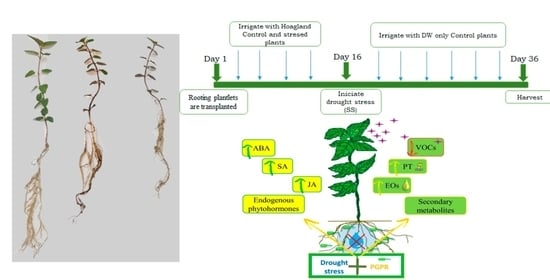
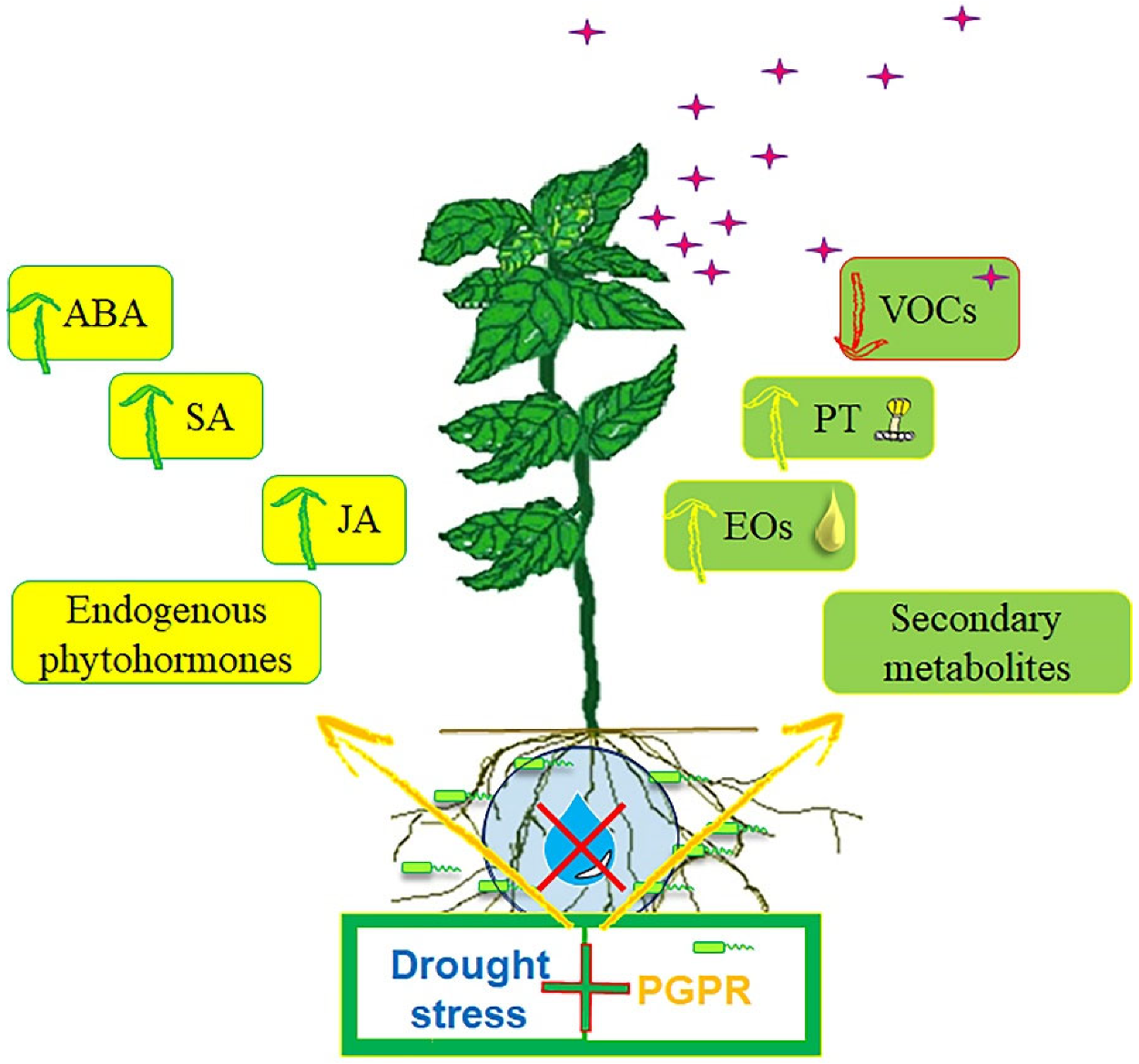
Influence of Drought Stress and PGPR Inoculation on Essential Oil Yield and Volatile Organic Compound Emissions in Mentha piperita
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant, Bacterial Inoculation, and Treatments
2.2. EO Extraction
2.3. Plant VOC (Volatile Organic Compounds) Collection
2.4. Trichome Density
2.5. Quantification of Endogenous Plant Hormones
2.6. RNA Extraction, Expression Analysis and Gene Copy Number Determination
2.7. Statistical Analyses
3. Results
3.1. Essential Oil Content and Main Constituents
3.2. VOC Emissions
3.3. Trichome Density
3.4. Endogenous Phytohormones
3.5. Gene Expression of Key Enzymes of EO Main Components
3.6. Principal Components Analysis (PCA)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Selmar, D.; Kleinwächter, M.; Abouzeid, S.; Yahyazadeh, M.; Nowak, M. The impact of drought stress on the quality of spice and medicinal plants. In Medicinal Plants and Environmental Challenges; Ghorbanpour, M., Varma, A., Eds.; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Gholamipourfard, K.; Salehi, M.; Banchio, E. Mentha piperita phytochemicals in agriculture, food industry and medicine: Features and applications. S. Afr. J. Bot. 2021, 141, 183–195. [Google Scholar] [CrossRef]
- Mittler, R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Gershenzon, J.; McConkey, M.E.; Croteau, R.B. Regulation of monoterpene accumulation in leaves of peppermint. Plant Physiol. 2000, 122, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Bhatla, S.C. Jasmonic Acid. In Plant Physiology, Development and Metabolism; Bhatla, S.C., Lal, M.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar] [CrossRef]
- Lv, Z.Y.; Sun, W.J.; Jiang, R.; Chen, J.F.; Ying, X.; Zhang, L.; Chen, W.S. Phytohormones jasmonic acid, salicylic acid, gibberellins, and abscisic acid are key mediators of plant secondary metabolites. World J. Tradit. Chin. Med. 2021, 7, 307–325. [Google Scholar]
- Fatiha, B.A.; Ouafae, B.; Souad, S.; Jamila, D.; Allal, D.; Lahcen, Z. Ethnobotany study of medicinal plants used in the treatment of respiratory diseases in the middle region of Oum Rbai. Int. J. Environ. Agric. Biotechnol. 2017, 2, 1460–1468. [Google Scholar] [CrossRef]
- Singh, P.; Pandey, A.K. Prospective of essential oils of the genus Mentha as biopesticides: A review. Front. Plant Sci. 2018, 9, 1295. [Google Scholar] [CrossRef]
- Mahendran, G.; Rahman, L. Ethnomedicinal, phytochemical and pharmacological updates on Peppermint (Mentha × piperita L.) A review. Phytother. Res. 2020, 34, 2088–2139. [Google Scholar] [CrossRef]
- Gupta, G.; Parihar, S.S.; Ahirwar, N.K.; Snehi, S.K.; Singh, V. Plant Growth Promoting Rhizobacteria (PGPR): Current and future prospects for development of sustainable agriculture. J. Microb. Biochem. Technol. 2015, 7, 96–102. [Google Scholar] [CrossRef]
