Evaluation of the Acaricidal Activity of Lithium Chloride against Tetranychus urticae (Acari: Tetranychidae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Acaricidal Bio-Assay
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stumpf, N.; Zebitz, C.P.; Kraus, W.; Moores, G.D.; Nauen, R. Resistance to organophosphates and biochemical genotyping of acetylcholinesterases in Tetranychus urticae (Acari: Tetranychidae). Pestic. Biochem. Physiol. 2001, 69, 131–142. [Google Scholar] [CrossRef]
- Van Leeuwen, T.; Van Pottelberge, S.; Nauen, R.; Tirry, L. Organophosphate insecticides and acaricides antagonise bifenazate toxicity through esterase inhibition in Tetranychus urticae. Pest Manag. Sci. Former. Pestic. Sci. 2007, 63, 1172–1177. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.T.; Lyon, H.H. Insects That Feed on Trees and Shrubs; Comstock Publishing Associates: Ithaca, NY, USA, 1976. [Google Scholar]
- Schulz, J. Tetranychus telarius (L.) new vector of virus Y. Plant Dis. Rept. 1963, 47, 594–596. [Google Scholar]
- Orlob, G.B. Relationships between Tetranychus urticae Koch and some plant viruses. Virology 1968, 35, 121–133. [Google Scholar] [CrossRef]
- Leeuwen, T.V.; Vontas, J.; Tsagkarakou, A.; Tirry, L. Mechanisms of acaricide resistance in the two-spotted spider mite Tetranychus urticae. In Biorational Control of Arthropod Pests; Springer: Berlin/Heidelberg, Germany, 2009; pp. 347–393. [Google Scholar] [CrossRef]
- Vrie, M.v.d.; Mcmurtry, J.A.; Huffaker, C.B. Ecology of tetranychid mites and their natural enemies: A review III. Biology, ecology and pest status, and host plant relations of tetranychids. Hilgardia 1972, 41, 343–432. [Google Scholar] [CrossRef]
- Heuskin, S.; Verheggen, F.J.; Haubruge, E.; Wathelet, J.-P.; Lognay, G. The use of semiochemical slow-release devices in integrated pest management strategies. Biotechnol. Agron. Société Environ. 2011, 15, 459–470. [Google Scholar]
- Isman, M.B. Plant essential oils for pest and disease management. Crop. Prot. 2000, 19, 603–608. [Google Scholar] [CrossRef]
- Isman, M. Pesticides based on plant essential oils for management of plant pests and diseases. In Proceedings of the International Symposium on Development of Natural Pesticides from Forest Resources, Seoul, Republic of Korea, 2001; pp. 1–9. [Google Scholar]
- Isman, M.B. Plant Essential Oils as Green Pesticides for Pest and Disease Management; ACS Publications: Washington, DC, USA, 2004; Ser 887; pp. 41–51. [Google Scholar]
