Abstract
Pistachio crops have a great economic potential, as their global production has increased dramatically over the past few decades. Therefore, it is important to maintain the healthy phytosanitary status of pistachio crops. In a Chilean pistachio orchard, a dieback of the trees was observed, with blighting of twigs and severe necrosis in the trunk and twigs. Bacterial isolation, pathogenicity tests and molecular characterization were conducted to determine the causal agent of the observed disease. The bacterial isolation and analysis of 16S rRNA gene led to the identification of Pantoea genus bacteria. Pathogenicity tests carried out on fruits inoculated with Pantoea isolates induced large necrosis seven days post-inoculation. Further inoculations were carried out on pruning cuttings and on the trunk of 18-month-old pistachio plants. Thirty-one weeks after inoculation, necrotic lesions were observed in the wood of pistachio plants. Sequence analysis of housekeeping genes enabled the isolated bacterium to be identified as Pantoa agglomerans, and to verify its role as the causal agent of the pistachio dieback with necrotic lesions. This is the first report of an occurrence of P. agglomerans inducing dieback in pistachio.
1. Introduction
The pistachio tree (Pistacia vera L.), which originated in the Middle East, has been introduced to various countries that have warm climates that allow its growth, such as Australia, Spain, and the United States of America (California) [1]. The species has deep roots, which allow it to adapt to areas with reduced water availability or physico-chemical limitations. The pistachio tree can have high longevity and production [2]. The global production of pistachios has increased dramatically over the past few decades, from around 50 thousand tons in 1970 to more than one million tons in 2020 [3]. In Chile, the area planted with pistachios is also increasing, reaching 146.2 ha in 2021 [4]. The production is concentrated in the central part of the country, which has edaphoclimatic conditions appropriate for the crop. Some important diseases of this species are verticilliosis (Verticillium dahliae Kleb), neck rot (Phytophtora sp.), alternaria (Alternaria alternata (Fr.) Keissl.), and botrytis (Botrytis cinerea Pers) [2]. The bacterium Xanthomonas translucens pv. translucens caused a dieback in pistachio orchards in Australia [5], inducing injuries to the trunk and to the plant’s extremities, as well as excessive resin production, leading to the plant’s death [6].
In recent years, Pantoea agglomerans, a Gram-negative bacillus belonging to the Enterobacteriaceae family, has emerged as an important pathogenic bacterium [7,8]. P. agglomerans is widely distributed in agricultural and natural environments as a common epiphyte and endophyte of various plant species; it was also isolated from humans and animals [9,10]. Interestingly, this species was widely used as a biological control agent against fungal and bacterial plant pathogens [10]. However, it has been demonstrated that P. agglomerans strains become host-specific tumor-forming pathogens by acquiring a plasmid-borne pathogenicity island [9]. Furthermore, this bacterial species can cause some occupational diseases in humans, and it also infects animals [11]. This bacterium was identified as causal agent of leaf blight and bulb rot in onions in Georgia, USA [12] and of blight and vascular wilt in corn and sorghum farms in Mexico [13]. Recently, it has been demonstrated that P. agglomerans could also cause brown apical necrosis in walnut [14], bacterial soft rot in cabbage [15], necrotic disease in jujube [16], mango [10] and plum [17], shot-hole disease in the leaves of plum and peach [18], and blight on pepino melon (Solanum muricatum) [19].
In this study, necrotic symptoms and dieback were observed in pistachio trees growing in an orchard located in Pumanque, O’Higgins region (Chile). Near 70% of the plants showed strong decline, with death of shoots, deformation of leaves, and necrosis of trunk and of vascular tissues of the twigs of the year. Thus, the main goal of this work was to identify the pathogen that causes the symptoms of dieback in pistachio trees by isolation, molecular characterization, and pathogenicity tests.