- Singh, R.P.; Jha, P.N. The multifarious PGPR Serratia marcescens CDP-13 augments induced systemic resistance and enhanced salinity tolerance of wheat (Triticum aestivum L.). PLoS ONE 2016, 11, e0155026. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar, V.; Prasad, R. Phyto-Microbiome in Stress Regulation; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A.; Rahman, M.A.; Guo, J.; Kang, Z.; Babar, M. Comparative physiological and metabolic analysis reveals a complex mechanism involved in drought tolerance in chickpea (Cicer arietinum L.) induced by PGPR and PGRs. Sci. Rep. 2019, 9, 2097. [Google Scholar] [CrossRef]
- Chiappero, J.; Cappellari, L.; Sosa Alderete, L.G.; Palermo, T.B.; Banchio, E. Plant growth promoting rhizobacteria improve the antioxidant status in Mentha piperita grown under drought stress leading to an enhancement of plant growth and total phenolic content. Ind. Crops Prod. 2019, 139, 111553. [Google Scholar] [CrossRef]
- Luria, S.E.; Burrous, J.W. Hybridization between Escherichia coli and Shigella. J. Bacteriol. 1957, 74, 461–476. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.V.; Cappellari, L.; Giordano, W.; Banchio, E. Plant growth-promoting effects of native Pseudomonas strains on Mentha piperita (peppermint): An in vitro study. Plant Biol. 2015, 17, 1218–1226. [Google Scholar] [CrossRef] [PubMed]
- Cappellari, L.; Santoro, M.V.; Reinoso, H.; Travaglia, C.; Giordano, W.; Banchio, E. Anatomical, morphological, and phytochemical effects of inoculation with plant growth- promoting rhizobacteria on peppermint (Mentha piperita). J. Chem. Ecol. 2015, 41, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Cappellari, L.; Chiappero, J.; Santoro, M.V.; Giordano, W.; Banchio, E. Inducing phenolic production and volatile organic compounds emission by inoculating Mentha piperita with plant growth-promoting rhizobacteria. Sci. Hortic. 2017, 220, 193–198. [Google Scholar] [CrossRef]
- D’Ambriogio de Argueso, A. Manual de Técnicas en Histología Vegetal; Hemisferio sur: Buenos Aires, Argentina, 1986. [Google Scholar]
- Cappellari, L.; Santoro, M.V.; Schmidtb, A.; Gershenzonb, J.; Banchio, E. Induction of essential oil production in Mentha x piperita by plant growth promoting bacteria was correlated with an increase in jasmonate and salicylate levels and a higher density of glandular trichomes. Plant Physiol. Biochem. 2019, 141, 142–153. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Skirycz, A.; Inzé, D. More from less: Plant growth under limited water. Curr. Opin. Biotechnol. 2010, 21, 197–203. [Google Scholar] [CrossRef]
- Davis, E.M.; Ringer, K.L.; McConkey, M.E.; Croteau, R. Monoterpene metabolism. Cloning, expression, and characterization of menthone reductases from peppermint. Plant Physiol. 2005, 137, 873–881. [Google Scholar] [CrossRef]
