Effects of Aminoethoxyvinylglycine (AVG) and 1-Methylcyclopropene (1-MCP) on the Pre-Harvest Drop Rate, Fruit Quality, and Stem-End Splitting in ‘Gala’ Apples
Abstract
1. Introduction
2. Materials and Methods
2.1. Exp. 1: AVG Effects on Fruit Drop and Fruit Quality
2.2. Exp. 2: Effects of AVG and 1-MCP on Fruit Drop and Fruit Quality
2.3. Exp. 3: Effects of AVG, 1-MCP and GA4+7 on Fruit Drop and Fruit Quality
2.4. Exp. 4: Effects of AVGand GA4+7 on Fruit Drop and Fruit Quality
3. Results
3.1. Exp. 1. AVG Effects on Fruit Drop and Fruit Quality at Harvest and after Cold Storage
3.2. Exp. 2. Effects of AVG and 1-MCP on Fruit Drop and Fruit Quality at Harvest and after Cold Storage
3.3. Exp. 3. Effects of AVG, 1-MCP and GA4+7 on Fruit Drop, Fruit Quality and Stem End Splitting
3.4. Exp. 4. Effects of AVG and GA4+7 on Fruit Drop, Fruit Quality and Stem End Splitting
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arseneault, M.H.; Cline, J.A. A review of apple preharvest fruit drop and practices for horticultural management. Sci. Hort. 2016, 211, 40–52. [Google Scholar] [CrossRef]
- Byers, R.E.; Eno, D.R. Harvest date influences fruit size and yield of ‘York’ and ‘Golden Delicious’ apple trees. J. Tree Fruit Prod. 2022, 3, 63–79. [Google Scholar] [CrossRef]
- Klee, H.J. Ethylene Signal Transduction. Moving beyond Arabidopsis. Plant Physiol. 2004, 135, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, J.J. Genetic regulation of fruit development and ripening. Plant Cell 2004, 16 (Suppl. S1), S170–S180. [Google Scholar] [CrossRef] [PubMed]
- Bonghi, C.; Tonutti, P.; Ramina, A. Biochemical and molecular aspects of fruitlet abscission. Plant Growth Regulat. 2000, 31, 35–42. [Google Scholar] [CrossRef]
- Roberts, J.A.; Elliott, K.A.; Gonzalez-Carranza, Z.H. Abscission, dehiscence, and other cell separation processes. Annu. Rev. Plant Biol. 2002, 53, 131–158. [Google Scholar] [CrossRef]
- Chu, C.L. Internal ethylene concentration of ‘McIntosh’, ‘Northern Spy’, ‘Empire’, ‘Mutsu’, and ‘Idared’ apple during the harvest season. J. Am. Soc. Hortic. Sci. 1988, 133, 226–229. [Google Scholar] [CrossRef]
- Gussman, C.D.; Goffreda, J.C.; Gianfagna, T.J. Ethylene production and fruit-softening rates in several apple fruit ripening variants. HortScience 1993, 28, 135–137. [Google Scholar] [CrossRef]
- Li, J.; Zhu, H.; Yuan, C. Profiling the expression of genes related to ethylene biosynthesis, ethylene perception, and cell wall degradation during fruit abscission and fruit ripening in apple. J. Amer. Soc. Hort. 2010, 135, 391–401. [Google Scholar] [CrossRef]
