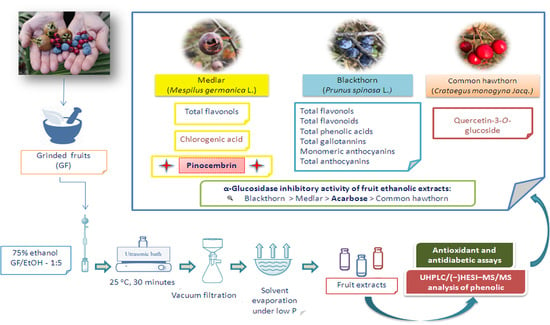
Polyphenolic Profile, Antioxidant and Antidiabetic Potential of Medlar (Mespilus germanica L.), Blackthorn (Prunus spinosa L.) and Common Hawthorn (Crataegus monogyna Jacq.) Fruit Extracts from Serbia
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Instruments
2.2. Plant Material
2.3. Preparation of the Extracts
2.4. Determination of Phenolic Compounds
2.4.1. Total Phenolic Content (TPC)
2.4.2. Total Flavonoid Content
2.4.3. Total Flavonol Content (TFlC)
2.4.4. Total Phenolic Acid Content (TPAC)
2.4.5. Total Gallotannin Content (TGC)
2.4.6. Monomeric and Total Anthocyanin Contents (MAC and TAC)
Total anthocyanins (mg/L) = (A′ × MW × DF × 1000)/(ε × l);
2.5. Phytochemical Composition Analysis
2.6. Antioxidant Activity
2.6.1. Total Antioxidant Capacity (TAC)
2.6.2. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Activity
2.6.3. 2,2′-Azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) Diammonium (ABTS) Radical-Cation Scavenging Activity
2.7. α-Glucosidase Inhibition
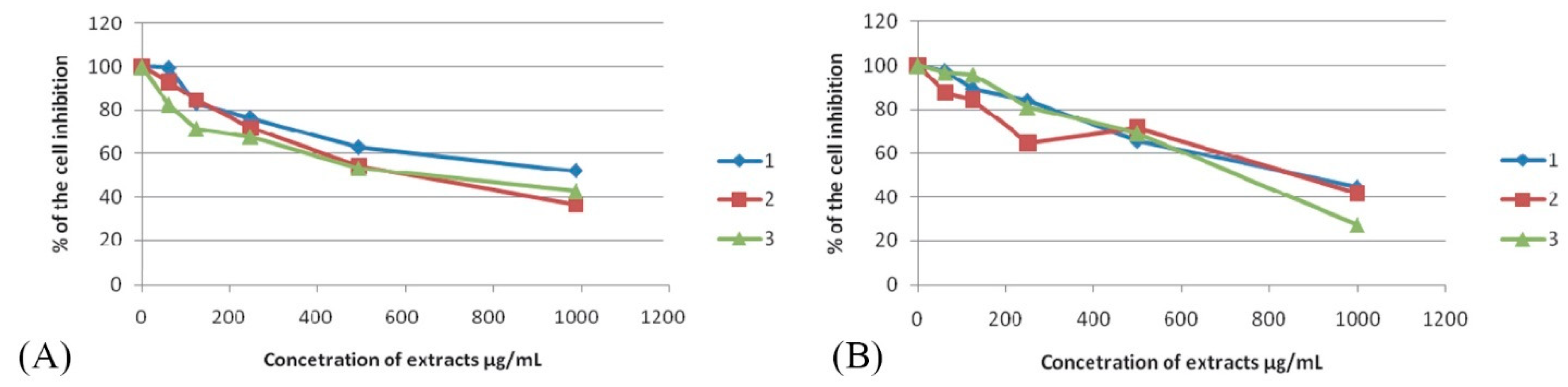
2.8. MTT Assay
2.9. Statistical Analysis
3. Results
3.1. Phytochemical Composition of Fruit Extracts
3.2. Antioxidant Activity
3.3. α-Glucosidase Inhibition and In Vitro Cytotoxicity
3.4. Pearson’s Correlation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of natural plant origins: From sources to food industry applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Kolašinac, S.P.; Dajić-Stevanović, Z.; Kilibarda, S.N.; Kostić, A.Ž. Carotenoids: New applications of “old” pigments. Phyton 2021, 90, 1041–1062. [Google Scholar] [CrossRef]
- Popović-Djordjević, J.B.; Fotirić-Akšić, M.; Katanić-Stanković, J.S.; Pantelić, N.Đ.; Mihailović, V. Wild-growing species in the service of medicine: Environmental challenges and sustainable production. In Environmental Challenges and Medicinal Plants, Environmental Challenges and Solutions, 1st ed.; Aftab, T., Ed.; Springer Nature: Cham, Switzerland, 2022; pp. 49–104. [Google Scholar] [CrossRef]
- Cevahir, G.; Bostan, S.Z. Organic acids, sugars and bioactive compounds of promising medlar (Mespilus germanica L.) genotypes selected from Turkey. Int. J. Fruit Sci. 2021, 21, 312–322. [Google Scholar] [CrossRef]
- Cosmulescu, S.N.; Trandafir, I.; Scrieciu, F.; Stonescu, A.M. Content in organic acids of Mespilus spp. and Crataegus spp. genotypes. Not. Bot. Horti. Agrobot. 2020, 48, 171–176. [Google Scholar] [CrossRef]
- Velickovic, M.; Radivojevic, D.; Oparnica, C.; Nikicevic, N.; Zivkovic, M.; Djordjevic, N.; Vajs, V.; Tesevic, V. Volatile compounds in medlar fruit (Mespilus germanica L.) at two ripening stages. Hem. Ind. 2013, 67, 437–441. [Google Scholar] [CrossRef]
