AgNO3: A Potential Regulator of Ethylene Activity and Plant Growth Modulator. Electron. J. Biotechnol. 2009, 12, 8–9. [Google Scholar] [CrossRef] [Green Version]
- Salem, J. Effects of Anti-Ethylene Compounds on Vitrification and Genome Fidelity of Stevia rebaudiana Bertoni. Egypt. J. Bot. 2020, 60, 519–535. [Google Scholar] [CrossRef]
- Strader, L.C.; Beisner, E.R.; Bartel, B. Silver Ions Increase Auxin Efflux Independently of Effects on Ethylene Response. Plant Cell 2009, 21, 3585–3590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, A.; Reddy, M.S.; Kumar, A. Efficient, One Step and Cultivar Independent Shoot Organogenesis of Potato. Physiol. Mol. Biol. Plants 2017, 23, 461–469. [Google Scholar] [CrossRef] [Green Version]
- Alva Ticona, S.; Oropeza, M. Effect of Culture Medium Consistence and Silver Nitrate on Micropropagation of Two Potato (Solanum tuberosum) Cultivars. Rev. Colomb. Biotecnol. 2013, 15, 55–62. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Merwad, A.-R.M.; Desoky, E.-S.M.; Rady, M.M. Response of Water Deficit-Stressed Vigna unguiculata Performances to Silicon, Proline or Methionine Foliar Application. Sci. Hortic. 2018, 228, 132–144. [Google Scholar] [CrossRef]
- Sestak, Z.; Catsky, J.; Jarvis, P. Determination of Chlorophylls a and b. Plant Photosynth. Prod. 1971, 672–701. [Google Scholar]
- Minocha, R.; Shortle, W.C.; Long, S.L.; Minocha, S.C. A Rapid and Reliable Procedure for Extraction of Cellular Polyamines and Inorganic Ions from Plant Tissues. J. Plant Growth Regul. 1994, 13, 187–193. [Google Scholar] [CrossRef] [Green Version]
- Federico, R.; Angelini, R.; Cogoni, A.; Floris, V. Enzymatic Methods for the Quantification of Polyamines Using Plant Amine Oxidases. Biochem. Physiol. Pflanz. 1991, 187, 113–119. [Google Scholar] [CrossRef]
- Loreto, F.; Velikova, V. Isoprene Produced by Leaves Protects the Photosynthetic Apparatus against Ozone Damage, Quenches Ozone Products, and Reduces Lipid Peroxidation of Cellular Membranes. Plant Physiol. 2001, 127, 1781–1787. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.R.; Bewley, J.D. Lipid Peroxidation Associated with Accelerated Aging of Soybean Axes. Plant Physiol. 1980, 65, 245–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tice, R.R.; Agurell, E.; Anderson, D.; Burlinson, B.; Hartmann, A.; Kobayashi, H.; Miyamae, Y.; Rojas, E.; Ryu, J.-C.; Sasaki, Y. Single Cell Gel/Comet Assay: Guidelines for in Vitro and in Vivo Genetic Toxicology Testing. Environ. Mol. Mutagen. 2000, 35, 206–221. [Google Scholar] [CrossRef]
- Ghosh, S.; Majumdar, S.; Sarkar, D.; Datta, K. An Efficient Adventitious Shoot Regeneration System for Potato (Solanum tuberosum L.) Using Leaf Discs. J. Plant Biochem. Biotechnol. 2015, 24, 298–304. [Google Scholar] [CrossRef]
- Xu, C.; Cao, H.; Zhang, Q.; Wang, H.; Xin, W.; Xu, E.; Zhang, S.; Yu, R.; Yu, D.; Hu, Y. Control of Auxin-Induced Callus Formation by BZIP59–LBD Complex in Arabidopsis Regeneration. Nat. Plants 2018, 4, 108–115. [Google Scholar] [CrossRef]
- Shin, J.; Seo, P.J. Varying Auxin Levels Induce Distinct Pluripotent States in Callus Cells. Front. Plant Sci. 2018, 9, 1653. [Google Scholar] [CrossRef] [Green Version]
- Bidabadi, S.S.; Jain, S.M. Cellular, Molecular, and Physiological Aspects of in Vitro Plant Regeneration. Plants 2020, 9, 702. [Google Scholar] [CrossRef]
- Kaur, A.; Kumar, A. The Effect of Gelling Agent, Medium PH and Silver Nitrate on Adventitious Shoot Regeneration in Solanum Tuberosum. bioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Shah, S.H.; Ali, S.; Jan, S.A.; Din, J.; Ali, G.M. Assessment of Silver Nitrate on Callus Induction and in Vitro Shoot Regeneration in Tomato (Solanum lycopersicum Mill.). Pak. J. Bot. 2014, 46, 2163–2172. [Google Scholar]