- Sun, L.; John, B.M.; Forsline, P.L.; Nocker, S. Natural variation in fruit abscission-related traits in apple (Malus). Euphytica 2009, 165, 55–67. [Google Scholar] [CrossRef]
- Greene, D.W. Time of aminoethoxyvinylglycine application influences preharvest drop and fruit quality of ‘McIntosh’ apples. HortScience 2005, 40, 2056–2060. [Google Scholar] [CrossRef]
- Yuan, R.; Carbaugh, D.H. Effects of NAA, AVG, and 1-MCP on ethylene biosynthesis, preharvest fruit drop, fruit maturity, and quality of ‘Golden Supreme’ and ‘Golden Delicious’ apples. HortScience 2007, 43, 1454–1460. [Google Scholar] [CrossRef]
- Robinson, T.L.; Hoying, S.; Iungerman, K.; Kviklys, D. AVG combined with NAA control pre-harvest drop of ‘McIntosh’ apples better than either chemical alone. Proc. XI International Symposium on Plant Bioregulators Fruit Production. Acta Hortic. 2010, 884, 343–350. [Google Scholar] [CrossRef]
- Yuan, R.; Li, J. Effect of sprayable 1-MCP, AVG, and NAA on ethylene biosynthesis preharvest fruit drop, fruit maturity, and quality of ‘Delicious’ apples. HortScience 2008, 43, 1454–1460. [Google Scholar] [CrossRef]
- Schupp, J.R.; Greene, D.W. Effect of aminoethoxyvinylglycine (AVG) on preharvest drop, fruit quality, and maturation of ‘McIntosh’ apples. I. Concentration and timing of dilute applications of AVG. HortScience 2004, 39, 1030–1035. [Google Scholar] [CrossRef]
- Yildiz, K.; Ozturk, B.; Ozkan, Y. Effects of aminoethoxyvinylglycine (AVG) on preharvest fruit drop, fruit maturity, and quality of ‘Red Chief’ apple. Sci. Hortic. 2012, 144, 121–124. [Google Scholar] [CrossRef]
- Greene, D.W.; Schupp, J.R. Effects of aminoethoxyvinylglycine (AVG) on pre-harvest fruit drop, fruit quality, and maturation of ‘McIntosh’ apples. II. Effect of timing and concentration relationships and spray volume. Hortscience 2004, 39, 1036–1041. [Google Scholar] [CrossRef]
- Ozturk, B.; Kucuker, E.; Karaman, S.; Ozkan, Y. The effect of cold storage and aminoethoxyvinylglycine (AVG) on bioactive compounds of plum (Prunus salic-ina Lindell cv. ‘Black Amber’). Postharvest Biol. Technol. 2012, 72, 35–41. [Google Scholar] [CrossRef]
- Opara, L.U.; Studman, C.J.; Banks, N.H. Sunlight affects the incidence of internal ring cracking and other physical attributes of ‘Gala’ apples. J. Tree Fruit Prod. 1997, 2, 45–52. [Google Scholar] [CrossRef]
- Opara, L.U.; Hodson, A.D.; Studman, S.P. Stem-end splitting and internal ring-cracking of ‘Gala’ apples as influenced by orchard management practices. J. Hortic. Sci. Biotechnol. 2000, 75, 465–469. [Google Scholar] [CrossRef]
