Potential of Debaryomyces hansenii Strains on the Inhibition of Botrytis cinerea in Blueberry Fruits (Vaccinium corymbosum L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Yeast Preparation
2.2. Pathogen and Fruit
2.3. Mycelial Growth Inhibition
2.4. Germination Percentage
2.5. Inhibition by Volatile Organic Compounds (VOCs)
2.6. Scanning Electron Microscopy (SEM)
2.7. Debaryomyces hansenii In Vivo Evaluation
2.8. Effect of D. hansenii on the Postharvest Quality of the Fruit
2.8.1. Soluble Solids
2.8.2. Titratable Acidity
2.8.3. pH
2.8.4. Color
2.8.5. Firmness
2.8.6. Physiological Weight Loss
2.9. Statistic Analysis
3. Results and Discussion
3.1. Mycelial Growth Inhibition
3.2. Germination Percentage
3.3. Inhibition by Volatile Compounds
3.4. Scanning Electron Microscopy
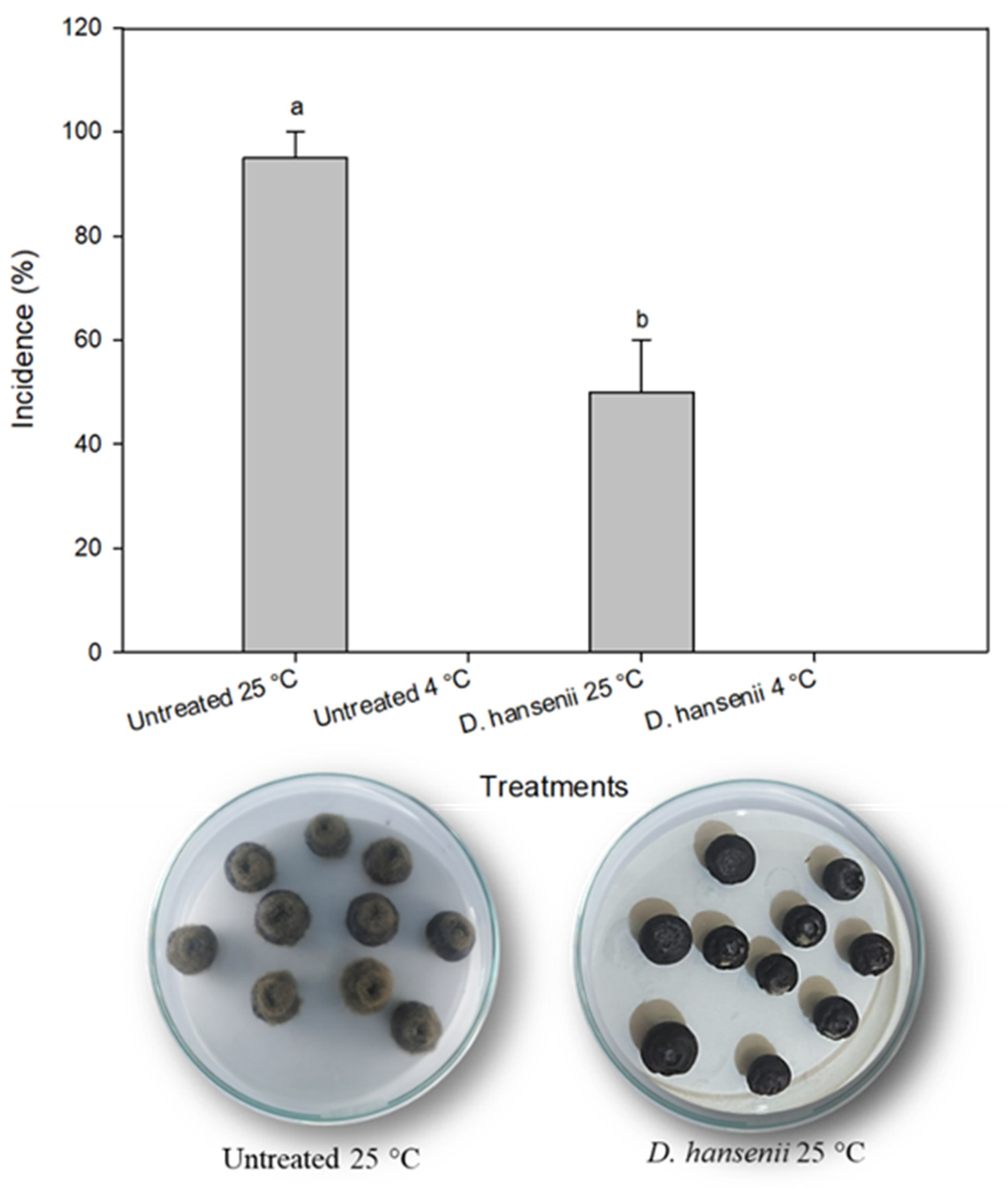
3.5. Debaryomyces Hansenii In Vivo Evaluation
3.6. Effect of D. hansenii on the Postharvest Quality of the Fruit
3.6.1. Soluble Solids, pH, Titratable Acidity
3.6.2. Firmness
3.6.3. Color
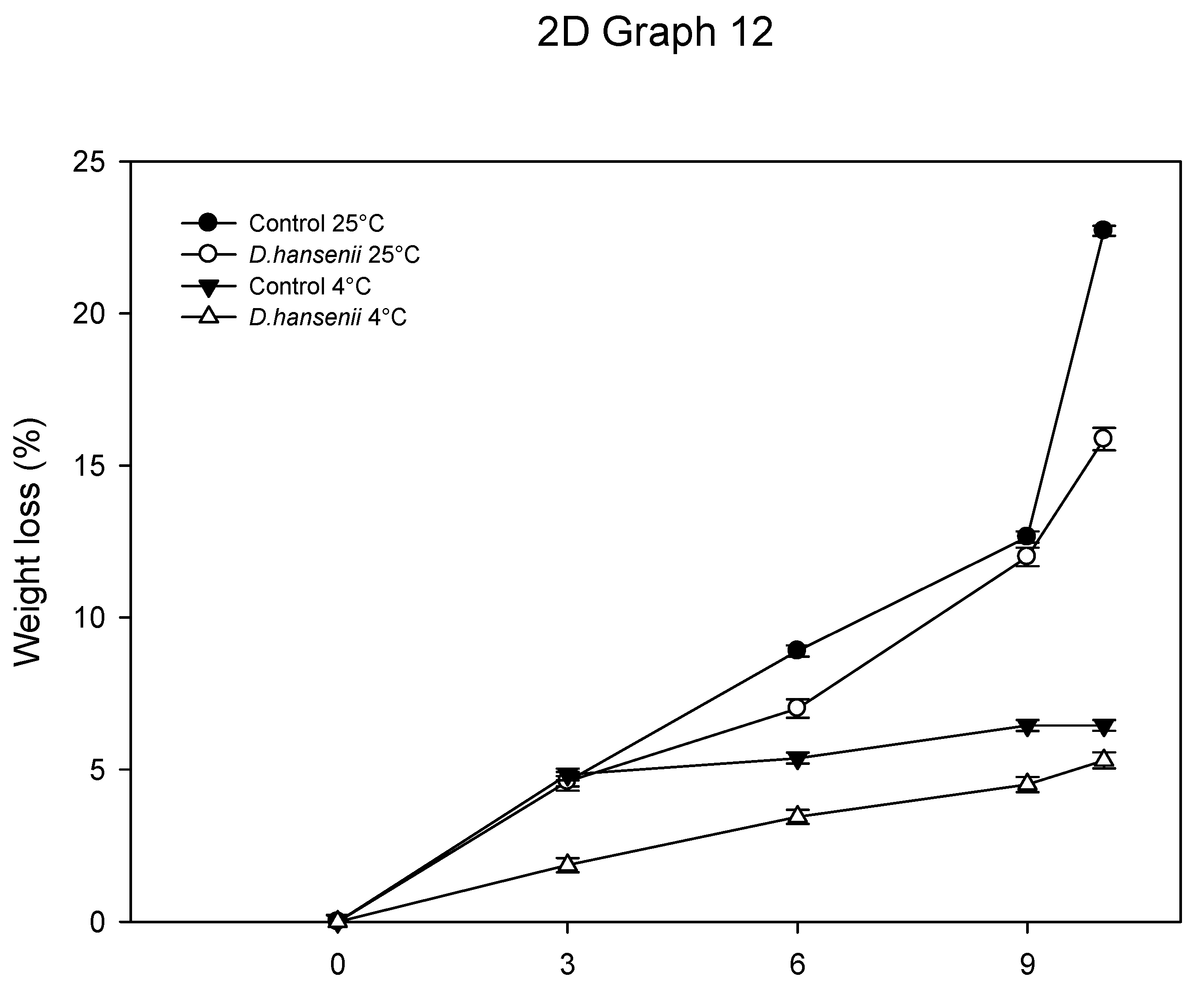
3.6.4. Physiological Weight Loss
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, B.; Wang, K.; Shu, X.; Liang, J.; Fan, X.; Sun, L. Changes in fruit firmness, quality traits and cell wall constituents of two highbush blueberries (Vaccinium corymbosum L.) during postharvest cold storage. Sci. Hortic. 2019, 246, 557–562. [Google Scholar] [CrossRef]
- Ramos-Bell, S.; Hernández-Montiel, L.G.; González-Estrada, R.R.; Gutiérrez-Martínez, P. Main diseases in postharvest blueberries, conventional and eco-friendly control methods: A review. LWT—Food Sci. Technol. 2021, 149, 7–12. [Google Scholar] [CrossRef]
- Spadaro, D.; Droby, S. Development of biocontrol products for postharvest diseases of fruit: The importance of elucidating the mechanisms of action of yeast antagonists. Trends Food Sci. Technol. 2016, 47, 39–49. [Google Scholar] [CrossRef]