- Alonso, W.R.; Rajaonarivony, J.I.M.; Gershenzon, J.; Croteau, R. Purification of 4S-limonene synthase, a monoterpene cyclase from the glandular trichomes of peppermint (Mentha x piperita) and Spearmint (M. spicata). J. Biol. Chem. 1992, 267, 7582–7587. [Google Scholar] [CrossRef]
- Lange, B.M.; Wildung, M.R.; Stauber, E.J.; Sanchez, C.; Pouchnik, D.; Croteau, R. Probing essential oil biosynthesis and secretion by functional evaluation of expressed sequence tags from mint glandular trichomes. Proc. Natl. Acad. Sci. USA 2000, 97, 2934–2939. [Google Scholar] [CrossRef] [PubMed]
- Turner, G.W.; Gershenzon, J.; Nielson, E.E.; Froehlich, J.E.; Croteau, R. Limonene synthase, the enzyme responsible for monoterpene biosynthesis in peppermint, is localized to leucoplasts of oil gland secretory cells. Plant Physiol. 1999, 120, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Croteau, R.B.; Davis, E.M.; Ringer, K.L.; Wildung, M.R. (–) Menthol biosynthesis and molecular genetics. Naturwissenschaften 2005, 92, 562–577. [Google Scholar] [CrossRef] [PubMed]
- Asghari, B.; Khademian, R.; Sedaghati, B. Plant growth promoting rhizobacteria (PGPR) confer drought resistance and stimulate biosynthesis of secondary metabolites in pennyroyal (Mentha pulegium L.) under water shortage condition. Sci. Hortic. 2019, 263, 109132. [Google Scholar] [CrossRef]
- Lange, B.M.; Turner, G.W. Terpenoid biosynthesis in trichomes-current status and future opportunities. Plant Biotechnol. J. 2012, 11, 2–22. [Google Scholar] [CrossRef] [PubMed]
- Schuurink, R.; Tissier, A. Glandular trichomes: Micro-organs with model status? New Phytol. 2019, 225, 2251–2266. [Google Scholar] [CrossRef]
- Kleinwächter, M.; Selmar, D. New insights explain that drought stress enhances the quality of spice and medicinal plants: Potential applications. Agron. Sustain. Dev. 2014, 35, 121–131. [Google Scholar] [CrossRef]
- Bettaieb, I.; Zakhama, N.; Aidi Wannes, W.; Kchouk, M.E.; Marzouk, B. Water deficit effects on Salvia officinalis fatty acids and essential oils composition. Sci. Hortic. 2009, 120, 271–275. [Google Scholar] [CrossRef]
- Kumar, I.; Kumar Sharma, R. Production of secondary metabolites in plants under abiotic stress: An overview. Significances Bioeng. Biosci. 2018, 2, 196–200. [Google Scholar] [CrossRef]
- Grace, S.C.; Logan, B.A. Energy dissipation and radical scavenging by the plant phenylpropanoid pathway. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2000, 355, 1499–1510. [Google Scholar] [CrossRef]
- Dudareva, N.; Negre, F.; Nagegowda, D.A.; Orlava, I. Plant volatiles: Recent advances and future perspectives. Crit. Rev. Plant Sci. 2006, 25, 417–440. [Google Scholar] [CrossRef]
- Peron, A.; Kaser, L.; Fitzky, A.C.; Graus, M.; Halbwirth, H.; Greiner, J.; Karl, T. Combined effects of ozone and drought stress on the emission of biogenic volatile organic compounds from Quercus robur L. Biogeosciences 2021, 18, 535–556. [Google Scholar] [CrossRef]