- Taylor, D.R.; Knight, J.N. Russeting and cracking of apple fruit and their control with plant growth regulators. In V International Symposium on Growth Regulators in Fruit Production; ISHS: Leuven, Belgium, 1985; Volume 179, pp. 819–820. [Google Scholar]
- Knoche, M.; Khanal, B.P.; Stopar, M. Russeting and microcracking of ‘Golden Delicious’ apple fruit concomitantly decline due to gibberellin A4+ 7 application. J. Am. Soc. Hortic. Sci. 2011, 136, 159–164. [Google Scholar] [CrossRef]
- Blanpied, G.D.; Silsby, K.J. Predicting Harvest Date Windows for Apples; Cornell Cooperative Extension: Ithaca, NY, USA, 1992. [Google Scholar]
- Alwan, T.F.; Watkins, C.B. Intermittent warming effects on superficial scald development of ‘Cortland’, ‘Delicious’ and ‘Law Rome’ apple fruit. Postharvest Biol Technol. 1999, 16, 203–212. [Google Scholar] [CrossRef]
- Byers, R.E. Effect of aminoethoxyvinylglycine (AVG) on preharvest fruit drop and maturity of “Delicious” apples. J. Tree Fruit Prod. 1997, 2, 53–75. [Google Scholar] [CrossRef]
- Byers, R.E. Effects of aminoethoxyvinylglycine (AVG) on preharvest fruit drop, maturity, and cracking of several apple cultivars. J. Tree Fruit Prod. 1997, 2, 77–97. [Google Scholar] [CrossRef]
- Boyaci, S. Effect of Aminoethoxyvinylglycine (AVG) Applications on Pre-Harvest Drop and Fruit Quality of ‘Red Delicious, Red Chief’Apple Cultivar. Erwerbs-Obstbau 2022, 64, 395–400. [Google Scholar] [CrossRef]
- Byers, R.E.; Carbaugh, D.H.; Combs, L.D. Ethylene inhibitors delay fruit drop, maturity, and increase fruit size of ‘Arlet’ apples. HortScience 2005, 40, 2061–2065. [Google Scholar] [CrossRef]
- Dias, C.; Ribeiro, T.; Rodrigues, A.C.; Ferrante, A.; Vasconcelos, M.W.; Pintado, M. Improving the ripening process after 1-MCP application: Implications and strategies. Trends Food Sci. Technol. 2021, 113, 382–396. [Google Scholar] [CrossRef]
- Yang, X.; Song, J.; Campbell-Palmer, L.; Fillmore, S.; Zhang, Z. Effect of ethylene and 1-MCP on expression of genes involved in ethylene biosynthesis and perception during ripening of apple fruit. Postharvest Biol. Technol. 2013, 78, 55–66. [Google Scholar] [CrossRef]
- Greene, D.W. Preharvest drop control and maturity of ‘Delicious’ apples as effected by aminoethoxyvinylglycine (AVG). J. Tree Fruit Prod. 2002, 3, 1–10. [Google Scholar] [CrossRef]
- Blankenship, S.M.; Unrath, C.R. PAL and ethylene content during maturation of red and golden delicious apples. Phytochemistry 1988, 27, 1001–1003. [Google Scholar] [CrossRef]