- Hernandez-Montiel, L.G.; Zulueta-Rodriguez, R.; Angulo, C.; Rueda-Puente, E.O.; Quiñonez-Aguilar, E.E.; Galicia, R. Marine yeasts and bacteria as biological control agents against anthracnose on mango. J. Phytopathol. 2017, 165, 833–840. [Google Scholar] [CrossRef]
- Medina-Córdova, N.; López-Aguilar, R.; Ascencio, F.; Castellanos, T.; Campa-Córdova, A.I.; Angulo, C. Biocontrol activity of the marine yeast Debaryomyces hansenii against phytopathogenic fungi and its ability to inhibit mycotoxins production in maize grain (Zea mays L.). Biol. Control 2016, 97, 70–79. [Google Scholar] [CrossRef]
- Mari, M.; Martini, C.; Guidarelli, M.; Neri, F. Postharvest biocontrol of Monilinia laxa, Monilinia fructicola and Monilinia fructigena on stone fruit by two Aureobasidium pullulans strains. Biol. Control 2012, 60, 132–140. [Google Scholar] [CrossRef]
- Palou, L.; Smilanick, J.L.; Droby, S. Alternatives to conventional fungicides for the control of citrus postharvest green and blue moulds. Stewart Postharvest Rev. 2008, 4, 1–16. [Google Scholar] [CrossRef]
- Hernández-Montiel, L.G.; Rivas-García, T.; Romero-Bastidas, M.C.; Chiquito-Contreras, J.; Ruiz-Espinoza, F.H.; Chiquito-Contreras, R.G. Potencial antagónico de bacterias y levaduras marinas para el control de hongos fitopatógenos. Rev. Mex. Cienc. Agrícolas 2018, 1, 4311–4321. [Google Scholar] [CrossRef]
- González-Estrada, R.R.; Carvajal-Millán, E.; Ragazzo-Sánchez, J.A.; Bautista-Rosales, P.U.; Calderón-Santoyo, M. Control of blue mold decay on Persian lime: Application of covalently cross-linked arabinoxylans bioactive coatings with antagonistic yeast entrapped. LWT—Food Sci. Technol. 2017, 85, 187–196. [Google Scholar] [CrossRef]
- Hernandez-Montiel, L.G.; Gutierrez-Perez, E.D.; Murillo-Amador, B.; Vero, S.; Chiquito-Contreras, R.G.; Rincon-Enriquez, G. Mechanisms employed by Debaryomyces hansenii in biological control of anthracnose disease on papaya fruit. Postharvest Biol. Technol. 2018, 139, 31–37. [Google Scholar] [CrossRef]