2. Materials and Methods
2.1. Bacterial Isolation and Culture Conditions
A total of 24 bacterial isolates was obtained from diseased pistachio trees of variety Kerman, grafted on the rootstock UCB-1, from an orchard located in Pumanque, O´Higgins region, Chile (34°34′54″ S–71°38′38″ W). For bacterial isolation, six whole pistachio trees with symptoms of disease (decline, death of shoots, deformation of leaves, or necrosis in the trunk and twigs) were transferred to the laboratory. Necrotic twigs and pieces of trunk were processed: the tissues were surface sterilized by immersion in 95% ethanol, followed by flaming of the cut region and bark remotion with a sterilized scalpel. The healthy wood adjacent to that necrotic area was scraped under sterile conditions and used for bacteria isolation. The resultant vegetal tissue fragments were homogenized in a laboratory blender for 3 min with sterile phosphate-buffered saline (1 M, pH 7.2), using 10 mL/g of vegetal tissue, after which 100 µL were spread in both YDC (2% dextrose, 1% yeast extract, 2% CaCO3, and 2% agar) and Kings-B (KB) (2% protease peptone, 0.15% K2HPO4, 0.15% MgSO2 x7H2O, 1% glycerol, and 1.5% agar, pH 7.0) [20] media containing plates. In parallel, samples of fragmented plant tissue were incubated overnight in 9 mL of PBS buffer on a rotary shaker at 28 °C and the suspension was then spread on YDC and KB agar plates. For re-isolation of bacteria from inoculated plants (pathogenicity tests), one twig per plant was cut, while fragments of 25 cm in length were analyzed in the case of trunks. In plants with necrosis, adjacent asymptomatic wood was scraped, and where there was no necrosis, the wood was scraped from the area near to the inoculation point. The samples were macerated in 1 mL of sterile bidistilled water, and 50 μL were streaked on KB medium containing plates incubated for 48 h at 28 °C. Subsequently, individual colonies were transferred two times to KB medium plates using the streak plate technique to obtain pure cultures. The initial selection of bacterial isolates was based on the macroscopic characteristics of the colonies, such as color, shape, brightness, and mucosity. Then, the genus of each sampled isolate was confirmed by amplifying and sequencing the 16S rRNA gene, which was then compared with the sequences available in the GenBank database. The isolated strains were grown in solid KB medium and incubated at 28 °C; for long-term maintenance, they were cryopreserved and stored at −80 °C in LB broth with glycerol, in a 1:1 ratio [21].
2.2. Pathogenicity Tests on Fruits and Pistachio Trees
To determine whether the identified isolates of P. agglomerans were the causal agent of the diseases in pistachio trees, pathogenicity tests ex planta and in planta were carried out by inoculating fruits and pistachio trees, respectively. Two isolates, OF148 and OF151, were used for both pathogenicity tests.
As a first approach in evaluating the bacterial isolates ability to cause necrosis in pistachio, a preliminary pathogenicity test was performed by inoculating immature pistachio fruits, Kerman variety, of approximately 2 cm in length. Prior to inoculation, the fruits were washed with sterile water before and after being surface sterilized with ethanol 70%. Next, fruits were punctured using a sterile needle and drops of 10 µL of a bacterial suspension at the concentration of 1 × 108 CFU/mL were injected into each fruit. Eight fruits were inoculated with each isolate. The inoculated pistachio fruits were then placed on sterile wet paper inside empty Petri dishes and incubated at 28 °C for one week. Punctured fruits inoculated with sterile water were used as negative controls.
For the in planta pathogenicity tests, inoculation was carried out in trunks and twigs of healthy 18-month-old pistachio plants, variety Kerman, grafted in Pioneer Gold, growing in pots, and obtained from a nursery. Before the inoculation, the plants were tested for the presence of bacterial pathogens; all test results were negative. In total, 26 plants were used: 16 inoculated with the two bacteria isolates (eight plants for each isolate, of which four were inoculated in the trunks and four in twigs). The control treatments were distributed as follows: four plants were inoculated with the culture medium (two in the trunks and two in twigs); four plants were inoculated with Escherichia coli DH5α (two in trunks and two in twigs); and two plants were only wounded (one in trunk and one in twig) (Table 1).

Table 1.
Description of treatments for in-planta pathogenicity test.
The inoculum for the pathogenicity assays was prepared from bacterial colonies with 24 h of growth at 28 °C in KB agar medium. A colony was collected with a sterile needle and introduced into a 15 mL tube containing 5 mL of LB broth (1% tryptone, 0.5% yeast extract, and 0.5% NaCl) and incubated at 28 °C overnight, with agitation (180 rpm) until it reached the stationary phase. A new bacterial culture was produced using an inoculum of 250 µL of the overnight culture grown in 50 mL of LB medium (ratio 1:250). It was incubated for approximately 3 h to reach the optical density of 0.1 measured at 600 nm (OD 600) in a spectrophotometer, equivalent to a bacterial suspension of 108 CFU/mL. As a means of verification, a plate count was performed [22]. The final culture (OD 600:0.1) was used as the inoculum for the pathogenicity tests in pistachio plants.
For the inoculation of the twigs, a cross-section cut was made at the tip of two twigs of each plant, leaving the woody part exposed. At that site, 100 μL of the bacterial suspension was slowly added, while being absorbed by the wound. Then, at the same point, a drop of glycerol was placed and finally sealed with a flexible sterile film to avoid dehydration. For the inoculation of the trunk, a 4 mm diameter hole was drilled with a sterilized drill bit and the same procedure that was used to inoculate the twigs was repeated. The plants were maintained under greenhouse conditions (temperature: 20–26 °C; humidity: 20–50%) and were observed every week to determine the presence of symptoms. Samples were taken at weeks 17 and 31 after inoculation, cutting the first layers of tissue until necrosis was observed (when present) and bacterial isolation and molecular identification was performed. In both types of inoculation, the film was maintained for four weeks.