- Whale, S.K.; Singh, Z. Endogenous ethylene and color development in the skin of ‘Pink Lady’ apple. J. Am. Soc. Hortic. Sci. 2007, 132, 20–28. [Google Scholar] [CrossRef]
- do Amarante, C.V.T.; Argenta, L.C.; de Freitas, S.T.; Steffens, C.A. Efficiency of pre-harvest application of 1-MCP (Harvista™ 1.3 SC) to delay maturation of ‘Cripps Pink’ apple fruit. Sci. Hort. 2022, 293, 110715. [Google Scholar] [CrossRef]
- Fan, X.; Mattheis, J.P. Methyl jasmonate promotes apple fruit degreening independently of ethylene action. HortScience 1999, 34, 310–312. [Google Scholar] [CrossRef]
- Rudell, D.R.; Mattheis, J.P. Synergism exists between ethylene and methyl jasmonate in artificial light-induced pigment enhancement of ‘Fuji’ apple fruit peel. Postharvest Biol. Technol. 2008, 47, 136–140. [Google Scholar] [CrossRef]
- An, J.P.; Wang, X.F.; Li, Y.Y.; Song, L.Q.; Zhao, L.L.; You, C.X.; Hao, Y.J. EIN3-LIKE1, MYB1, and ETHYLENE RESPONSE FACTOR3 act in a regulatory loop that synergistically modulates ethylene biosynthesis and anthocyanin accumulation. Plant Physiol. 2018, 178, 808–823. [Google Scholar] [CrossRef]
- Keyes, G.; Sorrells, M.E.; Setter, T.L. Gibberellic Acid Regulates Cell Wall Extensibility in Wheat (Triticum aestivum L.). Plant Physiol. 1990, 92, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Taiz, L. Plant cell expansion: Regulation of cell wall mechanical properties. Annu. Rev. Plant Biol. 1984, 35, 585–657. [Google Scholar] [CrossRef]
- Curry, E. Increase in epidermal planar cell density accompanies decreased russeting of ‘Golden Delicious’ apples treated with gibberellin A4+ 7. HortScience 2012, 47, 232–237. [Google Scholar] [CrossRef]
- Stern, R.; Ben-Arie, R.; Ginzberg, I. Reducing the incidence of calyx cracking in ‘Pink Lady’ apple using a combination of cytokinin (6-benzyladenine) and gibberellins (GA4+ 7). Hortic. Sci. Biotechnol. 2013, 88, 147–153. [Google Scholar]
- Ginzberg, I.; Stern, R.A. Strengthening fruit-skin resistance to growth strain by application of plant growth regulators. Sci. Hortic. 2016, 198, 150–153. [Google Scholar] [CrossRef]
- Fallahi, E.; Fallahi, B.; Shafii, B. Irrigation and rootstock influence on water use, tree growth, yield, and fruit quality at harvest at different ages of trees in ‘Pacific Gala’apple. HortScience 2013, 48, 588–593. [Google Scholar] [CrossRef]
- Jan, I.; Rab, A.; Sajid, M. Storage performance of apple cultivars harvested at different stages of maturity. J. Anim. Plant Sci. 2012, 22, 438–447. [Google Scholar]