- Voronkov, A.S.; Ivanova, T.V.; Kumachova, T.K.; Kozhevnikova, A.D.; Tsydendambaev, V.D. Polyunsaturated and very long chain fatty acids are involved in the adaptation of Maloideae (Rosaceae) to combined stress in the mountains. Chem. Biodivers. 2020, 17, e1900588. [Google Scholar] [CrossRef]
- Guimarães, R.; Barros, L.; Dueñas, M.; Carvalho, A.M.; Queiroz, M.J.R.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Characterisation of phenolic compounds in wild fruits from Northeastern Portugal. Food Chem. 2013, 141, 3721–3730. [Google Scholar] [CrossRef]
- Ruiz-Rodríguez, B.M.; De Ancos, B.; Sánchez-Moreno, C.; Fernández-Ruiz, V.; De Cortes Sánchez-Mata, M.; Cámara, M.; Tardío, J. Wild blackthorn (Prunus spinosa L.) and hawthorn (Crataegus monogyna Jacq.) fruits as valuable sources of antioxidants. Fruits 2014, 69, 61–73. [Google Scholar] [CrossRef]
- Ganhão, R.; Estévez, M.; Kylli, P.; Heinonen, M.; Morcuende, D. Characterization of selected wild Mediterranean fruits and comparative efficacy as inhibitors of oxidative reactions in emulsified raw pork burger patties. J. Agric. Food Chem. 2010, 58, 8854–8861. [Google Scholar] [CrossRef]
- Kondrashev, S.; Nesterova, N.; Luzin, A.; Kochanov, V.; Luzina, A.; Matyushin, A. Qualitative and quantitative assay of hydroxycinnamates of Prunus spinosa L. Pharmacogn. J. 2020, 12, 157–161. [Google Scholar] [CrossRef]
- Sikora, E.; Bieniek, M.I.; Borczak, B. Composition and antioxidant properties of fresh and frozen stored blackthorn fruits (Prunus spinosa L.). Acta Sci. Pol. Technol. Aliment. 2013, 12, 365–372. [Google Scholar]
- Marakoğlu, T.; Arslan, D.; Özcan, M.; Hacıseferoğulları, H. Proximate composition and technological properties of fresh blackthorn (Prunus spinosa L. subsp dasyphylla (Schur.)) fruits. J. Food Eng. 2005, 68, 137–142. [Google Scholar] [CrossRef]
- Sabatini, L.; Fraternale, D.; Di Giacomo, B.; Mari, M.; Albertini, M.C.; Gordillo, B.; Rocchi, M.B.L.; Sisti, D.; Coppari, S.; Semprucci, F.; et al. Chemical composition, antioxidant, antimicrobial and anti-inflammatory activity of Prunus spinosa L. fruit ethanol extract. J. Funct. Foods 2020, 67, 103885. [Google Scholar] [CrossRef]
- Abuashwashi, M.A.; Palomino, O.M.; Gómez-Serranillos, M.P. Geographic origin influences the phenolic composition and antioxidant potential of wild Crataegus monogyna from Spain. Pharm. Biol. 2016, 54, 2708–2713. [Google Scholar] [CrossRef]
- Wyspiańska, D.; Kucharska, A.Z.; Sokół-Łętowska, A.; Kolniak-Ostek, J. Physico-chemical, antioxidant, and anti-inflammatory properties and stability of hawthorn (Crataegus monogyna Jacq.) procyanidins microcapsules with inulin and maltodextrin. J. Sci. Food Agric. 2017, 97, 669–678. [Google Scholar] [CrossRef]
- Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. Comparing the composition and bioactivity of Crataegus monogyna flowers and fruits used in folk medicine. Phytochem. Anal. 2011, 22, 181–188. [Google Scholar] [CrossRef]
- Özderin, S.; Fakir, H.; Dönmez, E. Chemical properties of hawthorn (Crataegus L. spp.) taxa naturally distributed in Western Anatolia part of Turkey. Šumarski List 2016, 140, 369–375. [Google Scholar] [CrossRef]
- Yalçın-Dokumacı, K.; Uslu, N.; Hacıseferoğulları, H.; Örnek, M.N. Determination of some physical and chemical properties of common hawthorn (Crataegus monogyna Jacq. Var. Monogyna). Erwerbs-Obstbau 2021, 63, 99–106. [Google Scholar] [CrossRef]
- Assefa, S.T.; Yang, E.Y.; Chae, S.Y.; Song, M.; Lee, J.; Cho, M.C.; Jang, S. Alpha glucosidase inhibitory activities of plants with focus on common vegetables. Plants 2019, 9, 2. [Google Scholar] [CrossRef]
- Rasouli, H.; Yarani, R.; Pociot, F.; Popović-Djordjević, J. Anti-diabetic potential of plant alkaloids: Revisiting current findings and future perspectives. Pharm. Res. 2020, 155, 104723. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols andother oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Brighente, I.M.C.; Dias, M.; Verdi, L.G.; Pizzolatti, M.G. Antioxidant activity andtotalphenolic content of some Brazilian species. Pharm. Biol. 2007, 45, 156–161. [Google Scholar] [CrossRef]
- Yermakov, A.I.; Arasimov, V.V.; Yarosh, N.P. Methods of Biochemical Analysis of Plants; Agropromizdat: Leningrad, Russia, 1987. [Google Scholar]
- Matkowski, A.; Zielińska, S.; Oszmiański, J.; Lamer-Zarawska, E. Antioxidant activity of extracts from leaves and roots of Salvia miltiorrhiza Bunge, S. przewalskii Maxim., and S. verticillata L. Bioresour. Technol. 2008, 99, 7892–7896. [Google Scholar] [CrossRef]
- Haslam, E. Galloyl esters in the Aceraceae. Phytochemistry 1965, 4, 495–498. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and measurement of anthocyanins by UV-visible spectroscopy. In Current Protocols in Food Analytical Chemistry; Wiley and Sons: New York, NY, USA, 2003. [Google Scholar]
- Katanić-Stanković, J.; Srećković, N.; Mišić, D.; Gašić, U.; Imbimbo, P.; Monti, D.M.; Mihailović, V. Bioactivity, biocompatibility and phytochemical assessment of lilac sage, Salvia verticillata L. (Lamiaceae)—A plant rich in rosmarinic acid. Ind. Crops Prod. 2020, 143, 111932. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Kumarasamy, Y.; Byres, M.; Cox, P.J.; Jaspars, M.; Nahar, L.; Sarker, S.D. Screening seeds of some Scottish plants for free radical scavenging activity. Phyther. Res. 2007, 21, 615–621. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Matsui, T.; Yoshimoto, C.; Osajima, K.; Oki, T.; Osajima, Y. In vitro survey of α-glucosidase inhibitory food components. Biosci. Biotechnol. Biochem. 1996, 60, 2019–2022. [Google Scholar] [CrossRef]