2.3. Molecular Characterization of Bacterial Isolates
For the molecular characterization of the bacterial isolates, 16S rRNA gene sequencing and multilocus sequence analysis (MLSA) were conducted. Genomic DNA of bacterial isolates was extracted using the Wizard® Genomic DNA Purification Kit (Promega, Madison, WI, USA) according to the manufacturer’s instructions. PCR amplification of the 16S rRNA gene was conducted using primers 27F and 1492R [23]. PCR was prepared according to the Taq DNA polymerase Taq Platinum™ (Thermo Fisher Scientific, Waltham, MA, USA) standard protocol. Amplification conditions consisted of initial denaturation for 3 min at 95 °C, followed by thirty cycles of 30 s at 95 °C, 1 min at 58 °C, and 1 min at 72 °C, plus a final extension of 7 min at 72 °C. The PCR product obtained was checked by electrophoresis in a 1.2% agarose gel along with a 1 kb DNA ladder (Promega, Madison, WI, USA) following a standard protocol (120 V, 20 min). Subsequently, the amplicons were purified and sequenced at Macrogen (Seoul, Korea) using the same 16S rRNA universal primers that were used in the amplification and provided by the company. Partial 16S sequences of 24 bacterial isolates were obtained. Each sequence was compared against the nonredundant nucleotide database of the National Center for Biotechnology Information (NCBI) using the BLAST tool. The bacteria that were isolated were indistinguishable from each other, both phenotypically and in their 16S rRNA gene sequence; therefore, two bacterial isolates, OF148 and OF151, were selected for pathogenicity tests and multilocus analysis by PCR amplification and sequencing. The housekeeping genes encoding RNA polymerase b subunit (rpoB), ATP synthase b subunit (atpD), and initiation translation factor 2 (infB) were amplified and sequenced using specific primers [24]. Genomic DNA was prepared using the Wizard® Genomic DNA Purification Kit protocol (Promega, WI, USA) according to the manufacturer’s instructions. PCR amplification was performed on 250 ng of template DNA by using an Agilent thermal cycler. PCR was prepared according to the GoTaq® DNA polymerase (Promega, WI, USA) standard protocol. The amplification conditions, described previously [24], included denaturation at 95 °C for 5 min, 3 cycles of denaturation at 95 °C for 1 min, annealing at 54–58 °C (depending on the gene) for 2 min 15 s, and elongation at 72 C for 1 min 15 s, followed by 30 cycles of denaturation at 95 °C for 35 s, annealing at 55 °C for 1 min 15 s, and elongation at 72 °C for 1 min 15 s, and a further 7 min of elongation at 72 °C. PCR products were separated on 1% agarose gels at 75 V for 45 min. The amplicons were sequenced as reported above.
The sequences of the housekeeping genes, in addition to 16S rRNA gene, were manually concatenated and aligned following the alphabetical order of the genes, ending in a sequence of 2,760 bp (1050 bp for the 16S rRNA gene; 657 bp for the atpD gene; 615 bp for the infB gene; and 438 bp for the rpoB gene) and deposited in GenBank (accession numbers OP646280 to OP646287 for the 16S rRNA gene and OP649600 to OP649623 for the other genes). Representative sequences from the genus Pantoea included for comparative analysis were P. agglomerans, P. eucalypti, P. vagans, P. stewartii, and P. ananatis. The sequences were aligned to build a phylogenetic tree, using Bioedit Sequence Alignment Editor v7.2 [25] and MEGA v6.0 [26]. For genetic relationship analyses, maximum parsimony trees were generated in MEGA v6.0 software using 500 bootstrap replicates.
3. Results
3.1. Isolation and Identification of Pantoea Isolates from Diseased Pistachio Trees
The isolation of the possible causal agent of the diseases in the pistachio samples was performed from six trees with symptoms of strong decay, death of shoots, leaf deformation, and necrosis of vascular tissues in the trunk (Figure 1). In all cases, there was an abundant growth of a single type of colony, yellow and mucoid. Based on macroscopic characteristics, 24 colonies were selected and streaked on KB medium plates to obtain pure isolates. Through 16S rRNA gene analyses, it was established that the bacterium isolates belonged to the genus Pantoea, presenting 99.9% of nucleotide identity with a reference isolate of the species P. agglomerans (isolate C410P1; GenBank accession number CP016889).

Figure 1.
Symptoms of dieback and necrosis in pistachio trees: (A) diseased tree; (B) necrosis of twigs and leaves; (C) necrosis of the woody tissue in the trunk.
3.2. Pathogenicity of Pantoea Strains Isolated from Pistachio Trees
As most of the isolates were indistinguishable from each other, both phenotypically and in their 16S rRNA gene sequence, two isolates, OF148 and OF151, were selected for pathogenicity tests. Isolate OF148 was obtained from tissue from a twig with apical necrosis, while isolate OF151 was obtained from the xylem of a necrotic branch.