- Chiasson, H.; Bélanger, A.; Bostanian, N.; Vincent, C.; Poliquin, A. Acaricidal properties of Artemisia absinthium and Tanacetum vulgare (Asteraceae) essential oils obtained by three methods of extraction. J. Econ. Entomol. 2001, 94, 167–171. [Google Scholar] [CrossRef]
- Hay, R.K.; Waterman, P.G. Volatile Oil Crops: Their Biology, Biochemistry and Production; Longman Scientific and Technical: London, UK, 1993. [Google Scholar]
- Basta, A.; Spooner-Hart, R. Efficacy of an extract of Dorrigo pepper against two-spotted mite and greenhouse thrips. In Spray Oils beyond 2000, 25–29 October 1999; University of Western Sydney Australia: Penrith, Australia, 2002; pp. 471–476. [Google Scholar]
- Habashi, F. Handbook of Extractive Metallurgy; Wiley-Vch: Weinheim, Germany, 1997; Volume 3, p. 288. [Google Scholar]
- Aral, H.; Vecchio-Sadus, A. Toxicity of lithium to humans and the environment—A literature review. Ecotoxicol. Environ. Saf. 2008, 70, 349–356. [Google Scholar] [CrossRef]
- Shahzad, B.; Tanveer, M.; Hassan, W.; Shah, A.N.; Anjum, S.A.; Cheema, S.A.; Ali, I. Lithium toxicity in plants: Reasons, mechanisms and remediation possibilities–A review. Plant Physiol. Biochem. 2016, 107, 104–115. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, L.; Mu, S.-Y.; Tian, C.-Y. Apocynum venetum: A newly found lithium accumulator. Flora-Morphol. Distrib. Funct. Ecol. Plants 2014, 209, 285–289. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Mukherjee, A.B. Trace elements of group 12 (Previously group IIb). Trace Elem. Soil Hum. 2007, 283–319. [Google Scholar] [CrossRef]
- Hawrylak-Nowak, B.; Kalinowska, M.; Szymańska, M. A study on selected physiological parameters of plants grown under lithium supplementation. Biol. Trace Elem. Res. 2012, 149, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gao, P.; Gjetvaj, B.; Westcott, N.; Gruber, M.Y. Analysis of the metabolome and transcriptome of Brassica carinata seedlings after lithium chloride exposure. Plant Sci. 2009, 177, 68–80. [Google Scholar] [CrossRef]
- Vlasiuk, P.; Okhrimenko, M.; Kuz’menko, L. Fractional and amino acid composition of proteins and content of free amino acids in potato under the effect of lithium. Fiziol Biokhim Kult Rast 1975, 7, 115–120. [Google Scholar]
- Vlasyuk, P.; Kuzmenko, L.; Okhrimenko, M. Content and fractional composition of potato proteins and nucleic-acids under lithium effect. Dopovidi Akad. Nauk. Ukr. Rsr Seriya B-Geol. Khimichni Ta Biol. Nauk. 1975, 8, 742–748. [Google Scholar]