- Grozdanić, N.; Zdunić, G.; Šavikin, K.; Đuričić, I.; Kosanić, M.; Mačić, V.; Matić, I.; Stanojković, T. Seasonal variation in biopharmaceutical activity and fatty acid content of endemic Fucus virsoides algae from Adriatic sea. Acta Pol. Pharm. 2019, 76, 833–844. [Google Scholar] [CrossRef]
- Damjanovic, A.D.; Zdunic, G.; Savikin, K.; Mandic, B.; Jadranin, M.; Matic, I.Z.; Stanojkovic, T.P. Evaluation of the anti-cancer potential of Mahonia aquifolium extracts via apoptosis and anti-angiogenesis. Bangladesh J. Pharmacol. 2016, 11, 741–749. [Google Scholar] [CrossRef]
- Jarić, S.; Mačukanović-Jocić, M.; Djurdjević, L.; Mitrović, M.; Kostić, O.; Karadžić, B.; Pavlović, P. An ethnobotanical survey of traditionally used plants on Suva planina mountain (south-eastern Serbia). J. Ethnopharmacol. 2015, 175, 93–108. [Google Scholar] [CrossRef]
- Balta, V.; Đikić, D.; Crnić, I.; Odeh, D.; Oršolić, N.; Kmetić, I.; Murati, T.; Uzelac, V.D.; Jurčević, I.L. Effects of four-week intake of blackthorn flower extract on mice tissue antioxidant status and phenolic content. Pol. J. Food Nutr. Sci. 2020, 70, 361–375. [Google Scholar] [CrossRef]
- Marchelak, A.; Owczarek, A.; Matczak, M.; Pawlak, A.; Kolodziejczyk-Czepas, J.; Nowak, P.; Olszewska, M.A. Bioactivity potential of Prunus spinosa L. flower extracts: Phytochemical profiling, cellular safety, pro-inflammatory enzymes inhibition and protective effects against oxidative stress in vitro. Front. Pharmacol. 2017, 8, 680. [Google Scholar] [CrossRef]
- Mitic, V.; Stankov-Jovanovic, V.; Dimitrijevic, M.; Cvetkovic, J.; Simonovic, S.; Nikolic Mandic, S. Chemometric analysis of antioxidant activity and anthocyanin content of selected wild and cultivated small fruit from Serbia. Fruits 2014, 69, 413–422. [Google Scholar] [CrossRef]
- Radovanović, B.C.; Andelković, A.S.M.; Radovanović, A.B.; Andelković, M.Z. Antioxidant and antimicrobial activity of polyphenol extracts from wild berry fruits grown in Southeast Serbia. Trop. J. Pharm. Res. 2013, 12, 813–819. [Google Scholar] [CrossRef]
- Popović, B.M.; Blagojević, B.; Ždero Pavlović, R.; Mićić, N.; Bijelić, S.; Bogdanović, B.; Mišan, A.; Duarte, C.M.M.; Serra, A.T. Comparison between polyphenol profile and bioactive response in blackthorn (Prunus spinosa L.) genotypes from north Serbia-from raw data to PCA analysis. Food Chem. 2020, 302, 125373. [Google Scholar] [CrossRef]
- Veličković, J.M.; Kostić, D.A.; Stojanović, G.S.; Mitić, S.S.; Mitić, M.N.; Randelović, S.S.; Dordević, A.S. Phenolic composition, antioxidant and antimicrobial activity of the extracts from Prunus spinosa L. fruit. Hem. Ind. 2014, 68, 297–303. [Google Scholar] [CrossRef]