In the pathogenicity ex planta, after one week of incubation at 28 °C, all the fruits inoculated with both Pantoea isolates showed symptoms of necrosis progressing from the point of inoculation to the surrounding area, mainly through the vascular system of the fruit. No tissue damage was observed in control fruits inoculated with sterile water (Figure 2).

Figure 2.
Inoculated pistachio fruits after incubation at 28 °C for one week: (A) healthy control fruit inoculated with sterile water; (B) necrotic symptoms one week after inoculation with the bacterial isolate OF148; (C) necrotic symptoms after incubation for one week with isolate OF151.
The tissues from the zone adjacent to the necrosis and from the control fruits were ground and plated in YDC medium. Only the fruits inoculated with Pantoea isolates produced an abundant growth of yellow mucoid colonies. The 16S rRNA gene sequence analyses from these colonies had 99.9% identity with several P. agglomerans GenBank accessions (data not shown). In the in planta pathogenicity test, the initial evaluation of trunk and branches of each plant did not show necrosis or signs of bacterial infection (Figure 3A,B). The plating of the samples did not show bacterial growth after 48 h of incubation.

Figure 3.
(A) Longitudinal section and (B) cross-section of healthy twig before inoculation. (C) Twig seventeen weeks after inoculation with isolate OF148 (T1). (D) Twig seventeen weeks after inoculation with isolate OF151 (T3). Black arrows indicate the point where the first necrotic tissue appeared. (E) Necrosis in the cross- and (F) longitudinal sections of trunk seventeen weeks after inoculation with isolate OF148 (T2). (G) Necrosis in the cross- and (H) longitudinal sections of trunk seventeen weeks after inoculation with isolate OF151 (T4).
Within seventeen weeks after inoculation, in the twig samples (T1 and T3) it was observed that the yolk closest to the inoculation zone showed initial necrosis (Figure 3C,D). Two plants with trunk inoculation (Figure 3E–H) showed a necrotic area in cross-section (Figure 3E,G). Subsequently, the progress of the lesion was measured in a longitudinal cut, up and down the inoculation point. The trunk of the T2 plant (Figure 3F) had a necrosis progression of 4.5 cm, while that of the T4 treatment (Figure 3H) was 7 cm. Thirty-one weeks after inoculation, both control and plants inoculated with the bacterial isolates were sampled. Table 2 shows the average progress of the lesions.

Table 2.
Necrosis progression in the different treatments thirty-one weeks post-inoculation.
In Table 2, a statistically significant difference can be observed between the average length of necrosis between treatments with bacterial isolates (T1 to T4) and controls. In both twigs and trunks inoculated with the isolated bacteria, the length of the necrosis was more than double compared with that of the controls. When comparing twig treatments with each other, it was observed that those twigs that were inoculated with isolates OF148 and OF151 showed necrosis in the wood, while in the controls with culture medium (CM), E. coli and wound, only healthy tissue was observed in the trunks (Figure 4A–E). Figure 4 shows the symptoms obtained with T1 and T3 treatments. It was possible to observe, in both cases, a clear and defined necrosis due to the bacterial inoculation in twigs (Figure 4A,B) being slightly larger in treatments with isolate OF148; that was, however, without statistically significant differences. On the other hand, all of the plants inoculated in the trunk showed necrosis, independently of the treatment (Figure 4F–J). However, as with the twigs, plants inoculated with Pantoea isolates had a larger development of necrosis, with an average length ranging from 10.7 to 17.3 cm (Figure 4F,G), while necrosis in the control plants did not exceed 2.25 cm in length (Figure 4H–J).

Figure 4.
Experimentally inoculated pistachio plants after thirty-one weeks. Necrosis in longitudinal section of a twig inoculated (A) with isolate OF148 (T1) and (B) with isolate OF151 (T3). Necrosis in longitudinal section of a trunk sample (F) with isolate OF148 (T2) and (G) with isolate OF151 (T4). (C,H) Control twig inoculated with culture medium. (D,I) Control twig inoculated with E. coli. (E,J) twig wound control.
The trunk inoculation treatment with the isolate OF151 showed, on average, 6.6 cm more progression of the necrotic lesion than treatment with the isolate OF148, a statistically significant difference. The average length of necrosis for Pantoea treatments was 14 cm, while for controls it was 1.72 cm (Table 2).
