A Comparison of IPM and Organic Farming Systems Based on the Efficiency of Oophagous Predation on the Olive Moth (Prays oleae Bernard) in Olive Groves of Southern Iberia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of the Study Area
2.2. Experimental Design
2.2.1. Sampling of Fruit and Determination of the Attack Parameters of P. oleae
- Population Index (POP): Total number of eggs/100 fruits. This density value reflects the relative density of ovideponent females in the cultivated area.
- Potential Attack (%PA): Number of fruits with any type of eggs (live, predated or hatched) × 100/total number of fruits observed. It represents a value equivalent to the fruit drop that P. oleae would cause in case of the total absence of oophagous predation activity.
- Hatching rate: Number of hatched eggs × 100/(sum of live and hatched eggs).
- Predation rate (%PRED): Number of predated eggs/(sum of live eggs + predated + hatched). This allows for the assessment of predatory activity, an index of the activity of predatory eggs.
- Final Attack (%FA): Number of fruits that contain at least one hatched egg × 100/(number of fruits observed). Given that the loss of fruit caused by P. oleae is exclusively due to the emergence of the larvae inside the attacked fruits once their development is complete, only hatched eggs are considered for its calculation. Therefore, it may be considered that the latter have survived the predatory action of natural enemies. For the calculation of the %FA value, therefore, live eggs were not taken into