- Lara-Capistran, L.; Zulueta-Rodriguez, R.; Castellanos-Cervantes, T.; Reyes-Perez, J.J.; Preciado-Rangel, P.; Hernandez-Montiel, L.G. Efficiency of marine bacteria and yeasts on the biocontrol activity of pythium ultimum in ancho-type pepper seedlings. Agronomy 2020, 10, 408. [Google Scholar] [CrossRef]
- Hernández-Montiel, L.G.; Holguín-Peña, R.J.; Larralde-Corona, C.P.; Zulueta-Rodríguez, R.; Rueda-Puente, E.; Moreno-Legorreta, M. Effect of inoculum size of yeast Debaryomyces hansenii to control Penicillium italicum on Mexican lime (Citrus aurantiifolia) during storage. J. Food 2012, 10, 235–242. [Google Scholar] [CrossRef]
- Parafati, L.; Vitale, A.; Restuccia, C.; Cirvilleri, G. Biocontrol ability and action mechanism of food-isolated yeast strains against Botrytis cinerea causing post-harvest bunch rot of table grape. Food Microbiol. 2015, 47, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Guerrero, A.; González-Estrada, R.R.; Hanako-Rosas, G.; Bautista-Baños, S.; Acevedo-Hernández, G.; Tiznado-Hernández, M.E.; Gutiérrez-Martínez, P. Use of inductors in the control of Colletotrichum gloeosporioides and Rhizopus stolonifer isolated from soursop fruits: In vitro tests. Food Sci. Biotechnol. 2018, 27, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Çorbacı, C.; Uçar, F.B. Purification, characterization and in vivo biocontrol efficiency of killer toxins from Debaryomyces hansenii strains. Int. J. Biol. Macromol. 2018, 119, 1077–1082. [Google Scholar] [CrossRef]
- Fernandez-San Millan, A.; Larraya, L.; Farran, I.; Ancin, M.; Veramendi, J. Successful biocontrol of major postharvest and soil-borne plant pathogenic fungi by antagonistic yeasts. Biol. Control. 2021, 160, 104683. [Google Scholar] [CrossRef]
- Shen, H.; Wei, Y.; Wang, X.; Xu, C.; Shao, X. The marine yeast Sporidiobolus pararoseus ZMY-1 has antagonistic properties against Botrytis cinerea in vitro and in strawberry fruit. Postharvest Biol. Technol. 2019, 150, 1–8. [Google Scholar] [CrossRef]
- Vázquez-Vázquez, M.L.; Hernández-Montiel, L.G.; Sanchez-Viveros, G.; Reyes-Pérez, J.; Martinez-Hernandez, M.; Chiquito-Contreras, R. Efecto de levaduras de origen marino y ulvan en el control poscosecha de Penicillium italicum agente causal del moho azul en limón persa. Biotecnia 2021, 23, 78–88. [Google Scholar] [CrossRef]