- Barros, L.; Carvalho, A.M.; Morais, J.S.; Ferreira, I.C.F.R. Strawberry-tree, blackthorn and rose fruits: Detailed characterisation in nutrients and phytochemicals with antioxidant properties. Food Chem. 2010, 120, 247–254. [Google Scholar] [CrossRef]
- Natić, M.; Pavlović, A.; Lo Bosco, F.; Stanisavljević, N.; Zagorac, D.D.; Akšić, M.F.; Papetti, A. Nutraceutical properties and phytochemical characterization of wild Serbian fruits. Eur. Food Res. Technol. 2019, 245, 469–478. [Google Scholar] [CrossRef]
- Morales, P.; Ferreira, I.C.F.R.; Carvalho, A.M.; Fernández-Ruiz, V.; Sánchez-Mata, M.S.O.S.C.C.; Cámara, M.; Morales, R.; Tardío, J. Wild edible fruits as a potential source of phytochemicals with capacity to inhibit lipid peroxidation. Eur. J. Lipid Sci. Technol. 2013, 115, 176–185. [Google Scholar] [CrossRef]
- Haciseferoğullari, H.; Özcan, M.; Sonmete, M.H.; Özbek, O. Some physical and chemical parameters of wild medlar (Mespilus germanica L.) fruit grown in Turkey. J. Food Eng. 2005, 69, 1–7. [Google Scholar] [CrossRef]
- Żołnierczyk, A.K.; Ciałek, S.; Styczyńska, M.; Oziembłowski, M. Functional properties of fruits of common medlar (Mespilus germanica L.) extract. Appl. Sci. 2021, 11, 7528. [Google Scholar] [CrossRef]
- Gruz, J.; Ayaz, F.A.; Torun, H.; Strnad, M. Phenolic acid content and radical scavenging activity of extracts from medlar (Mespilus germanica L.) fruit at different stages of ripening. Food Chem. 2011, 124, 271–277. [Google Scholar] [CrossRef]
- Tessa, G.; Donno, D.; Gamba, G.; Mellano, M.G.; Beccaro, G.L. Local and underutilised fruits as a source of nutraceutical molecules: Bioactive compounds in Mespilus germanica L. Eur. Food Res. Technol. 2021, 247, 2861–2868. [Google Scholar] [CrossRef]
- Isbilir, S.S.; Kabala, S.I.; Yagar, H. Assessment of in vitro antioxidant and antidiabetic capacities of medlar (Mespilus germanica). Not. Bot. Horti. Agrobot. Cluj Napoca 2019, 47, 384–389. [Google Scholar] [CrossRef]
- Akbulut, M.; Ercisli, S.; Jurikova, T.; Mlcek, J.; Gozlekci, S. Phenotypic and bioactive diversity on medlar fruits (Mespilus germanica L.). Erwerbs-Obstbau 2016, 58, 185–191. [Google Scholar] [CrossRef]
- Popović-Đorđević, B.J.; Jevtić, I.I.; Stanojković, P.T. Antidiabetics: Structural diversity of molecules with a common aim. Curr. Med. Chem. 2018, 25, 2140–2165. [Google Scholar] [CrossRef]
- Dirir, A.M.; Daou, M.; Yousef, A.F.; Yousef, L.F. A review of alpha-glucosidase inhibitors from plants as potential candidates for the treatment of type-2 diabetes. Phytochem. Rev. 2022, 21, 1049–1079. [Google Scholar] [CrossRef]
- AL-Ishaq, R.K.; Abotaleb, M.; Kubatka, P.; Kajo, K.; Büsselberg, D. Flavonoids and their anti-diabetic effects: Cellular mechanisms and effects to improve blood sugar levels. Biomolecules 2019, 9, 430. [Google Scholar] [CrossRef]