- Johnstone, J.W.; Hewett, E.W.; Hertog, M.L. Post-harvest softening of apple (Malus domestica) fruit: A review. N. Z. J. Crop Hort. Sci. 2002, 30, 145–160. [Google Scholar] [CrossRef]
- Halder-Doll, H.; Bangerth, F. Inhibition of autocatalytic C2H4-biosynthesis by AVG applications and consequences on the physiological behaviour and quality of apple fruits in cool storage. Sci. Hortic. 1987, 33, 87–96. [Google Scholar] [CrossRef]
- Lee, J.; Kang, I.K.; Nock, J.F.; Watkins, C.B. Effects of preharvest and postharvest applications of 1-Methylcyclopropene on fruit quality and physiological disorders of ‘Fuji’ apples during storage at warm and cold Temperatures. HortScience 2019, 54, 1375–1383. [Google Scholar] [CrossRef]
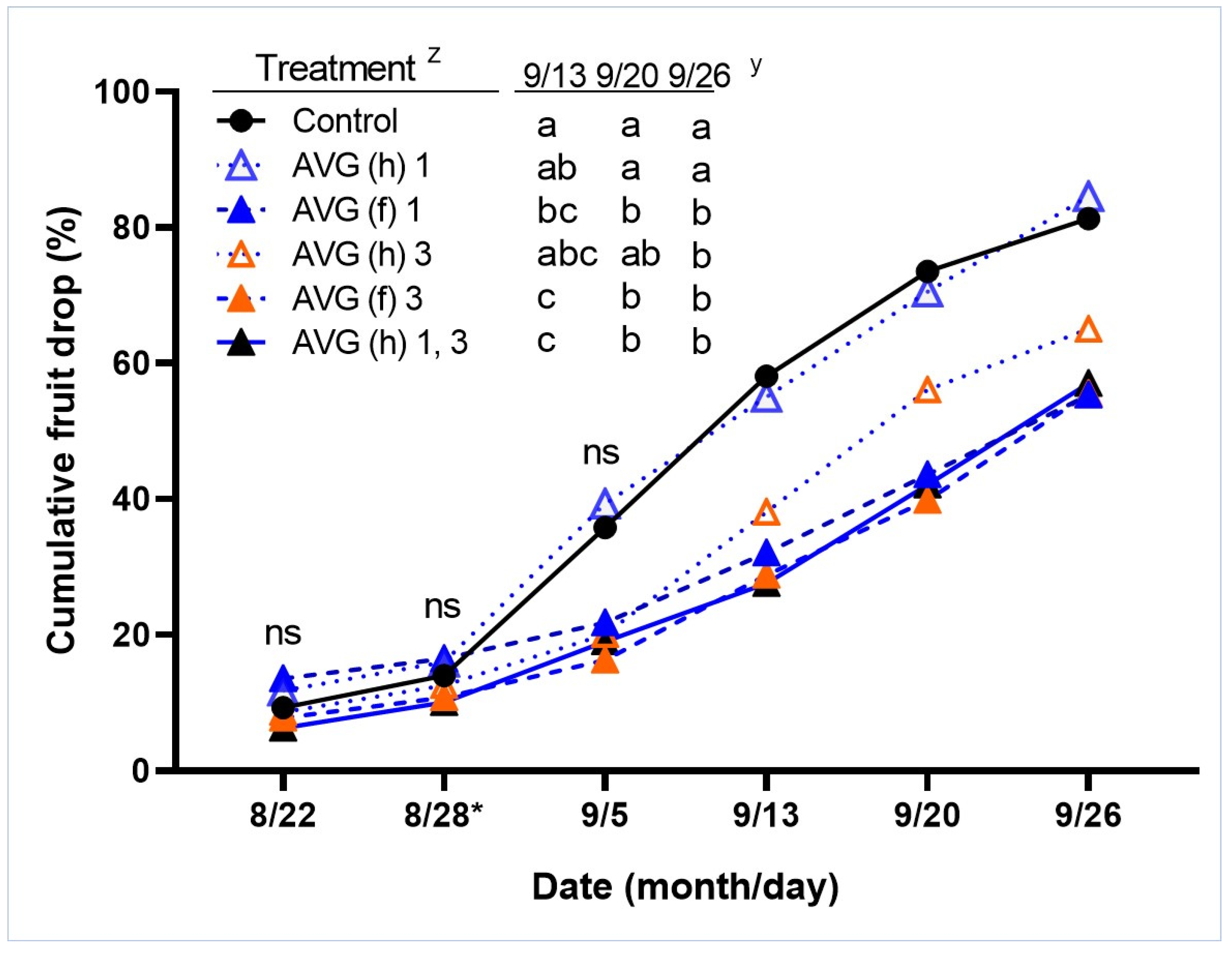
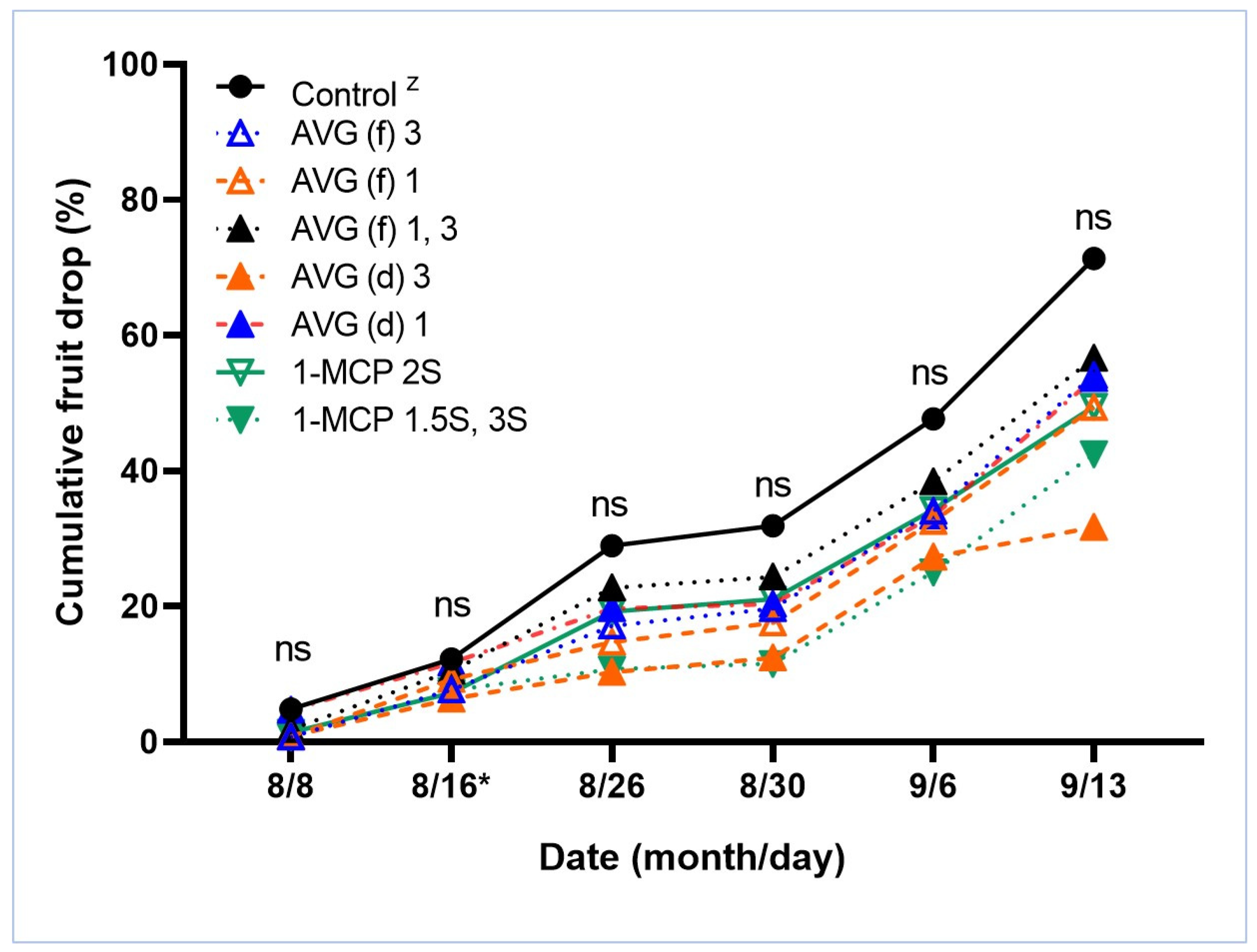


| Treatment | Diameter (mm) | Weight (g) | Firmness (N) | SPI (1 to 8) | TSS (Brix) | TA (g/L) | pH | Color (IAD) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H1 | H2 | H1 | H2 | H1 | H2 | H1 | H2 | H1 | H2 | H1 | H2 | H1 | H2 | H1 | H2 | |
| At harvest | ||||||||||||||||
| Control | 64.0 ab | 64.3 a | 114.7 ab | 117.5 a | 72.5 b | 69.8 a | 7.4 a | 7.6 a | 12.1 a | 13.4 a | 2.5 a | 2.4 a | 3.8 b | 4.0 a | 0.16 c | 0.14 d |
| AVG (h) 1 W | 67.7 a | 65.9 a | 134.7 a | 125.3 a | 76.0 ab | 73.4 a | 6.7 ab | 7.4 ab | 9.2 c | 13.0 ab | 2.0 b | 2.4 a | 4.0 a | 4.0 a | 0.28 bc | 0.29 bc |
| AVG (f) 1 W | 64.2 ab | 64.6 a | 118.3 ab | 118.5 a | 79.2 ab | 72.5 a | 5.9 ab | 7.7 a | 10.3 bc | 12.4 ab | 2.5 a | 2.5 a | 4.0 a | 4.0 a | 0.37 ab | 0.25 cd |
| AVG (h) 3 W | 67.1 ab | 65.0 a | 129.7 ab | 120.4 a | 80.9 ab | 78.3 a | 5.1 b | 7.2 ab | 10.2 bc | 12.2 ab | 2.5 a | 2.3 a | 3.9 a | 4.0 a | 0.55 a | 0.48 a |
| AVG (f) 3 W | 62.4 b | 64.4 a | 106.0 b | 117.0 a | 88.1 a | 80.5 a | 5.0 b | 6.6 b | 10.9 b | 12.8 ab | 2.5 a | 2.4 a | 4.0 a | 4.0 a | 0.48 a | 0.42 ab |
| AVG (h) 1 + 3 W | 66.6 ab | 67.7 a | 126.3 ab | 134.2 a | 79.2 ab | 76.0 a | 5.7 ab | 7.1 ab | 9.3 c | 12.0 b | 2.0 b | 2.2 a | 3.9 a | 4.0 a | 0.39 ab | 0.38 ab |
| After cold storage | ||||||||||||||||
| Control | 64.8 a | 65.1 ab | 113.8 a | 117.7 ab | 52.9 b | 49.8 c | 8.0 a | 8.0 a | 8.7 ab | 7.0 b | 1.4 a | 1.1 a | 3.7 a | 3.8 a | 0.10 b | 0.09 d |
| AVG (h) 1 W | 66.0 a | 65.5 a | 124.5 a | 120.1 ab | 57.4 ab | 56.0 b | 8.0 a | 8.0 a | 9.3 a | 8.5 ab | 1.5 a | 1.5 a | 3.5 a | 3.7 a | 0.18 a | 0.16 bc |