- Di Francesco, A.; Ugolini, L.; Lazzeri, L.; Mari, M. Production of volatile organic compounds by Aureobasidium pullulans as a potential mechanism of action against postharvest fruit pathogens. Biol. Control. 2015, 81, 8–14. [Google Scholar] [CrossRef]
- Ferreira, E.M.S.; Resende, D.A.; Vero, S.; Pimenta, R.S. The Use of Psychrophilic Antarctic Yeast in the Biological Control of Post-harvest Diseases of Fruits Stored at Low Temperatures. Fungi Antarct. 2019, 243–263. [Google Scholar] [CrossRef]
- Widyastuti, S.R.I. Physical Interactions between Yeast Pichia guilliermondii and Post-Harvest Fruit Pathogen Penicillium expansum. HAYATI J. Biosci. 2008, 15, 27–31. [Google Scholar] [CrossRef][Green Version]
- Freimoser, F.M.; Rueda-Mejia, M.P.; Tilocca, B.; Migheli, Q. Biocontrol yeasts: Mechanisms and applications. World J. Microbiol. Biotechnol. 2019, 35, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Chiabrando, V.; Peano, C.; Giacalone, G. The efficacy of different postharvest treatments on physico-chemical characteristics, bioactive components and microbiological quality of fresh blueberries during storage period. Food Res. 2017, 1, 240–248. [Google Scholar] [CrossRef]
- Ahima, J.; Zhang, X.; Yang, Q.; Zhao, L.; Tibiru, A.M.; Zhang, H. Biocontrol activity of Rhodotorula mucilaginosa combined with salicylic acid against Penicillium digitatum infection in oranges. Biol. Control. 2019, 135, 23–32. [Google Scholar] [CrossRef]
- Li, Y.; Rokayya, S.; Jia, F.; Nie, X.; Xu, J.; Elhakem, A.; Almatrafi, M.; Benajiba, N.; Helal, M. Shelf-life, quality, safety evaluations of blueberry fruits coated with chitosan nano-material films. Sci. Rep. 2021, 11, 55. [Google Scholar] [CrossRef]
- Vieira, J.M.; Flores-lópez, M.L.; Jasso, D.; Rodríguez, D.; Sousa, M.C.; Vicente, A.A.; Martins, J.T. Effect of chitosan—Aloe vera coating on postharvest quality of blueberry (Vaccinium corymbosum) fruit. Postharvest Biol. Technol. 2016, 116, 88–97. [Google Scholar] [CrossRef]