- Veličković, I.; Žižak, Ž.; Rajčević, N.; Ivanov, M.; Soković, M.; Marin, P.; Grujić, S. Examination of the polyphenol content and bioactivities of Prunus spinosa L. fruit extracts. Arch. Biol. Sci. 2020, 72, 105–115. [Google Scholar] [CrossRef]
- Karakas, N.; Okur, M.E.; Ozturk, I.; Ayla, S.; Karadag, A.E.; Polat, D.C. Antioxidant activity of blackthorn (Prunus spinosa L.) fruit extract and cytotoxic effects on various cancer cell lines. Medeni. Med. J. 2019, 34, 297–304. [Google Scholar]
- Meschini, S.; Pellegrini, E.; Condello, M.; Occhionero, G.; Delfine, S.; Condello, G.; Mastrodonato, F. Cytotoxic and apoptotic activities of Prunus spinosa Trigno Ecotype extract on human cancer cells. Molecules 2017, 22, 1578. [Google Scholar] [CrossRef]
- Sönmez, M.; Önder, F.C.; Tokay, E.; Çelik, A.; Feray-Köçkar, F.; Ay, M. Investigation of antioxidant, enzyme inhibition and antiproliferative activities of blackthorn (Prunus spinosa L.) extracts. Int. J. Life Sci. Biotechnol. 2021, 4, 360–380. [Google Scholar] [CrossRef]
| Extract | Total Phenolic Content (mg GAE/g) | Total Flavonoid Content (mg RUE/g) | Total Flavonol Content (mg RUE/g) | Total Phenolic Acid Content (mg CAE/g) | Total Gallotannin Content (mg GAE/g) | Monomeric Anthocyanin Content (mg Mv-3-glc/g) | Total Anthocyanin Content (mg Mv-3-glc/g) |
|---|---|---|---|---|---|---|---|
| M. germanica | 16.7 ± 0.3 * a | 2.30 ± 0.07 c | 0.99 ± 0.13 b | 3.20 ± 0.11 b | 1.47 ± 0.07 c | 0.02 ± 0.001 b | 0.03 ± 0.004 b |
| P. spinosa | 25.9 ± 0.2 a | 5.09 ± 0.12 a | 2.14 ± 0.19 a | 4.13 ± 0.22 a | 2.54 ± 0.03 a | 0.13 ± 0.01 a | 0.16 ± 0.001 a |
| C. monogyna | 14.9 ± 0.7 a | 3.51 ± 0.05 b | 1.84 ± 0.23 a | 2.05 ± 0.07 c | 2.04 ± 0.13 b | 0.02 ± 0.001 b | 0.03 ± 0.001 b |
| mg/kg dw | M. germanica | P. spinosa | C. monogyna |
|---|---|---|---|
| Protocatechuic acid | 12.91 ± 0.39 * | 14.38 ± 0.29 | 50.75 ± 0.93 |
| Syringic acid | 10.24 ± 0.33 | 11.38 ± 1.86 | 40.29 ± 4.84 |
| Chlorogenic acid | 78.81 ± 1.45 | 22.32 ± 0.47 | 12.19 ± 0.19 |
| Caffeic acid | 8.55 ± 0.40 | 1.82 ± 0.01 | 0.48 ± 0.10 |
| Aesculetin | 4.65 ± 0.34 | 0.86 ± 0.10 | 0.11 ± 0.03 |
| Rutin | 2.92 ± 0.25 | 104.12 ± 13.11 | 3.54 ± 0.24 |
| p-Coumaric acid | 3.53 ± 0.18 | 1.53 ± 0.42 | 1.41 ± 0.17 |
| Quercetin-3-O-glucoside | 11.71 ± 0.41 | 22.86 ± 0.46 | 148.86 ± 0.61 |
| Kaempferol-3-O-glucoside | 3.12 ± 0.06 | 5.94 ± 0.14 | 42.58 ± 0.68 |
| Quercetin-3-O-rhamnoside | 14.04 ± 1.22 | 79.66 ± 4.33 | 1.23 ± 0.09 |
| Quercetin | 2.00 ± 0.11 | 13.71 ± 0.91 | 3.13 ± 0.47 |
| Pinocembrin | 0.44 ± 0.03 | ND ** | ND |
| Fruit Extracts | Total Antioxidant Capacity (mg AAE/g) | IC50 (µg/mL) | |
|---|---|---|---|
| DPPH· Scavenging Activity | ABTS·+ Scavenging Activity | ||