| AVG (f) 1 W | 63.6 a | 64.6 ab | 110.0 a | 118.3 ab | 58.2 a | 55.6 b | 8.0 a | 8.0 a | 6.8 c | 8.1 ab | 1.4 a | 1.4 a | 3.7 a | 4.0 a | 0.16 ab | 0.15 c |
| AVG (h) 3 W | 66.2 a | 66.4 a | 123.3 a | 124.0 a | 58.7 a | 54.7 bc | 8.0 a | 8.0 a | 7.7 abc | 7.7 ab | 1.4 a | 1.4 a | 3.6 a | 3.6 a | 0.20 a | 0.22 ab |
| AVG (f) 3 W | 63.3 a | 61.9 b | 106.3 a | 101.4 b | 59.6 a | 61.4 a | 8.0 a | 8.0 a | 6.9 bc | 9.2 a | 1.5 a | 1.3 a | 3.3 a | 3.5 a | 0.23 a | 0.26 a |
| AVG (h) 1 + 3 W | 66.3 a | 67.2 a | 122.4 a | 130.4 a | 58.2 a | 54.2 bc | 8.0 a | 8.0 a | 8.5 abc | 7.2 b | 1.4 a | 1.2 a | 3.7 a | 3.8 a | 0.21 a | 0.19 bc |
| Treatment | Diameter (mm) | Weight (g) | Firmness (N) | Color (IAD) | SPI (1–8) | IEC (ppm) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H1 | H2 | H1 | H2 | H1 | H2 | H1 | H2 | H1 | H2 | H1 | H2 | |
| At harvest | ||||||||||||
| Control | 75.8 a | 77.5 a | 195.9 ab | 215.1 a | 73.6 c | 62.7 a | 0.12 b | 0.03 b | 6.2 a | 7.2 a | 3.3 a | 11.0 a |
| AVG (f) 1 W | 71.3 b | 74.3 a | 170.8 b | 194.5 a | 85.6 a | 65.4 a | 0.26 ab | 0.06 ab | 4.5 ab | 6.8 ab | 2.3 ab | 6.5 a |
| A VG (f) 3 W | 73.6 ab | 74.2 a | 183.6 ab | 193.3 a | 81.6 abc | 67.1 a | 0.47 a | 0.11 a | 3.9 b | 5.8 b | 1.2 b | 6.1 a |
| AVG (d) 1 W | 73.6 ab | 75.4 a | 188.1 ab | 207.1 a | 79.1 abc | 66.3 a | 0.35 ab | 0.10 a | 5.7 ab | 6.3 ab | 1.6 b | 6.0 a |
| AVG (d) 3 W | 73.0 ab | 76.2 a | 182.8 ab | 208.4 a | 85.4 ab | 66.7 a | 0.54 a | 0.12 a | 3.5 b | 6.0 ab | 2.3 ab | 6.4 a |
| AVG (f) 1, 3 W | 74.2 ab | 74.3 a | 187.1 ab | 191.5 a | 73.8 c | 63.6 a | 0.41 ab | 0.11 a | 4.2 ab | 6.7 ab | 1.2 b | 6.4 a |
| 1-MCP 1.5 + 3 SPI | 75.6 a | 74.7 a | 193.5 ab | 195.3 a | 76.5 bc | 62.7 a | 0.28 ab | 0.11 a | 5.1 ab | 6.7 ab | 1.8 ab | 11. a |
| 1-MCP 2 SPI | 75.6 a | 75.1 a | 198.9 a | 203.3 a | 78.2 abc | 67.6 a | 0.25 ab | 0.09 ab | 4.8 ab | 6.2 ab | 2.5 ab | 9.8 a |
| After cold storage | ||||||||||||
| Control | 75.3 a | 74.1 a | 193.1 a | 185.1 a | 56.9 a | 48.9 a | 0.06 b | 0.02 a | 8.00 a | 8.0 a | 81.4 a | 127.7 a |
| AVG (f) 1 W | 70.1 b | 74.8 a | 162.1 b | 176.2 a | 62.7 a | 47.1 a | 0.16 ab | 0.03 a | 7.70 a | 8.0 a | 100.0 a | 85.7 a |
| AVG (f) 3 W | 72.3 ab | 76.6 a | 172.6 ab | 194.2 a | 60.5 a | 47.1 a | 0.19 ab | 0.03 a | 7.86 a | 8.0 a | 84.1 a | 80.0 a |
| AVG (d) 1 W | 73.0 ab | 77.1 a | 177.1 ab | 197.0 a | 59.1 a | 43.3 a | 0.12 ab | 0.03 a | 8.00 a | 8.0 a | 74.0 a | 91.8 a |
| AVG (d) 3 W | 73.4 ab | 74.6 a | 179.2 ab | 181.4 a | 60.9 a | 48.9 a | 0.20 a | 0.03 a | 7.66 a | 8.0 a | 77.9 a | 106.8 a |