- Mannozzi, C.; Cecchini, J.P.; Tylewicz, U.; Siroli, L.; Patrignani, F.; Lanciotti, R.; Rocculi, P.; Dalla Rosa, M.; Romani, S. Study on the efficacy of edible coatings on quality of blueberry fruits during shelf-life. LWT—Food Sci. Technol. 2017, 85, 440–444. [Google Scholar] [CrossRef]
- Jiang, H.; Sun, Z.; Jia, R.; Wang, X.; Huang, J. Effect of Chitosan as an Antifungal and Preservative Agent on Postharvest Blueberry. J. Food Qual. 2016, 39, 516–523. [Google Scholar] [CrossRef]
- Djioua, T.; Charles, F.; Lopez-Lauri, F.; Filgueiras, H.; Coudret, A.; Freire, M., Jr.; Ducamp-Collin, M.N.; Sallanon, H. Improving the storage of minimally processed mangoes (Mangifera indica L.) by hot water treatments. Postharvest Biol. Technol. 2009, 52, 221–226. [Google Scholar] [CrossRef]
- Zhi Shao, Y.; Ke Zeng, J.; Tang, H.; Zhou, Y.; Li, W. The chemical treatments combined with antagonistic yeast control anthracnose and maintain the quality of postharvest mango fruit. J. Integr. Agric. 2019, 18, 1159–1169. [Google Scholar] [CrossRef]
- Chiabrando, V.; Giacalone, G. Quality evaluation of blueberries coated with chitosan and sodium alginate during postharvest storage. Int. Food Res. J. 2017, 24, 1553–1561. [Google Scholar]
- Xu, F.; Wang, S.; Xu, J.; Liu, S.; Li, G. Effects of combined aqueous chlorine dioxide and UV-C on shelf-life quality of blueberries. Postharvest Biol. Technol. 2016, 117, 125–131. [Google Scholar] [CrossRef]
- Kumari, P.; Barman, K.; Patel, V.B.; Siddiqui, M.W.; Kole, B. Reducing postharvest pericarp browning and preserving health promoting compounds of litchi fruit by combination treatment of salicylic acid and chitosan. Sci. Hortic. 2015, 197, 555–563. [Google Scholar] [CrossRef]
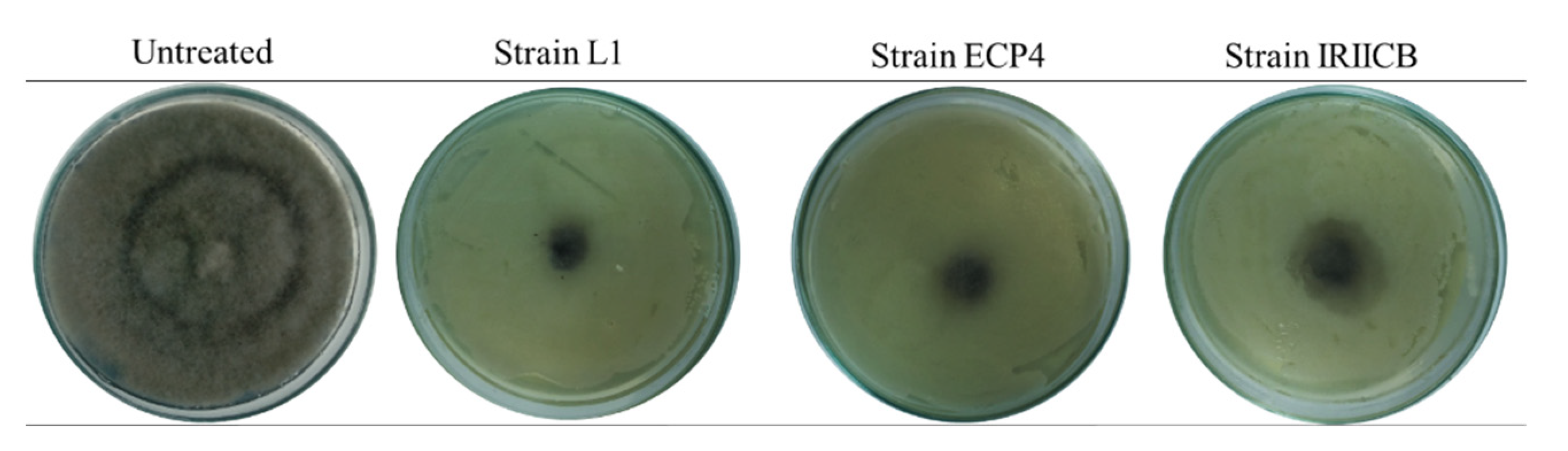




| Treatments | Strains (108 cel/mL) | Growth (mm) | Inhibition (%) |
|---|---|---|---|
| D. hansenii | L1 IRIICB ECP4 | 15.86 ± 3.76 a 23.21 ± 2.36 c 17.62 ± 4.75 b | 90.41 ± 4.59 d 81.44 ± 2.88 b 88.26 ± 5.79 c |