| M. germanica | 238.2 ± 12.3 * a | 884 ± 12 d | 2048 ± 144 c |
| P. spinosa | 159.0 ± 23.1 b | 610 ± 21 b | 1153 ± 23 b |
| C. monogyna | 100.4 ± 6.7 c | 718 ± 31 c | 2964 ± 375 d |
| Standards | |||
| Gallic acid | - | 0.91 ± 0.10 a | 4.42 ± 0.09 a |
| Ellagic acid | - | 0.46 ± 0.05 a | 5.74 ± 0.28 a |
| Caffeic acid | - | 2.84 ± 0.19 a | 10.9 ± 1.7 a |
| Quercetin | - | 1.51 ± 0.14 a | 8.15 ± 0.31 a |
| Rutin | - | 4.95 ± 0.26 a | 56.6 ± 1.5 a |
| Ascorbic acid | - | 3.16 ± 0.09 a | 16.7 ± 1.9 a |
| BHT | - | 12.9 ± 0.9 a | 24.0 ± 2.9 a |
| Fruit Extract | α-Glucosidase Inhibition | HeLa * | FemX | LS174T |
|---|---|---|---|---|
| M. germanica | 199.84 ± 0.18 b,** | 624.83 ± 4.96 | 854.98 ± 9.97 b | >1000 |
| P. spinosa | 129.46 ± 0.73 a | >1000 | 868.25 ± 8.45 b | >1000 |
| C. monogyna | 335.71 ± 6.68 c | 651.80 ± 6.80 | 724.30 ± 9.42 a | >1000 |
| Acarbose *** | 201.38 ± 0.50 b |
| TPC * | TFC | TPAC | TAC | DPPH· | ABTS·+ | TAntioxC | |
|---|---|---|---|---|---|---|---|
| TPC | 1.000 | ||||||
| TFC | 0.825 ** | 1.000 | |||||
| TPAC | 0.908 | 0.513 | 1.000 | ||||
| TAC | 0.988 | 0.902 | 0.834 | 1.000 | |||
| DPPH· | −0.698 | −0.980 | −0.334 | −0.799 | 1.000 | ||
| ABTS·+ | −0.930 | −0.559 | −0.999 | −0.863 | 0.385 | 1.000 | |
| TAntioxC | 0.067 | −0.508 | 0.478 | −0.086 | 0.668 | −0.430 | 1.000 |
| TPC * | TFC | TPAC | TAC | DPPH· | ABTS·+ | TAntioxC | Protocatechuic acid | Syringic acid | Chlorogenic acid | Caffeic acid | Aesculetin | Rutin | p-Coumaric acid | Quercetin-3-O-glucoside | Kaempferol-3-O-glucoside | Quercetin-3-O-rhamnoside | Quercetin | α-GlcI | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TPC | 1.000 | ||||||||||||||||||
| TFC | 0.825 ** | 1.000 | |||||||||||||||||
| TPAC | 0.908 | 0.513 | 1.000 | ||||||||||||||||
| TAC | 0.988 | 0.902 | 0.834 | 1.000 | |||||||||||||||
| DPPH· | −0.698 | −0.980 | −0.334 | −0.799 | 1.000 | ||||||||||||||
| ABTS·+ | −0.930 | −0.559 | −0.999 | −0.863 | 0.385 | 1.000 | |||||||||||||
| TAntioxC | 0.067 | −0.508 | 0.478 | −0.086 | 0.668 | −0.430 | 1.000 | ||||||||||||
| Protocatechuic acid | −0.599 | −0.042 | −0.879 | −0.470 | −0.155 | 0.852 | −0.839 | 1.000 | |||||||||||
| Syringic acid | −0.600 | −0.043 | −0.879 | −0.471 | −0.154 | 0.852 | −0.839 | 1.000 | 1.000 | ||||||||||
| Chlorogenic acid | −0.227 | −0.737 | 0.201 | −0.373 | 0.856 | −0.148 | 0.957 | −0.644 | −0.643 | 1.000 | |||||||||
| Caffeic acid | −0.213 | −0.728 | 0.215 | −0.360 | 0.849 | −0.162 | 0.961 | −0.654 | −0.654 | 1.000 | 1.000 | ||||||||
| Aesculetin | −0.214 | −0.728 | 0.214 | −0.361 | 0.849 | −0.161 | 0.960 | −0.654 | −0.653 | 1.000 | 1.000 | 1.000 | |||||||
| Rutin | 0.987 | 0.904 | 0.831 | 1.000 | −0.802 | −0.860 | −0.091 | −0.465 | −0.466 | −0.378 | −0.365 | −0.366 | 1.000 | ||||||