- Hayta, S.; Smedley, M.A.; Li, J.; Harwood, W.A.; Gilmartin, P.M. Plant Regeneration from Leaf-Derived Callus Cultures of Primrose (Primula vulgaris). HortScience 2016, 51, 558–562. [Google Scholar] [CrossRef] [Green Version]
- Warchol, M.; Juzoń, K.; Dziurka, K.; Czyczylo-Mysza, I.; Kaploniak, K.; Marcińska, I.; Skrzypek, E. The Effect of Zinc, Copper, and Silver Ions on Oat (Avena sativa L.) Androgenesis. Plants 2021, 10, 248. [Google Scholar] [CrossRef] [PubMed]
- Malik, W.A.; Mahmood, I.; Razzaq, A.; Afzal, M.; Shah, G.A.; Iqbal, A.; Zain, M.; Ditta, A.; Asad, S.A.; Ahmad, I.; et al. Exploring Potential of Copper and Silver Nano Particles to Establish Efficient Callogenesis and Regeneration System for Wheat (Triticum aestivum L.). GM Crops Food 2021, 12, 564–585. [Google Scholar] [CrossRef] [PubMed]
- Giridhar, P.; Indu, E.; Vijaya Ramu, D.; Ravishankar, G. Effect of Silver Nitrate on in Vitro Shoot Growth of Coffee. Trop. Sci. 2003, 43, 144–146. [Google Scholar] [CrossRef]
- Kaur, A.; Reddy, M.S.; Kumar, A. Direct Somatic Embryogenesis of Potato [Solanum tuberosum (L.)] Cultivar ‘Kufri Chipsona 2’. Plant Cell Tissue Organ. Cult. 2018, 134, 457–466. [Google Scholar] [CrossRef]
- Turhan, H. The Effect of Silver Nitrate (Ethylene Inhibitor) on in Vitro Shoot Development in Potato (Solanum tuberosum L.). Biotechnology 2004, 3, 72–74. [Google Scholar]
- Vaseva, I.I.; Qudeimat, E.; Potuschak, T.; Du, Y.; Genschik, P.; Vandenbussche, F.; Van Der Straeten, D. The Plant Hormone Ethylene Restricts Arabidopsis Growth via the Epidermis. Proc. Natl. Acad. Sci. USA 2018, 115, E4130–E4139. [Google Scholar] [CrossRef] [Green Version]
- Feray, A.; Hourmant, A.; Brun, A.; Penot, M. Effect of Polyamines on Morphogenesis of in Vitro Potato Plants (Solanum tuberosum Cv. Bintje). C. R. Acad. Sci. Sér. 3 Sci. Vie 1993, 316, 1446–1451. [Google Scholar]
- Vondráková, Z.; Eliášová, K.; Vágner, M.; Martincová, O.; Cvikrová, M. Exogenous Putrescine Affects Endogenous Polyamine Levels and the Development of Picea abies Somatic Embryos. Plant Growth Regul. 2015, 75, 405–414. [Google Scholar] [CrossRef]
- Aragão, V.P.M.; Reis, R.S.; Silveira, V.; Santa-Catarina, C. Putrescine Promotes Changes in the Endogenous Polyamine Levels and Proteomic Profiles to Regulate Organogenesis in Cedrela fissilis Vellozo (Meliaceae). Plant Cell Tissue Organ. Cult. 2017, 130, 495–505. [Google Scholar] [CrossRef]
- Ahlawat, J.; Sehrawat, A.R.; Choudhary, R.; Samarina, L.; Bandaralage, J.-H.; Chaudhary, R. Quantifying Synergy of Plant Growth Hormones, Anti-Oxidants, Polyamines and Silver Nitrate for Optimizing the Micro Propagation of Capparis decidua: An Underutilised Medicinal Shrub. Nucleus 2020, 63, 313–325. [Google Scholar] [CrossRef]
- Khoshbakht, D.; Asghari, M.; Haghighi, M. Influence of Foliar Application of Polyamines on Growth, Gas-Exchange Characteristics, and Chlorophyll Fluorescence in Bakraii citrus under Saline Conditions. Photosynthetica 2018, 56, 731–742. [Google Scholar] [CrossRef]
- Yousefi, F.; Jabbarzadeh, Z.; Amiri, J.; Rasouli-Sadaghiani, M.H. Response of Roses (Rosa hybrida L.‘Herbert Stevens’) to Foliar Application of Polyamines on Root Development, Flowering, Photosynthetic Pigments, Antioxidant Enzymes Activity and NPK. Sci. Rep. 2019, 9, 1–11. [Google Scholar]
- Kim, J.K.; Baskar, T.B.; Park, S.U. Silver Nitrate and Putrescine Enhance In vitro Shoot Organogenesis in Polygonum tinctorium. Biosci. Biotechnol. Res. Asia 2016, 13, 53–58. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, A.; Liu, S.; Singh, P.K.; Kumar, N.; Pandey, A.C.; Tripathi, D.K.; Chauhan, D.K.; Sahi, S. Differential Phytotoxic Responses of Silver Nitrate (AgNO3) and Silver Nanoparticle (AgNps) in Cucumis sativus L. Plant Gene 2017, 11, 255–264. [Google Scholar] [CrossRef]