| Untreated | - | 90 ± 0 d | 0.0 ± 0 a |
| Parameters | Treatments | Storage (25 °C) | Storage (4 °C) | ||
|---|---|---|---|---|---|
| Day 0 | Day 9 | Day 0 | Day 9 | ||
| L* | Control D. hansenii | 35.01 ± 0.65 a 35.23 ± 0.58 a | 31.30 ± 0.70 a 32.77 ± 0.67 a | 35.24 ± 0.69 a 32.24 ± 0.62 b | 32.07 ± 0.70 a 27.53 ± 0.66 a |
| a* | Control D. hansenii | −3.89 ± 0.12 a −3.80 ± 0.10 a | −4.70 ± 0.07 a −4.94 ± 0.11 a | −3.79 ± 0.11 a −3.66 ± 0.16 a | −5.62 ± 0.18 a −5.88 ± 0.41 a |
| b* | Control D. hansenii | −7.04 ± 0.23 a −7.17 ± 0.19 a | −5.77 ± 0.21 a −5.45 ± 0.14 a | −6.67 ± 0.23 a −5.58 ± 0.31 a | −6.63 ± 0.23 a −4.55 ± 0.25 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos-Bell, S.; Hernández-Montiel, L.G.; Velázquez-Estrada, R.M.; Herrera-González, J.A.; Gutiérrez-Martínez, P. Potential of Debaryomyces hansenii Strains on the Inhibition of Botrytis cinerea in Blueberry Fruits (Vaccinium corymbosum L.). Horticulturae 2022, 8, 1125. https://doi.org/10.3390/horticulturae8121125
Ramos-Bell S, Hernández-Montiel LG, Velázquez-Estrada RM, Herrera-González JA, Gutiérrez-Martínez P. Potential of Debaryomyces hansenii Strains on the Inhibition of Botrytis cinerea in Blueberry Fruits (Vaccinium corymbosum L.). Horticulturae. 2022; 8(12):1125. https://doi.org/10.3390/horticulturae8121125
Chicago/Turabian StyleRamos-Bell, Surelys, Luis G. Hernández-Montiel, Rita M. Velázquez-Estrada, Juan A. Herrera-González, and Porfirio Gutiérrez-Martínez. 2022. "Potential of Debaryomyces hansenii Strains on the Inhibition of Botrytis cinerea in Blueberry Fruits (Vaccinium corymbosum L.)" Horticulturae 8, no. 12: 1125. https://doi.org/10.3390/horticulturae8121125
APA StyleRamos-Bell, S., Hernández-Montiel, L. G., Velázquez-Estrada, R. M., Herrera-González, J. A., & Gutiérrez-Martínez, P. (2022). Potential of Debaryomyces hansenii Strains on the Inhibition of Botrytis cinerea in Blueberry Fruits (Vaccinium corymbosum L.). Horticulturae, 8(12), 1125. https://doi.org/10.3390/horticulturae8121125