| AVG (f) 1 + 3 W | 74.7 a | 74.1 a | 190.5 ab | 177.6 a | 60.9 a | 47.1 a | 0.20 ab | 0.04 a | 7.80 a | 8.0 a | 66.5 a | 152.5 a |
| 1-MCP 1.5 + 3 SPI | 75.5 a | 71.3 a | 192.3 a | 166.7 a | 57.4 a | 50.7 a | 0.14 ab | 0.04 a | 7.86 a | 8.0 a | 69.6 a | 127.7 a |
| 1-MCP 2 SPI | 74.2 ab | 71.8 a | 188.3 ab | 170.4 a | 59.6 a | 49.8 a | 0.13 ab | 0.03 a | 7.86 a | 8.0 a | 91.8 a | 94.1 a |
| Treatment | Diameter (mm) | Weight (g) | Firmness (N) | Color (IAD) | TSS (Brix) | SPI (1–8) | IEC (PPM) | SES (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H1 | H2 | H1 | H2 | H1 | H2 | H1 | H2 | H1 | H2 | H1 | H2 | H1 | H2 | H1 | H2 | |
| After Harvest | ||||||||||||||||
| Control | 60.1 ab | 62.7 ab | 158.7 ab | 163.2 ab | 73.8 a | 61.8 a | 0.19 c | 0.120 c | 13.9 a | 13.5 ab | 7.1 a | 7.5 a | 10.3 a | 2.1 ab | 33.3 a | |
| AVG (f) 3 W | 59.9 ab | 60.9 ab | 154.2 ab | 163.2 ab | 82.9 a | 75.4 a | 0.66 a | 0.52 a | 12.1 b | 11.5 b | 4.0 cd | 5.1 b | 1.3 b | 1.3 ab | 0 d | |
| AVG (f) + GA 3 W | 60.7 ab | 62.4 ab | 158.7 ab | 176.9 ab | 74.6 a | 72.6 a | 0.59 ab | 0.37 ab | 11.9 b | 12.5 b | 4.8 bcd | 5.7 b | 1.5 b | 1.2 ab | 6.66 cd | |
| AVG (h) 3 W, 1-MCP 1.5 SPI | 60.9 a | 63.5 a | 167.8 a | 185.9 a | 78.7 a | 70.1 a | 0.58 ab | 0.24 bc | 12.6 ab | 13.3 ab | 5.1 bc | 6.9 a | 1.3 b | 2.6 ab | 20.0 abc | |
| AVG (h) 3 W, 1-MCP 1.5 + 3 SPI | 58.1 ab | 60.4 b | 136.0 b | 154.2 b | 79.8 a | 66.4 a | 0.72 a | 0.52 a | 12.9 ab | 12.2 b | 4.0 d | 4.7 b | 0.84 b | 0.7 b | 0 d | |
| 1-MCP 3 SPI | 59.6 ab | 62.9 ab | 149.6 ab | 167.8 ab | 74.5 a | 60.4 a | 0.39 bc | 0.26 bc | 13.9 a | 15.9 a | 5.8 b | 6.9 a | 4.7 b | 7.3 a | 30.0 ab | |
| 1-MCP 1.5+3 SPI | 57.6 b | 61.2 ab | 136.0 b | 163.2 b | 88.3 a | 79.2 a | 0.62 a | 0.33 abc | 14.1 a | 14.3 ab | 4.2 cd | 5.6 b | 2.4 b | 2.5 ab | 13.3 bcd | |
| After cold storage | ||||||||||||||||
| Control | 57.6 abc | 131.5 ab | 57.9 b | 0.16 c | 13.9 ab | 8.0 a | ||||||||||
| AVG (f) 3 W | 56.2 bc | 122.4 b | 62.0 ab | 0.43 a | 12.9 b | 7.9 a | ||||||||||
| AVG (f) + GA 3 W | 58.6 abc | 154.2 a | 60.8 b | 0.35 ab | 13.6 ab | 8.0 a | ||||||||||
| AVG (h) 3 W, 1-MCP 1.5 SPI | 60.2 a | 154.2 a | 58.5 b | 0.26 abc | 17.4 a | 8.0 a | ||||||||||
| AVG (h) 3 W, 1-MCP 1.5 + 3 SPI | 55.4 c | 113.3 b | 70.3 a | 0.43 a | 14.0 ab | 8.0 a | ||||||||||
| 1-MCP 3 SPI | 58.7 ab | 140.6 ab | 63.4 ab | 0.25 bc | 14.9 ab | 8.0 a | ||||||||||
| 1-MCP 1.5 + 3 SPI | 56.9 bc | 127.0 ab | 70.3 a | 0.35 ab | 15.3 ab | 7.9 a | ||||||||||