- Noori, A.; Donnelly, T.; Colbert, J.; Cai, W.; Newman, L.A.; White, J.C. Exposure of Tomato (Lycopersicon esculentum) to Silver Nanoparticles and Silver Nitrate: Physiological and Molecular Response. Int. J. Phytoremediation 2020, 22, 40–51. [Google Scholar] [CrossRef] [PubMed]
- De Veylder, L.; Beeckman, T.; Inzé, D. The Ins and Outs of the Plant Cell Cycle. Nat. Rev. Mol. Cell Biol. 2007, 8, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Hafez, R.; Fouad, A. Mitigation of Genotoxic and Cytotoxic Effects of Silver Nanoparticles on Onion Root Tips Using Some Antioxidant Scavengers. Egypt. J. Bot. 2020, 60, 133–145. [Google Scholar] [CrossRef]
- Chen, X.-B.; Zhao, X.-H.; Zhu, Y.; Gong, Y.-D.; Li, L.-B.; Zhang, J.-P.; Kuang, T.-Y. Hydrogen Peroxide-Induced Chlorophyll a Bleaching in the Cytochrome b 6 f Complex: A Simple and Effective Assay for Stability of the Complex in Detergent Solutions. Photosynth. Res. 2006, 90, 205–214. [Google Scholar] [CrossRef]
- Dai, H.; Shan, C. Others Effects of Lanthanum on the Antioxidant Capacity of Chloroplasts and Chlorophyll Fluorescence Parameters of Maize Seedlings under Chromium Stress. Photosynthetica 2019, 57, 27–31. [Google Scholar] [CrossRef]
- Santos, C.L.; Pourrut, B.; Oliveira, J.M.P. The Use of Comet Assay in Plant Toxicology: Recent Advances. Front. Genet. 2015, 6, 216. [Google Scholar] [CrossRef]
- Szpunar-Krok, E.; Jańczak-Pieniążek, M.; Skrobacz, K.; Bobrecka-Jamro, D.; Balawejder, M. Response of Potato (Solanum tuberosum L.) Plants to Spraying by Hydrogen Peroxide. Sustainability 2020, 12, 2469. [Google Scholar] [CrossRef] [Green Version]
- Dikilitas, M.; Collins, A.R.; Kocyigit, A.; El Yamani, N.; Karakas, S. DNA Damage in Potato Plants Exposed to High Level of NaCl Stress. In Proceedings of the Frontiers in Genetics, Conference Abstract: ICAW, Antwerpen, Belgium, 1–4 September 2015. [Google Scholar]
- Vishwakarma, K.; Upadhyay, N.; Singh, J.; Liu, S.; Singh, V.P.; Prasad, S.M.; Chauhan, D.K.; Tripathi, D.K.; Sharma, S. Differential Phytotoxic Impact of Plant Mediated Silver Nanoparticles (AgNPs) and Silver Nitrate (AgNO3) on Brassica Sp. Front. Plant Sci. 2017, 8, 1501. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.Y.; Kim, Y.J.; Park, K.Y. Increasing Polyamine Contents Enhances the Stress Tolerance via Reinforcement of Antioxidative Properties. Front. Plant Sci. 2019, 10, 1331. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adly, W.M.R.M.; Mazrou, Y.S.A.; EL-Denary, M.E.; Mohamed, M.A.; Abd El-Salam, E.-S.T.; Fouad, A.S. Boosting Polyamines to Enhance Shoot Regeneration in Potato (Solanum tuberosum L.) Using AgNO3. Horticulturae 2022, 8, 113. https://doi.org/10.3390/horticulturae8020113
Adly WMRM, Mazrou YSA, EL-Denary ME, Mohamed MA, Abd El-Salam E-ST, Fouad AS. Boosting Polyamines to Enhance Shoot Regeneration in Potato (Solanum tuberosum L.) Using AgNO3. Horticulturae. 2022; 8(2):113. https://doi.org/10.3390/horticulturae8020113
Chicago/Turabian StyleAdly, Walaa M. R. M., Yasser S. A. Mazrou, Mohammad E. EL-Denary, Mahasen A. Mohamed, El-Sayed T. Abd El-Salam, and Ahmed S. Fouad. 2022. "Boosting Polyamines to Enhance Shoot Regeneration in Potato (Solanum tuberosum L.) Using AgNO3" Horticulturae 8, no. 2: 113. https://doi.org/10.3390/horticulturae8020113
APA StyleAdly, W. M. R. M., Mazrou, Y. S. A., EL-Denary, M. E., Mohamed, M. A., Abd El-Salam, E.-S. T., & Fouad, A. S. (2022). Boosting Polyamines to Enhance Shoot Regeneration in Potato (Solanum tuberosum L.) Using AgNO3. Horticulturae, 8(2), 113. https://doi.org/10.3390/horticulturae8020113