- Selmar, D.; Kleinwächter, M. Stress enhances the synthesis of secondary plant products: The impact of the stress-related over-reduction on the accumulation of natural products. Plant Cell Physiol. 2013, 54, 817–826. [Google Scholar] [CrossRef]
- Turner, G.W.; Gershenzon, J.; Croteau, R.B. Distribution of peltate glandular trichomes on developing leaves of peppermint. Plant Physiol. 2000, 124, 655–664. [Google Scholar] [CrossRef]
- Fortelny, N.; Overall, C.M.; Pavlidis, P.; Freue, G.V.C. Can we predict protein from mRNA levels? Nature 2017, 547, E19–E20. [Google Scholar] [CrossRef]
- Zade, N.S.E.; Sadeghi, A.; Moradi, P. Streptomyces strains alleviate water stress and increase peppermint (Mentha piperita) yield and essential oils. Plant Soil 2018, 434, 441–452. [Google Scholar] [CrossRef]
- Karamzadeh, S. Drought and production of second metabolites in medicinal and aromatic plants. Drought J. 2003, 7, 90–95. [Google Scholar]
- Petropoulos, S.A.; Daferera, D.; Polissiou, M.G.; Passam, H.C. The effect of water deficit stress on the growth, yield and composition of essential oils of parsley. Sci. Hortic. 2007, 115, 393–397. [Google Scholar] [CrossRef]
- Aziz, E.A.; Hendawi, S.T.; Azza, E.E.D.; Omer, E.A. Effect of soil type and irrigation intervals on plant growth, essential oil and constituents of Thymus vulgaris plant. Am.-Eurasian J. Agric. Environ. Sci. 2008, 4, 443–450. [Google Scholar]
- García-Caparrós, P.; Romero, M.J.; Llanderal, A.; Cermeño, P.; Lao, M.T.; Segura, M.L. Effects of drought stress on biomass, essential oil content, nutritional parameters, and costs of production in six Lamiaceae species. Water 2019, 11, 573. [Google Scholar] [CrossRef]
- Misra, A.; Srivastava, N.K. Influence of water stress on Japanese mint. J. Herbs Spices Med. Plants 2000, 7, 51–58. [Google Scholar] [CrossRef]
- Singh, M.; Ramesh, S. Effect of irrigation and nitrogen on herbage, oil yield and water-use efficiency in rosemary grown under semi-arid tropical conditions. J. Med. Aromat. Plant Sci. 2000, 22, 659–662. [Google Scholar]
- Govahi, M.; Ghalavand, A.; Nadjafi, F.; Sorooshzadeh, A. Comparing different soil fertility systems in Sage (Salvia officinalis) under water deficiency. Ind. Crops Prod. 2015, 74, 20–27. [Google Scholar] [CrossRef]
- Barbieri, N.; Costamagna, M.; Gilabert, M.; Perotti, M.; Schuff, C.; Isla, M.I.; Benavente, A. Antioxidant activity and chemical composition of essential oils of three aromatic plants from La Rioja province. Pharm. Biol. 2009, 120, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Arpanahi, A.; Feizian, M.; Mehdipourian, G. Plant growth promoting rhizobacteria enhance oil content and physiological status of Thymus daenensis Celak under drought stress. J. Med. Herbs 2019, 9, 223–231. [Google Scholar]
- Caser, M.; Chitarra, W.; D’Angiolillo, F.; Perrone, I.; Demasi, S.; Lovisolo, C.; Scariot, V. Drought stress adaptation modulates plant secondary metabolite production in Salvia dolomitica Codd. Ind. Crops Prod. 2018, 129, 85–96. [Google Scholar] [CrossRef]