3.3. Re-Isolation of Pantoea from Inoculated Plants and Their Molecular Identification by Housekeeping Gene Sequencing
The results of the in-planta pathogenicity tests enabled the verification of the pathogenicity of Pantoea isolates OF148 and OF151. After the plating of ground tissue from the inoculated twigs, colonies with a very similar growth were obtained. Both T1 and T3 isolates showed colonies of an intense yellow color and a mucoid texture. At the same time, a slight difference was observed between the colonies of both isolates. Colonies of the T1 treatment (isolate OF148) showed mucoid texture, with defined borders, while the colonies of the T3 treatment (isolate OF151) developed as a homogeneous mucus layer, with no ability to distinguish individual colonies. In the inoculum with culture medium there was no bacterial growth, while bacterial colonies were observed in the case of control with E. coli and wounds. In these last two cases, the observed colonies had a light white-yellow color and were smaller than those from isolates OF148 and OF151. The treatment with only the wounds presented a large type of colony, with white and soft pink colors and a texture comparable to the other colonies that were observed. In the trunk inoculation assays, a bacterial growth was also observed in the controls. Bacterial growth observed in the samples obtained from trunks with T2 and T4 treatments and colonies were very similar to those from inoculated twigs (T1 and T3), presenting an intense yellow color and mucous texture. In all of the samples of the control treatments, the observed bacterial growth was represented by colonies lacking the typical coloration and/or morphology of the Pantoea isolates. The 16SrRNA analyses indicated that all of them belonged to genera different than Pantoea (data not shown).
When performing PCR for the specific genes of Pantoea (atpD, infB and rpoB), positive results were obtained only from samples from twigs or trunks that were inoculated with isolates OF148 and OF151 (T1–T4). The original isolates OF148 and OF151, used for pathogenicity tests, were also included in the multilocus analyses. The sequences obtained were analyzed individually in BLASTn (NCBI) and showed 99.0 to 99.8% nucleotide identity with P. agglomerans isolates from different areas of the world. The sequences of the three housekeeping genes were concatenated, including the 16S rRNA gene sequence, and ended in a total sequence length of 2760 nt. The concatenated sequence was aligned and compared with sequences belonging to different species of the genus Pantoea. Based on the alignment of the concatenated sequences, a phylogenetic tree was constructed using the maximum parsimony method. The phylogenetic distribution obtained (Figure 5) shows that both the original isolates OF148 and OF151 and those recovered from necrotic tissue of the inoculated plants (pathogenicity tests) are grouped in the same clade with the reference strains CFSAN047153, C410P1, and CFSAN047154 of P. agglomerans species, with identities ranging between 98.4–99.6%. In particular, the isolates used in this study form a monophyletic group within the clade of P. agglomerans, having between 99.8% and 100% of nucleotide identity among them. As expected, sequences belonging to species P. eucalypti, P. vagans, P. stewartii, and P. ananatis formed four clades that were clearly distinguishable from the P. agglomerans clade. The original isolates OF148 and OF151, and those isolated from the lesions obtained after experimental inoculation, were all identified as P. agglomerans by the MLSA on the amplified genes.
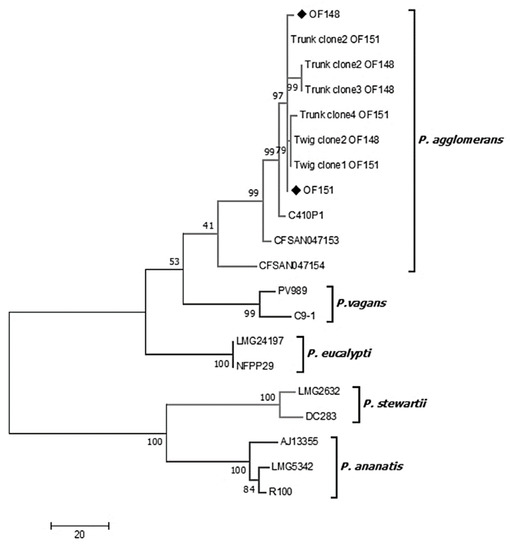
Figure 5.
Phylogenetic distribution by the method of maximum parsimony, obtained from the multilocus sequence analysis of the atpD, infB, rpoB, and 16S rRNA genes (2760 bp) of P. agglomerans isolated from pistachio. The values indicated on the nodes represent a bootstrap of 500 repetitions. OF148 and OF151: original isolates, (♦) bacterial isolates from infected plants in the orchard. Sequences from the genus Pantoea are listed below, along with their geographic origins and GenBank accession numbers in square brackets: P. agglomerans CFSAN047153 [USA-NZ_CP034469], P. agglomerans C410P1 [China-NZ_CP016889], P. agglomerans CFSAN047154 [USA-NZ_CP034474], P. eucalypti LMG24197 [Uruguay-NZ_CP045720], P. eucalypti [USA-NZ_FUWI00000000.1], P. vagans PV989 [China-NZ_CP028349], P. vagans C9-1 [Switzerland-NC_014562], P. stewartii LMG2632 [India-NZ_JPKO00000000], P. stewartii DC283 [USA-AHIE00000000], P. ananatis AJ13355 [Japan-AP012032], P. ananatis LMG5342 [Germany-NC_016816], P. ananatis R100 [China-NZ_CP014207].