- Vlasiuk, P.; Kuz’menko, L.; Okhrimenko, M. Role of lithium in protein-nucleic metabolism of plants. Fiziol. I Biokhimiia Kul’turnykh Rastenii. Physiol. Biochem. Cultiv. Plants 1979, 11, 438–447. [Google Scholar]
- Kent, N. Absorption, translocation and ultimate fate of lithium in the wheat plant. New Phytol. 1941, 40, 291–298. [Google Scholar] [CrossRef]
- Abood, J.; Lösel, D.; Ayres, P. Lithium chloride and cucumber powdery mildew infection. Plant Pathol. 1991, 40, 108–117. [Google Scholar] [CrossRef]
- Kavanagh, L.; Keohane, J.; Cabellos, G.G.; Lloyd, A.; Cleary, J. Induced Plant Accumulation of Lithium. Geosciences 2018, 8, 56. [Google Scholar] [CrossRef]
- Price, L.H.; Heninger, G.R. Lithium in the treatment of mood disorders. N. Engl. J. Med. 1994, 331, 591–598. [Google Scholar] [PubMed]
- Ohgami, H.; Terao, T.; Shiotsuki, I.; Ishii, N.; Iwata, N. Lithium levels in drinking water and risk of suicide. Br. J. Psychiatry 2009, 194, 464–465. [Google Scholar] [CrossRef] [PubMed]
- Schrauzer, G.N.; Shrestha, K.P. Lithium in drinking water and the incidences of crimes, suicides, and arrests related to drug addictions. Biol. Trace Elem. Res. 1990, 25, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Kapusta, N.D.; Mossaheb, N.; Etzersdorfer, E.; Hlavin, G.; Thau, K.; Willeit, M.; Praschak-Rieder, N.; Sonneck, G.; Leithner-Dziubas, K. Lithium in drinking water and suicide mortality. Br. J. Psychiatry 2011, 198, 346–350. [Google Scholar] [CrossRef]
- Sugawara, N.; Yasui-Furukori, N.; Ishii, N.; Iwata, N.; Terao, T. Lithium in tap water and suicide mortality in Japan. Int. J. Environ. Res. Public Health 2013, 10, 6044–6048. [Google Scholar] [CrossRef]
- Giotakos, O.; Nisianakis, P.; Tsouvelas, G.; Giakalou, V.-V. Lithium in the public water supply and suicide mortality in Greece. Biol. Trace Elem. Res. 2013, 156, 376–379. [Google Scholar] [CrossRef]
- Liaugaudaite, V.; Mickuviene, N.; Raskauskiene, N.; Naginiene, R.; Sher, L. Lithium levels in the public drinking water supply and risk of suicide: A pilot study. J. Trace Elem. Med. Biol. 2017, 43, 197–201. [Google Scholar] [CrossRef]
- Terao, T. Is lithium potentially a trace element? World J. Psychiatry 2015, 5, 1. [Google Scholar] [CrossRef]
- Marshall, T.M. Lithium as a nutrient. J. Am. Physicians Surg. 2015, 20, 104–109. [Google Scholar]
- Schrauzer, G.N. Lithium: Occurrence, dietary intakes, nutritional essentiality. J. Am. Coll. Nutr. 2002, 21, 14–21. [Google Scholar] [CrossRef]
- Jacobson, S.; Ceolin, L.; Kaur, P.; Pastuszak, A.; Einarson, T.; Koren, G.; Jones, K.; Johnson, K.; Sahn, D.; Donnenfeld, A. Prospective multicentre study of pregnancy outcome after lithium exposure during first trimester. Lancet 1992, 339, 530–533. [Google Scholar] [CrossRef] [PubMed]