- Hammerbacher, A.; Coutinho, T.A.; Gershenzon, J. Roles of plant volatiles in defence against microbial pathogens and microbial exploitation of volatiles. Plant Cell Environ. 2019, 42, 2827–2843. [Google Scholar] [CrossRef]
- Karimi, A.; Meiners, T. Antifungal activity of Zataria multiflora Boiss. Essential oils and changes in volatile compound composition under abiotic stress conditions. Ind. Crops Prod. 2021, 171, 113888. [Google Scholar] [CrossRef]
- Mahdavi, A.; Moradi, P.; Mastinu, A. Variation in Terpene Profiles of Thymus vulgaris in Water Deficit Stress Response. Molecules 2020, 25, 1091. [Google Scholar] [CrossRef]
- Spinelli, F.; Cellini, A.; Marchetti, L.; Nagesh, K.M.; Piovene, C. Emission and Function of Volatile Organic Compounds in Response to Abiotic Stress. In Abiotic Stress in Plants—Mechanisms and Adaptations; Shanker, A., Venkateswarlu, B., Eds.; IntechOpen: London, UK, 2011; pp. 367–394. [Google Scholar] [CrossRef]
- Copolovici, L.; Niinemets, U. Environmental impacts on plant volatile emission. In Deciphering Chemical Language of Plant Communication, Signaling and Communication in Plants; Blande, J.D., Glinwood, R., Eds.; Springer: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Werker, E. Trichome diversity and development. In Advances in Botanical Research; Hallahan, D.L., Gray, J.C., Eds.; Academic: San Diego, CA, USA, 2000. [Google Scholar] [CrossRef]
- Banchio, E.; Valladares, G.; Zygadlo, J.; Bogino, P.C.; Rinaudi, L.V.; Giordano, W. Changes in composition of essential oils and volatile emissions of Minthostachys mollis, induced by leaf punctures of Liriomyza huidobrensis. Biochem. Syst. Ecol. 2007, 35, 68–74. [Google Scholar] [CrossRef]
- Eberl, F.; Gershenzon, J. Releasing plant volatiles, as simple as ABC. A protein actively expels volatile compounds from plants. Science 2017, 356, 1334–1335. [Google Scholar] [CrossRef] [PubMed]
- Bouwmeester, H.; Schuurink, R.C.; Bleeker, P.M.; Schiestl, F. The role of volatiles in plant communication. Plant J. 2019, 100, 892–907. [Google Scholar] [CrossRef] [PubMed]
- Tissier, A.; Morgan, J.A.; Dudareva, N. Plant volatiles: Going ‘in’ but not ‘out’ of trichome cavities. Trends Plant Sci. 2017, 22, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Laxmi, A. Transcriptional regulation of drought response: A tortuous network of transcriptional factors. Front. Plant Sci. 2015, 6, 895. [Google Scholar] [CrossRef] [PubMed]





| Gene | Forward Primer Sequence (5′-3′) | Reverse Primer Sequence (5′-3′) |
|---|---|---|
| Act | GCTCCAAGGGCTGTGTTCC | TCTTTCTGTCCCATGCCAAC |
| LimS | TTGTGGCGAATTCTCTCGCT | GGCTTCTGAGCTGGTCACTT |
| Pr | GCATGGAGATCCCAGATGGC | AGTAGAGCCAGGAAGGATGGA |
| Treatment | Limonene | Menthone | Menthofurane | Menthol | Pulegone |
|---|---|---|---|---|---|
| Regularly watered | 0.57 ± 0.10 a | 0.48 ± 0.09 a | 0.33 ± 0.09 a | 0.41 ± 0.10 a | 3.89 ± 0.39 a |