4. Discussion
In this study, it was possible to identify the causal agent of necrotic disease and dieback of pistachio plants in the O’Higgins region of Chile. In the bacterial isolation from diseased pistachio trees, we expected the isolation of Xanthomonas bacteria based on the diseases described in pistachio [5]; however, only one type of non-fluorescent and yellow colony was observed, and fungi were never isolated (data not shown). The first identification, based on the 16S rRNA gene sequence, revealed that all isolates belonged to the Pantoea genus. Pistachio plants that were experimentally inoculated with two of the bacterial isolates showed a clear necrosis in the inoculation areas, confirming that both Pantoea isolates, OF148 and OF151, were able to cause necrotic symptoms in both the trunk and the twigs. Although the symptomatology in the experimentally inoculated trees did not show the dieback as in the trees from orchards, the symptoms observed agreed with the symptomatology observed in the field in the initial stage of the disease, represented by necrotic lesions in twigs and necrosis progressing in the wood. In this work, the plants were observed only during the 31 weeks after bacterial inoculation, so the time was not sufficient for the typical dieback to appear in the plants inoculated in the trunk. The two analyzed isolates caused different symptoms in twigs and trunks. Isolate OF148 affected twigs more severely, while isolate OF151 showed a necrotic progression in trunks. The twigs that were inoculated with the bacteria showed symptoms, but treatment with isolate OF148 had, on average, 1.5 cm more lesion progression than treatment with isolate OF151. On the other hand, all of the plants inoculated in the trunk presented necrosis; however, those that were inoculated with Pantoea isolates had a stronger expression of this symptom, even exceeding 20 cm of progression for lesions of isolate OF151. Although a necrotic growth could be observed in trunks inoculated with the controls, these lesions were considerably shorter than those observed in treatments with the Pantoea isolates. In addition, in cases in which bacteria were isolated from the control tissues, they did not correspond to the genus Pantoea.
It should be reported that the film that wrapped the hole of the trunk was removed four weeks after inoculation, and during irrigation the water soaked that area; therefore, it was possible that other agents with pathogenicity that was lower than that of the Pantoea isolates were entering (symptoms on controls). When observing the averages of the treatments, it was seen that, in the twigs, the treatment with isolate OF148 had a larger length of necrosis, unlike the trunk, where the highest average was the treatment with isolate OF151. This difference corresponds with the origin of the isolation from trees, being isolate OF148 obtained from symptomatic pistachio branches and isolate OF151 from the pistachio trunk.
Pathogenic isolates OF148 and OF151, as well as bacteria isolated from experimentally inoculated plants, were molecularly identified by housekeeping gene sequence analysis. As nucleotide sequence analysis of the 16S rRNA gene indicated taxonomic discrepancies in the Pantoea genus [27], the use of housekeeping genes shows a better resolution in identifying Pantoea species [24,28]. The phylogenetic tree based on the MLSA analysis showed clearly separate clades corresponding to different Pantoea species. Based on a previous study (24), genes rpoB, atpD, and infB, together and separately, delineate the seven validly published Pantoea species [24]. In this study, the MLSA of the housekeeping genes atpD, infB, and rpoB, in addition to the 16S rRNA gene, conclusively confirmed that the bacterial isolates from pistachio trees from Chilean orchards belong to P. agglomerans species. MLSA analysis also enabled the confirmation that re-isolated bacteria from experimentally inoculated and symptomatic trees corresponded to the same original isolates used in the inoculation, fulfilling Koch’s postulates. All the colonies obtained from the lesions, analyzed with the MLSA technique, were identified as P. agglomerans. Although isolates OF148 and OF151 could not be distinguished in the MLSA analysis, the difference observed in the symptoms caused suggest that the two isolates correspond to different pathotypes of P. agglomerans, which is, therefore, a new etiological agent causing necrosis and dieback in pistachio trees.
It is important to note that in a small hill next to the orchard where the declining pistachio trees were located, there was an onion field. Previous studies reported leaf blight and onion bulb rot that were caused by P. agglomerans in Georgia and Michigan [29,30]. Analysis and pathogenicity tests were performed, and the presence of this bacterium and not of P. ananatis, previously described in onions, was confirmed in the onions (unpublished data). This would indicate that P. agglomerans could have infected pistachio from the adjacent onion crop.
P. agglomerans is also used as a biocontroller, mainly in fruit post-harvest. In mandarins, it can control green mold (Penicillium digitatum) and in oranges, blue mold (Penicillium italicum) [31]. Furthermore, it has been shown to be effective for the control of Botrytis cinerea, Penicillium expansum, and Rhizopus stolonifer in pears and apples [32]. Therefore, it is extremely important to evaluate the isolates of this bacterial species and control their use as biocontrol agents, in order to avoid undesired effects.
The results of this study report the presence of pathogenic P. agglomerans in Chilean orchards, confirming the concern about environmental bacteria frequently associated with agricultural crops and used as a biocontroller that could cause plant and even human diseases. This situation is exacerbated by evidence suggesting the transfer of pathogenicity islands or plasmids between Pantoea species [10], indicating the need for identification of the genetic determinants responsible for the pathogenicity in P. agglomerans isolates. Further studies focusing on the infection process, disease spread, and genetic determinants of pathogenicity are necessary to develop an appropriate strategy for the control and surveillance of pathogenic P. agglomerans strains.