| p-Coumaric acid | −0.315 | −0.796 | 0.111 | −0.456 | 0.899 | −0.057 | 0.926 | −0.571 | −0.571 | 0.996 | 0.994 | 0.995 | −0.460 | 1.000 | |||||
| Quercetin-3-O-glucoside | −0.568 | −0.003 | −0.860 | −0.435 | −0.194 | 0.831 | −0.860 | 0.999 | 0.999 | −0.673 | −0.683 | −0.683 | −0.430 | −0.603 | 1.000 | ||||
| Kaempferol-3-O-glucoside | −0.575 | −0.012 | −0.864 | −0.444 | −0.185 | 0.836 | −0.855 | 1.000 | 1.000 | −0.666 | −0.677 | −0.676 | −0.439 | −0.596 | 1.000 | 1.000 | |||
| Quercetin-3-O-rhamnoside | 1.000 | 0.825 | 0.908 | 0.988 | −0.698 | −0.930 | 0.067 | −0.599 | −0.600 | −0.227 | −0.214 | −0.214 | 0.988 | −0.315 | −0.567 | −0.575 | 1.000 | ||
| Quercetin | 0.971 | 0.936 | 0.782 | 0.996 | −0.849 | −0.815 | −0.173 | −0.391 | −0.392 | −0.453 | −0.440 | −0.441 | 0.997 | −0.532 | −0.355 | −0.363 | 0.971 | 1.000 | |
| α-GlcI | −0.852 | −0.407 | −0.993 | −0.762 | 0.219 | 0.985 | −0.580 | 0.930 | 0.930 | −0.317 | −0.331 | −0.330 | −0.758 | −0.230 | 0.915 | 0.919 | −0.851 | −0.702 | 1.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katanić Stanković, J.S.; Mićanović, N.; Grozdanić, N.; Kostić, A.Ž.; Gašić, U.; Stanojković, T.; Popović-Djordjević, J.B. Polyphenolic Profile, Antioxidant and Antidiabetic Potential of Medlar (Mespilus germanica L.), Blackthorn (Prunus spinosa L.) and Common Hawthorn (Crataegus monogyna Jacq.) Fruit Extracts from Serbia. Horticulturae 2022, 8, 1053. https://doi.org/10.3390/horticulturae8111053
Katanić Stanković JS, Mićanović N, Grozdanić N, Kostić AŽ, Gašić U, Stanojković T, Popović-Djordjević JB. Polyphenolic Profile, Antioxidant and Antidiabetic Potential of Medlar (Mespilus germanica L.), Blackthorn (Prunus spinosa L.) and Common Hawthorn (Crataegus monogyna Jacq.) Fruit Extracts from Serbia. Horticulturae. 2022; 8(11):1053. https://doi.org/10.3390/horticulturae8111053
Chicago/Turabian StyleKatanić Stanković, Jelena S., Nenad Mićanović, Nadja Grozdanić, Aleksandar Ž. Kostić, Uroš Gašić, Tatjana Stanojković, and Jelena B. Popović-Djordjević. 2022. "Polyphenolic Profile, Antioxidant and Antidiabetic Potential of Medlar (Mespilus germanica L.), Blackthorn (Prunus spinosa L.) and Common Hawthorn (Crataegus monogyna Jacq.) Fruit Extracts from Serbia" Horticulturae 8, no. 11: 1053. https://doi.org/10.3390/horticulturae8111053
APA StyleKatanić Stanković, J. S., Mićanović, N., Grozdanić, N., Kostić, A. Ž., Gašić, U., Stanojković, T., & Popović-Djordjević, J. B. (2022). Polyphenolic Profile, Antioxidant and Antidiabetic Potential of Medlar (Mespilus germanica L.), Blackthorn (Prunus spinosa L.) and Common Hawthorn (Crataegus monogyna Jacq.) Fruit Extracts from Serbia. Horticulturae, 8(11), 1053. https://doi.org/10.3390/horticulturae8111053