| GB03 | 1.17 ± 0.13 a | 1.83 ± 0.28 b | 0.65 ± 0.19 a | 2.40 ± 0.66 bc | 12.51 ± 2.26 b |
| WCS417r | 1.35 ± 0.36 a | 2.01 ± 0.46 b | 0.67 ± 0.29 a | 1.85 ± 0.17 bc | 18.93 ± 2.53 b |
| MS | |||||
| Non-inoculated | 1.45 ± 0.27 a | 1.64 ± 0.22 b | 0.75 ± 0.24 a | 1.03 ± 0.46 ab | 12.36 ± 1.52 b |
| GB03 | 1.79 ± 0.42 a | 1.99 ± 0.41 b | 0.35 ± 0.12 a | 2.67 ± 0.40 c | 17.74 ± 4.44 b |
| WCS417r | 1.02 ± 0.22 a | 2.12 ± 0.27 b | 0.43 ± 0.14 a | 2.34 ± 1.06 bc | 13.01 ± 2.50 b |
| SS | |||||
| Non-inoculated | 1.53 ± 0.41 a | 1.49 ± 0.30 b | 0.74 ± 0.29 a | 1.12 ± 0.22 abc | 13.31 ± 2.46 b |
| GB03 | 0.92 ± 0.19 a | 1.53 ± 0.25 b | 0.38 ± 0.12 a | 1.62 ± 0.36 abc | 11.76 ± 3.64 b |
| WCS417r | 1.31 ± 0.26 a | 1.55 ± 0.24 b | 0.36 ± 0.07 a | 1.47 ± 0.58 abc | 10.48 ± 1.71 b |
| Treatment | Adaxial Face | Abaxial Face | ||
|---|---|---|---|---|
| Peltate (n per mm2) | Capitate (n per mm2) | Peltate (n per mm2) | Capitate (n per mm2) | |
| Regularly watered | 1.31± 0.09 a | 4.89 ± 0.24 a | 2.70 ± 0.21 a | 11.83 ± 0.69 a |
| GB03 | 1.65 ± 0.13 ab | 5.31 ± 0.30 a | 4.17 ± 0.24b cd | 12.90 ± 0.43 ab |
| WCS417r | 2.07 ± 0.16 c | 6.46 ± 0.35 b | 5.43 ± 0.34 e | 16.65 ± 0.58 c |
| MS | ||||
| Non-inoculated | 1.66 ± 0.13 ab | 7.06 ± 0.44 b | 4.20± 0.23 cd | 17.77 ± 0.68 c |
| GB03 | 1.97 ± 0.14 bc | 6.55± 0.41 b | 5.18 ± 0.18 de | 16.87 ± 0.65 c |
| WCS417r | 1.70 ± 0.14 b | 5.24 ± 0.28 a | 3.93 ± 0.20 bc | 14.12 ± 0.62 b |
| SS | ||||
| Non-inoculated | 1.61 ± 0.14 ab | 6.94 ± 0.41 b | 3.82 ± 0.26 bc | 17.05± 0.91 c |
| GB03 | 1.79 ± 0.12 bc | 6.68 ± 0.37 b | 4.08 ± 0.29 bc | 16.61± 0.86 c |
| WCS417r | 1.57 ± 0.09 ab | 5.14 ± 0.29 a | 3.39 ± 0.29 ab | 13.22 ± 0.57ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiappero, J.; Cappellari, L.d.R.; Palermo, T.B.; Giordano, W.; Banchio, E. Influence of Drought Stress and PGPR Inoculation on Essential Oil Yield and Volatile Organic Compound Emissions in Mentha piperita. Horticulturae 2022, 8, 1120. https://doi.org/10.3390/horticulturae8121120
Chiappero J, Cappellari LdR, Palermo TB, Giordano W, Banchio E. Influence of Drought Stress and PGPR Inoculation on Essential Oil Yield and Volatile Organic Compound Emissions in Mentha piperita. Horticulturae. 2022; 8(12):1120. https://doi.org/10.3390/horticulturae8121120
Chicago/Turabian StyleChiappero, Julieta, Lorena del Rosario Cappellari, Tamara Belén Palermo, Walter Giordano, and Erika Banchio. 2022. "Influence of Drought Stress and PGPR Inoculation on Essential Oil Yield and Volatile Organic Compound Emissions in Mentha piperita" Horticulturae 8, no. 12: 1120. https://doi.org/10.3390/horticulturae8121120
APA StyleChiappero, J., Cappellari, L. d. R., Palermo, T. B., Giordano, W., & Banchio, E. (2022). Influence of Drought Stress and PGPR Inoculation on Essential Oil Yield and Volatile Organic Compound Emissions in Mentha piperita. Horticulturae, 8(12), 1120. https://doi.org/10.3390/horticulturae8121120