5. Conclusions
The results obtained in this work show that P. agglomerans is the etiologic agent of necrotic lesions and subsequent pistachio dieback. To the best of our knowledge, this is the first report, worldwide, of this pathogen in pistachio plants. It should be considered that this bacterium could be infecting other crops in Chile, due to its polyphagous behavior. It is important to be informed about the pathogenic potential of P. agglomerans species, mainly due to its use as a biocontroller, to avoid undesired or dangerous effects.
Author Contributions
Conceptualization, A.Z., T.Z. and N.F.; methodology, A.Z., T.Z., P.C., G.H., A.B. and N.F.; investigation, A.Z., T.Z., P.C., G.H., A.B. and N.F.; resources, A.Z. and N.F.; data curation, A.Z., T.Z. and N.F.; writing—original draft preparation, A.Z., T.Z. and P.C.; writing—review and editing, A.Z., T.Z., P.C., G.H., A.B. and N.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mir-Makhamad, B.; Bjørn, R.; Stark, S.; Spengler, R.N. Pistachio (Pistachio vera) domestication and dispersal out of central Asia. Agronomy 2022, 12, 1758. [Google Scholar] [CrossRef]
- Saavedra, E. El Pistachero. Antecedentes Generales y Avances en el Manejo Agronómico del Cultivo del Pistachero en Chile; FIA (Fundación para la Innovación Agraria), Ministerio de Agricultura: Santiago, Chile, 2011; 108p. [Google Scholar]
- FAOSTAT. Food and Agriculture Organization of the United Nations Database. Available online: https://www.fao.org/faostat/en/#home (accessed on 31 August 2022).
- ODEPA. Oficina De Estudios y Políticas Agrarias Database. Available online: https://www.odepa.gob.cl/ (accessed on 31 August 2022).
- Giblot-Ducray, D.; Marefat, A.; Gillings, M.R.; Parkinson, N.M.; Bowman, J.P.; Ophel-Keller, K.; Taylor, C.; Facelli, E.; Scott, E.S. Proposal of Xanthomonas translucens pv. pistaciae pv. nov., pathogenic to pistachio (Pistacia vera). Syst. Appl. Microbiol. 2009, 32, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Marefat, A.; Ophel-Keller, K.; Scott, E.S.; Sedgley, M. The use of ARMS PCR in detection and identification of xanthomonads associated with pistachio dieback in Australia. Eur. J. Plant Pathol. 2006, 116, 57–68. [Google Scholar] [CrossRef]
- Cheng, A.; Liu, C.Y.; Tsai, H.Y.; Hsu, M.S.; Yang, C.J.; Huang, Y.T.; Liao, C.H.; Hsueh, P.R. Bacteremia caused by Pantoea agglomerans at a medical center in Taiwan, 2000–2010. J. Microbiol. Immunol. Infect. 2013, 46, 187–194. [Google Scholar] [CrossRef]
- Dutkiewicz, J.; Mackiewicz, B.; Lemieszek, M.K.; Golec, M.; Milanowski, J. Pantoea agglomerans: A mysterious bacterium of evil and good. Part IV. Beneficial effects. Ann. Agric. Environ. Med. 2016, 23, 206–222. [Google Scholar] [CrossRef]
- Barash, I.; Manulis-Sasson, S. Virulence mechanisms and host specificity of gall-forming Pantoea agglomerans. Trends Microbiol. 2007, 15, 538–545. [Google Scholar] [CrossRef]
- Gutiérrez-Barranquero, J.A.; Cazorla, F.M.; Torés, J.A.; de Vicente, A. Pantoea agglomerans as a new etiological agent of a bacterial necrotic disease of mango trees. Phytopathology 2019, 109, 17–26. [Google Scholar] [CrossRef]
- Shu, R.; Yin, X.; Long, Y.; Yuan, J.; Zhou, H. Detection and control of Pantoea agglomerans causing plum bacterial shot-hole disease by loop-mediated isothermal amplification technique. Front. Microbiol. 2022, 13, 896567. [Google Scholar] [CrossRef]
- Gitaitis, R.D.; Gay, J.D. First report of a leaf blight, seed stalk rot, and bulb decay of onion by Pantoea ananas in Georgia. Plant Dis. 1997, 81, 1096. [Google Scholar] [CrossRef]
- Morales-Valenzuela, G.; Silva-Rojas, H.V.; Ochoa-Martínez, D.; Valadez-Moctezuma, E.; Alarcon-Zuniga, B.; Zelaya-Molina, L.X.; Córdova-Téllez, L.; Mendoza-Onofre, L.; Vaquera-Huerta, H.; Carballo-Carballo, A.; et al. First report of Pantoea agglomerans causing leaf blight and vascular wilt in maize and sorghum in Mexico. Plant Dis. 2007, 91, 1365. [Google Scholar] [CrossRef]