- Won, E.; Kim, Y.-K. An oldie but goodie: Lithium in the treatment of bipolar disorder through neuroprotective and neurotrophic mechanisms. Int. J. Mol. Sci. 2017, 18, 2679. [Google Scholar] [CrossRef] [PubMed]
- Weiner, M.L. Overview of lithium toxicology. In Lithium in Biology and Medicine; Schrauzer, G.N., Klippel, K.F., Eds.; VCH Verlag: Weinheim, Germany, 1991; pp. 83–99. [Google Scholar]
- Tutun, H.; Kahraman, H.A.; Aluc, Y.; Avci, T.; Ekici, H. Investigation of some metals in honey samples from West Mediterranean region of Turkey. Vet. Res. Forum 2019, 10, 181–186. [Google Scholar] [PubMed]
- Bogdanov, S.; Jurendic, T.; Sieber, R.; Gallmann, P. Honey for nutrition and health: A review. J. Am. Coll. Nutr. 2008, 27, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Kolics, É.; Specziár, A.; Taller, J.; Mátyás, K.K.; Kolics, B. Lithium chloride outperformed oxalic acid sublimation in a preliminary experiment for Varroa mite control in pre-wintering honey bee colonies. Acta Vet. Hung. 2021, 68, 370–373. [Google Scholar] [CrossRef]
- Kolics, B.; Kolics, É.; Mátyás, K.; Taller, J.; Specziár, A. Comparison of Alternative Application Methods for Anti-Varroa Lithium Chloride Treatments. Insects 2022, 13, 633. [Google Scholar] [CrossRef]
- Kolics, B.; Kolics, É.; Solti, I.; Bacsi, Z.; Taller, J.; Specziár, A.; Mátyás, K. Lithium Chloride Shows Effectiveness against the Poultry Red Mite (Dermanyssus gallinae). Insects 2022, 13, 1005. [Google Scholar] [CrossRef]
- Dennehy, T.J.; Farnham, A.W.; Denholm, I. The microimmersion bioassay: A novel method for the topical application of pesticides to spider mites. Pestic. Sci. 1993, 39, 47–54. [Google Scholar] [CrossRef]
- Kolics, É.; Mátyás, K.; Taller, J.; Specziár, A.; Kolics, B. Contact Effect Contribution to the High Efficiency of Lithium Chloride Against the Mite Parasite of the Honey Bee. Insects 2020, 11, 333. [Google Scholar] [CrossRef]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Feyereisen, R.; Dermauw, W.; Van Leeuwen, T. Genotype to phenotype, the molecular and physiological dimensions of resistance in arthropods. Pestic. Biochem. Physiol. 2015, 121, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Ziegelmann, B.; Abele, E.; Hannus, S.; Beitzinger, M.; Berg, S.; Rosenkranz, P. Lithium chloride effectively kills the honey bee parasite Varroa destructor by a systemic mode of action. Sci. Rep. 2018, 8, 683. [Google Scholar] [CrossRef]