| Treatment | Diameter (mm) | Weight (g) | Firmness (N) | Color (IAD) | TSS (Brix) | SPI (1–8) | IEC (ppm) | SES (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H1 | H2 | H1 | H2 | H1 | H2 | H1 | H2 | H1 | H2 | H1 | H2 | H1 | H2 | H2 | |
| Control | 59.6 a | 60.4 a | 140.6 a | 154.2 a | 64.9 b | 59.6 b | 0.13 b | 0.10 b | 13.0 a | 12.8 a | 7.6 a | 7.7 a | 3.51 a | 1.69 a | 9.45 a |
| AVG 3 W | 59.1 a | 60.1 a | 136.0 a | 154.2 a | 79.1 a | 74.7 a | 0.52 a | 0.40 a | 12.3 a | 12.2 a | 4.1 b | 5.5 c | 0.96 b | 1.37 a | 1.08 b |
| AVG 3 W, GA 3 W | 58.9 a | 59.4 a | 140.6 a | 140.6 a | 78.7 a | 72.9 a | 0.63 a | 0.34 a | 11.8 a | 12.1 a | 3.8 b | 6.6 b | 0.16 b | 2.28 a | 7.51 a |
| AVG 3 W, GA 3 + 2+1 W | 57.9 a | 60.7 a | 131.5 a | 149.6 a | 78.7 a | 68.9 a | 0.56 a | 0.27 ab | 12.2 a | 12.1 a | 5.3 b | 6.8 ab | 0.97 b | 1.38 a | 4.34 ab |
| Variables | Fruit Drop | Splitting | Firmness | Diameter | Weight | Color | TSS | Starch | Ethylene |
|---|---|---|---|---|---|---|---|---|---|
| Fruit drop | |||||||||
| Splitting | 0.54 | ||||||||
| Firmness | −0.40 | −0.32 | |||||||
| Diameter | 0.45 | 0.46 | −0.29 | ||||||
| Weight | 0.24 | 0.27 | −0.11 | 0.88 | |||||
| Color | −0.55 | −0.67 | 0.32 | −0.66 | −0.44 | ||||
| Brix | 0.30 | 0.50 | −0.20 | 0.25 | 0.09 | −0.44 | |||
| Starch | 0.60 | 0.68 | −0.38 | 0.64 | 0.42 | −0.79 | 0.41 | ||
| Ethylene | −0.081 | 0.27 | −0.19 | 0.00 | −0.06 | −0.09 | 0.03 | 0.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Islam, M.T.; Sherif, S.M. Effects of Aminoethoxyvinylglycine (AVG) and 1-Methylcyclopropene (1-MCP) on the Pre-Harvest Drop Rate, Fruit Quality, and Stem-End Splitting in ‘Gala’ Apples. Horticulturae 2022, 8, 1100. https://doi.org/10.3390/horticulturae8121100
Liu J, Islam MT, Sherif SM. Effects of Aminoethoxyvinylglycine (AVG) and 1-Methylcyclopropene (1-MCP) on the Pre-Harvest Drop Rate, Fruit Quality, and Stem-End Splitting in ‘Gala’ Apples. Horticulturae. 2022; 8(12):1100. https://doi.org/10.3390/horticulturae8121100
Chicago/Turabian StyleLiu, Jianyang, Md Tabibul Islam, and Sherif M. Sherif. 2022. "Effects of Aminoethoxyvinylglycine (AVG) and 1-Methylcyclopropene (1-MCP) on the Pre-Harvest Drop Rate, Fruit Quality, and Stem-End Splitting in ‘Gala’ Apples" Horticulturae 8, no. 12: 1100. https://doi.org/10.3390/horticulturae8121100
APA StyleLiu, J., Islam, M. T., & Sherif, S. M. (2022). Effects of Aminoethoxyvinylglycine (AVG) and 1-Methylcyclopropene (1-MCP) on the Pre-Harvest Drop Rate, Fruit Quality, and Stem-End Splitting in ‘Gala’ Apples. Horticulturae, 8(12), 1100. https://doi.org/10.3390/horticulturae8121100