- Yang, K.Q.; Qu, W.W.; Liu, X.; Liu, H.X.; Hou, L.Q. First report of Pantoea agglomerans causing brown apical necrosis of walnut in China. Plant Dis. 2011, 95, 773. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Liu, Y.; Liu, S.; Qu, Z.; Zhang, Y. First report of bacterial soft rot caused by Pantoea agglomerans on Chinese cabbage in China. Plant Dis. 2019, 104, 277–278. [Google Scholar] [CrossRef]
- She, X.M.; Yu, L.; Lan, G.B.; Tang, Y.F.; Deng, M.G.; Li, Z.G.; He, Z.F. First report of necrotic disease caused by Pantoea agglomerans on Ziziphus jujuba in China. Plant Dis. 2019, 103, 1405. [Google Scholar] [CrossRef]
- Li, C.; Jia, Y.; Tian, Y.; Zhou, L.; Sun, W.; Deng, J.; Liu, F. First report of necrotic disease caused by Pantoea agglomerans on plum (Prunus salicina) in China. Plant Dis. 2020, 104, 1248. [Google Scholar] [CrossRef]
- Tong, Y.P.; Yang, B.Y.; Tian, Q.; Hu, F.P.; Cai, X.Q. Isolation and identification of a new pathogen causing bacterial leaf shot hole disease on peach and plum. J. Fujian Agric. For. Univ. 2020, 49, 300–306. [Google Scholar]
- She, X.; Yu, L.; Lan, G.; Tang, Y.; Deng, M.; Li, Z.; He, Z. Pantoea agglomerans causing blight disease on pepino melon (Solanum muricatum) in China. Crop Prot. 2021, 139, 105385. [Google Scholar] [CrossRef]
- King, E.O.; Ward, M.K.; Raney, D.E. Two simple media for the demonstration of pyocyanin and fluorescin. J. Lab. Clin. Med. 1954, 44, 301–307. [Google Scholar]
- Sambrook, J.; Russell, D.W. Molecular Cloning: A Laboratory Manual (3-Volume Set); Molecular cloning: A laboratory manual; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2001. [Google Scholar]
- Hoben, H.J.; Somasegaran, P. Comparison of the pour, spread, and drop plate methods for enumeration of Rhizobium spp. in inoculants made from presterilized peat. Appl. Environ. Microbiol. 1982, 44, 1246–1247. [Google Scholar] [CrossRef]
- Jiang, H.; Dong, H.; Zhang, G.; Yu, B.; Chapman, L.R.; Fields, M.W. Microbial diversity in water and sediment of Lake Chaka, an athalassohaline lake in northwestern China. Appl. Environ. Microbiol. 2006, 72, 3832–3845. [Google Scholar] [CrossRef]
- Brady, C.; Cleenwerck, I.; Venter, S.; Vancanneyt, M.; Swings, J.; Coutinho, T. Phylogeny and identification of Pantoea species associated with plants, humans and the natural environment based on multilocus sequence analysis (MLSA). Syst. Appl. Microbiol. 2008, 31, 447–460. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Rezzonico, F.; Smits, T.H.; Montesinos, E.; Frey, J.E.; Duffy, B. Genotypic comparison of Pantoea agglomerans plant and clinical strains. BMC Microbiol. 2009, 9, 204. [Google Scholar] [CrossRef] [PubMed]
- Delétoile, A.; Decré, D.; Courant, S.; Passet, V.; Audo, J.; Grimont, P.; Arlet, G.; Brisse, S. Phylogeny and identification of Pantoea species and typing of Pantoea agglomerans strains by multilocus gene sequencing. J. Clin. Microbiol. 2009, 47, 300–310. [Google Scholar] [CrossRef]
- Edens, D.G.; Gitaitis, R.D.; Sanders, F.H.; Nischwitz, C. First report of Pantoea agglomerans causing a leaf blight and bulb rot of onions in Georgia. Plant Dis. 2006, 90, 1551. [Google Scholar] [CrossRef]
- Tho, K.E.; Wiriyajitsomboon, P.; Hausbeck, M.K. First report of Pantoea agglomerans causing onion leaf blight and bulb rot in Michigan. Plant Dis. 2015, 99, 1034. [Google Scholar] [CrossRef]
- Soto, F.; Tramón, C.; Aqueveque, P.; de Bruijn, J. Microorganismos antagonistas que inhiben el desarrollo de patógenos en post-cosecha de limones (Citrus limon L.). Chil. J. Agric. Anim. Sci. 2018, 34, 173–184. [Google Scholar] [CrossRef]
- Nunes, C.; Usall, J.; Teixidó, N.; Viñas, I. Biological control of postharvest pear diseases using a bacterium, Pantoea agglomerans CPA-2. Int. J. Food Microbiol. 2001, 70, 53–61. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).