- Brun, L.; Maillet, J.; Hinsinger, P.; Pépin, M. Evaluation of copper availability to plants in copper-contaminated vineyard soils. Environ. Pollut. 2001, 111, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-Q. Mites of Greenhouses: Identification, Biology and Control; CABI: Wallingford, UK, 2003; 244p. [Google Scholar]
- Miles, M.; Eelen, H. The effects of spinosad to beneficial insects and mites and its use in IPM. Commun. Agric. Appl. Biol. Sci. 2006, 71, 275–284. [Google Scholar] [PubMed]
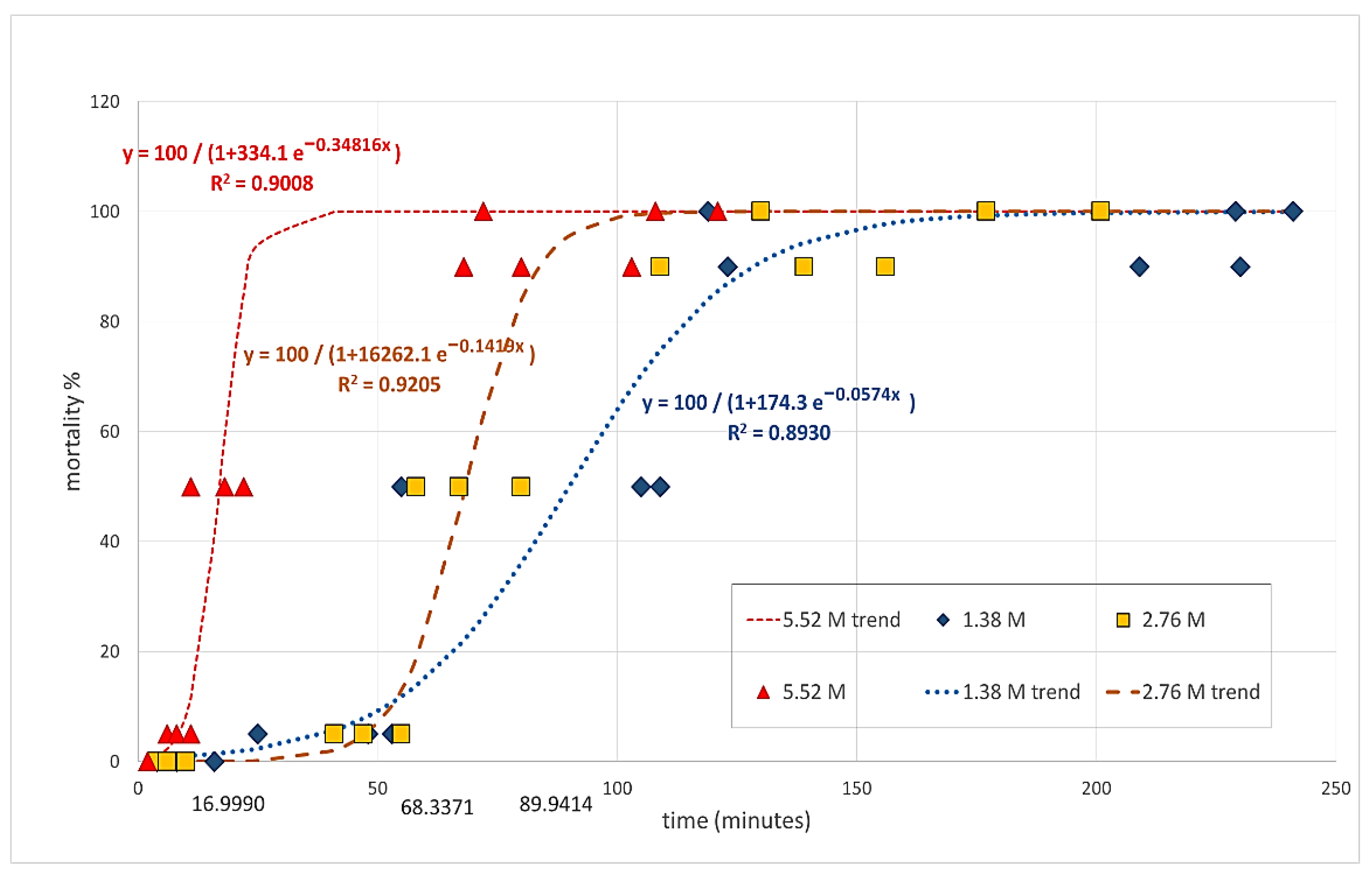
| Treatment | First Symptom * | First Dead * | LD50 * | LD90 * | LD100 |
|---|---|---|---|---|---|
| 1.38 M | 9.33 ± 3.528 a | 42.00 ± 8.622 a | 89.6 7± 17.372 a | 187.33 ± 32.733 a | 196.33 a ± 38.822 |
| 2.76 M | 6.67 ± 1.764 a | 47.67 ± 4.055 a | 68.33 ± 6.386 a | 134.67 ± 13.740 a,b | 169.33 a ± 20.851 |
| 5.52 M | 1.67 ± 0.333 b | 8.33 ± 1.453 b | 17.00 ± 3.215 b | 83.67 ± 10.269 b | 100.33 a ± 14.655 |
| The trend of mortality rates (y) by time elapsed after treatment in minutes (x) | |||||
| 1.38 M | y = 100/(1 + 16262.1 ± e−0.1419x); R2 = 0.9205 | ||||
| 2.76 M | y = 100/(1 + 174.3 ± e−0.0574x); R2 = 0.8930 | ||||
| 5.52 M | y = 100/(1 + 334.1 ± e −0.34816x); R2 = 0.9008 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solti, I.; Kolics, É.; Keszthelyi, S.; Bacsi, Z.; Staszny, Á.; Nagy, E.; Taller, J.; Mátyás, K.; Kolics, B. Evaluation of the Acaricidal Activity of Lithium Chloride against Tetranychus urticae (Acari: Tetranychidae). Horticulturae 2022, 8, 1127. https://doi.org/10.3390/horticulturae8121127
Solti I, Kolics É, Keszthelyi S, Bacsi Z, Staszny Á, Nagy E, Taller J, Mátyás K, Kolics B. Evaluation of the Acaricidal Activity of Lithium Chloride against Tetranychus urticae (Acari: Tetranychidae). Horticulturae. 2022; 8(12):1127. https://doi.org/10.3390/horticulturae8121127
Chicago/Turabian StyleSolti, Izabella, Éva Kolics, Sándor Keszthelyi, Zsuzsanna Bacsi, Ádám Staszny, Erzsébet Nagy, János Taller, Kinga Mátyás, and Balázs Kolics. 2022. "Evaluation of the Acaricidal Activity of Lithium Chloride against Tetranychus urticae (Acari: Tetranychidae)" Horticulturae 8, no. 12: 1127. https://doi.org/10.3390/horticulturae8121127
APA StyleSolti, I., Kolics, É., Keszthelyi, S., Bacsi, Z., Staszny, Á., Nagy, E., Taller, J., Mátyás, K., & Kolics, B. (2022). Evaluation of the Acaricidal Activity of Lithium Chloride against Tetranychus urticae (Acari: Tetranychidae). Horticulturae, 8(12), 1127. https://doi.org/10.3390/horticulturae8121127