account, since these are likely to hatch or be predated. The entomophagous action allows discarding a proportion of the population of P. oleae eggs; however, in the opposite case, its hatching involves the establishment of the larva in the endocarp of the fruit and his subsequent fall. %FA describes the magnitude of this drop, providing a realistic estimation of the impact of P. oleae on the harvest.
- Fruit Recovery (%REC): Number of fruits in which all the eggs have been predated × 100/(number of fruits that have contained eggs). This parameter indicates the real effectiveness of predation, since it corresponds to the percentage of fruits in which all the eggs have been predated. Once all P. oleae eggs have been eliminated, fruits are considered as recovered, so for practical purposes, the recovery percentage is a parameter that indicates the real effectiveness of oophagous predation by lacewings. It is important to note that this value does not always correspond to the %PRED, except in those cases in which the number of fruits attacked present infestation densities equal to 1 egg/fruit.
2.2.2. Sampling of Lacewings
2.3. Statistical Analysis
3. Results
3.1. Analysis of the Attack Parameters of P. oleae
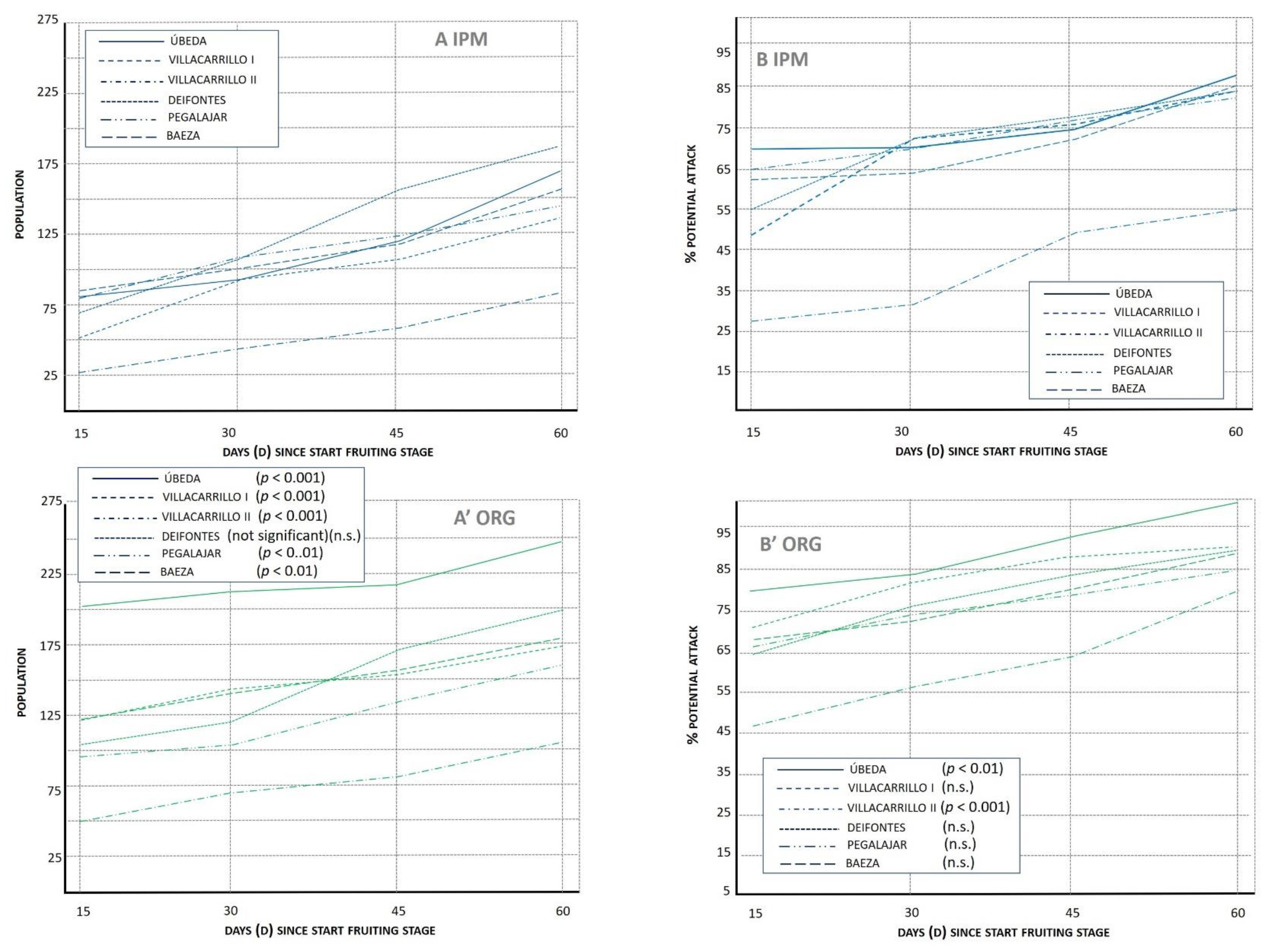
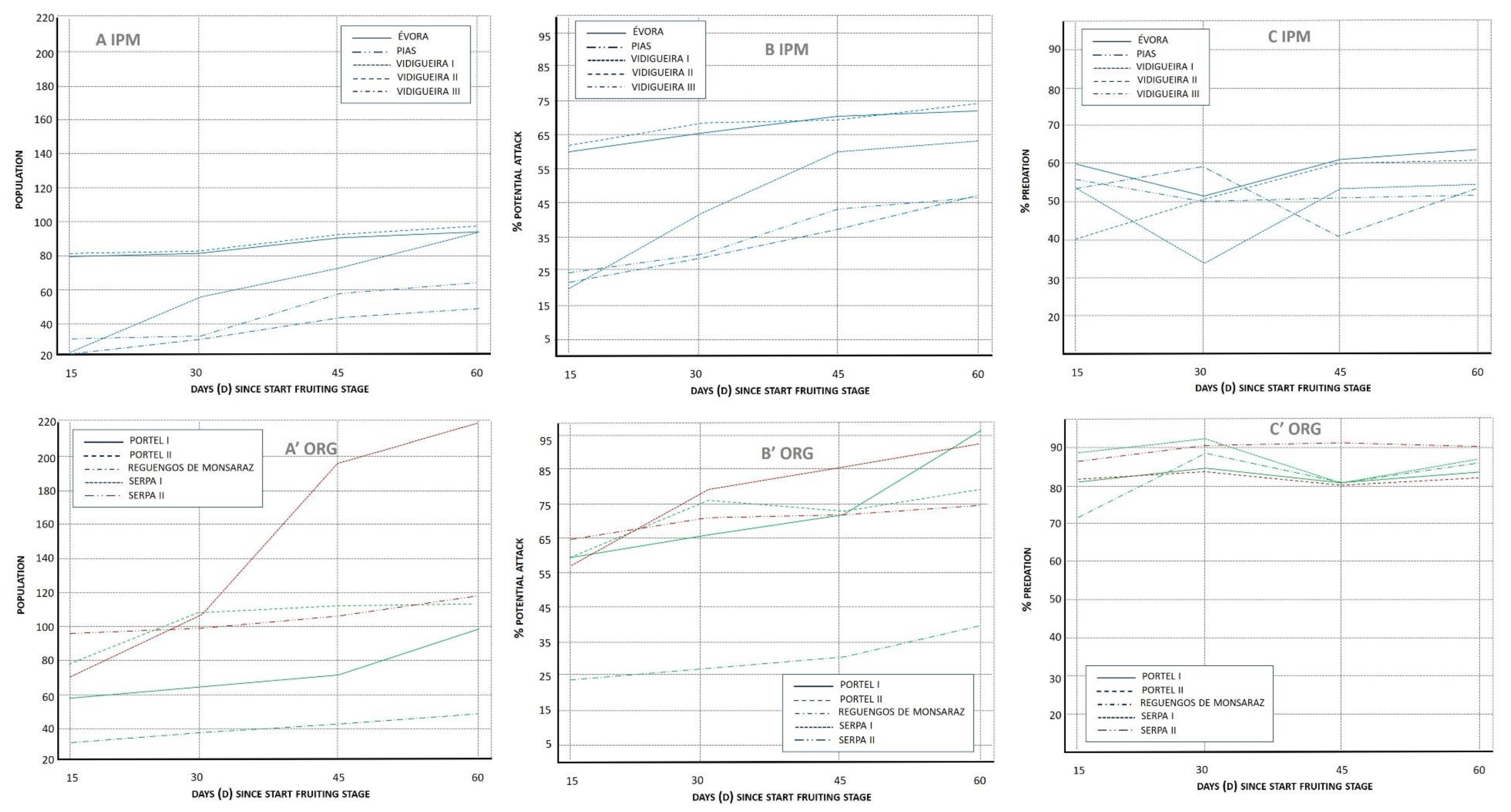
3.1.1. Population Index (POP)
3.1.2. Potential Attack (%PA)
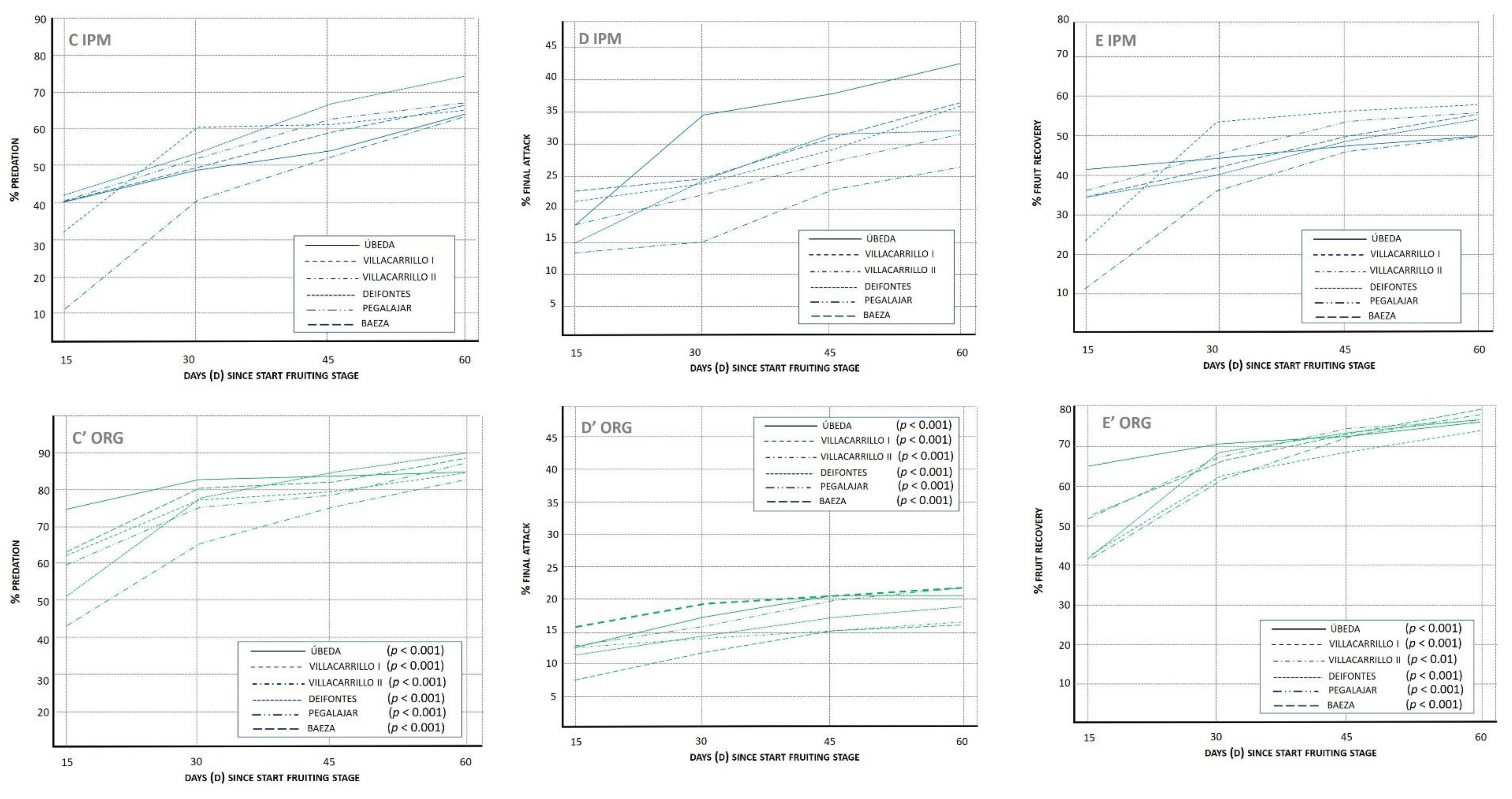
3.1.3. Lacewing Diversity and Egg Predation (%PRED)
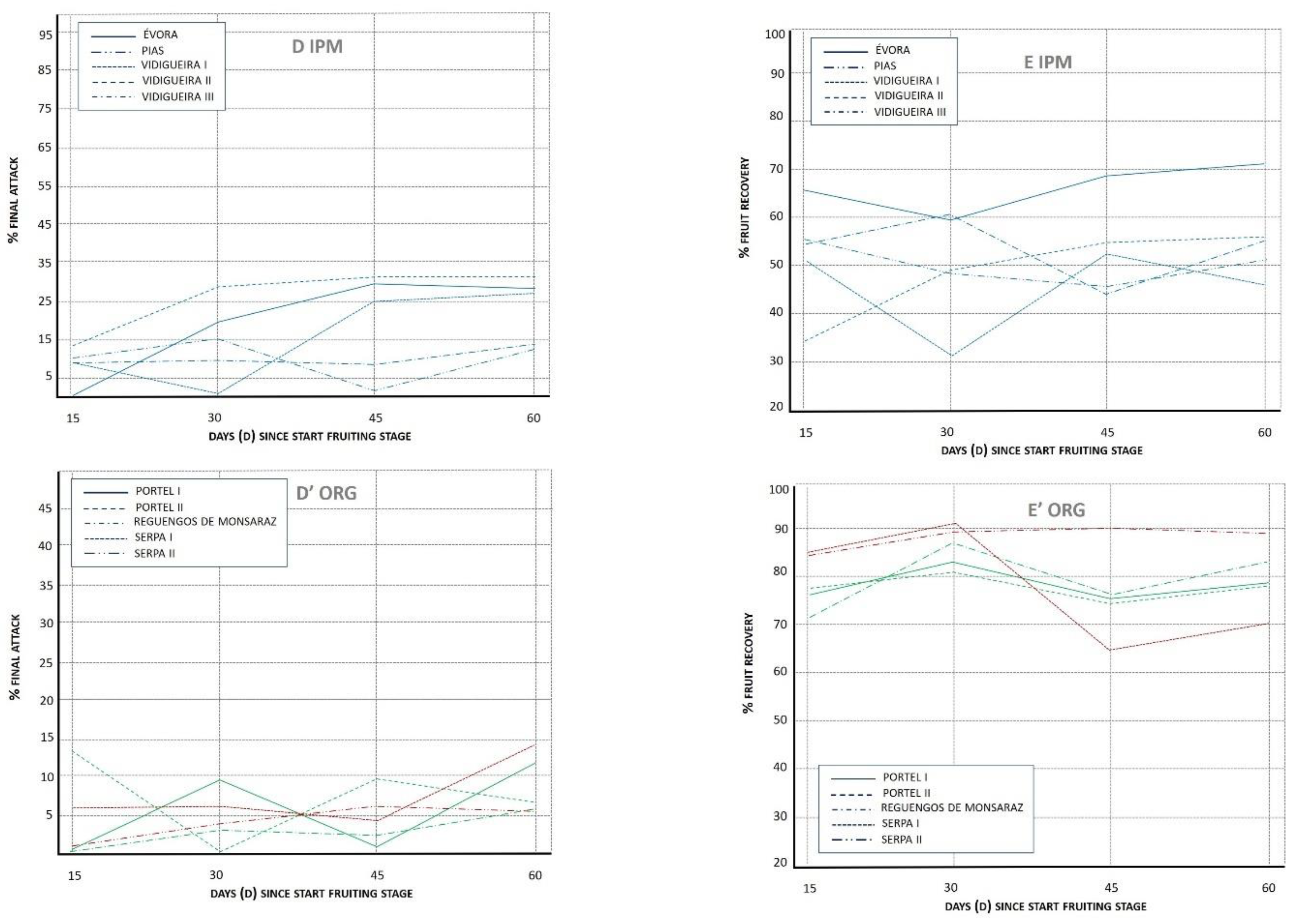
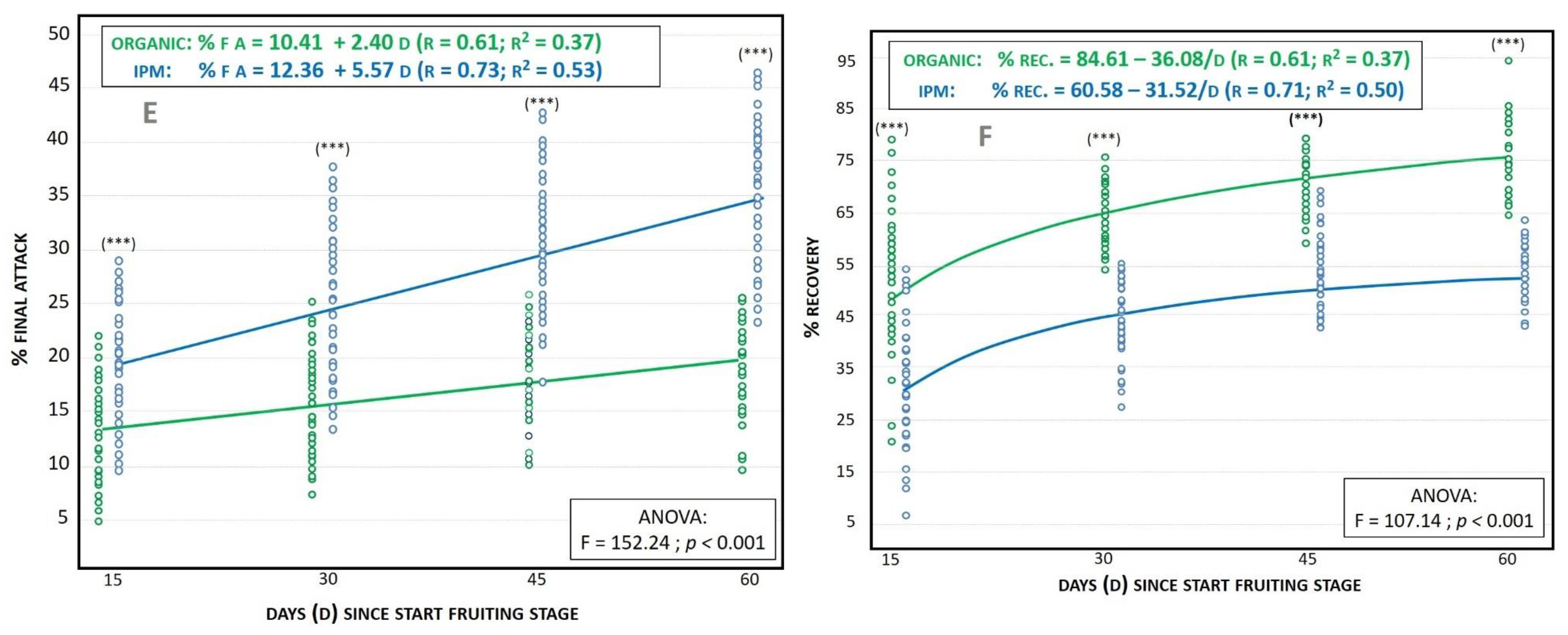
3.1.4. Final Attack (%FA)
3.1.5. Fruit Recovery (%REC)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arambourg, Y. Traité d’entomologie oléicole; Conseil oléicole International: Madrid, Spain, 1986. [Google Scholar]
- Ramos, P.; Campos, M.; Ramos, J.M. Long-term study on the evaluation of yield and economic losses caused by Prays oleae Bern. in the olive crop of Granada (southern Spain). Crop Prot. 1998, 17, 645–647. [Google Scholar] [CrossRef]
- Álvarez, H.A.; Jiménez-Muñoz, R.; Morente, M.; Campos, M.; Ruano, F. Ground cover presence in organic olive orchards affects the interaction of natural enemies against Prays oleae, promoting an effective egg predation. Agric. Ecosyst. Environ. 2021, 315, 107441. [Google Scholar] [CrossRef]
- Vilar, J.; Pereira, J.E. La Olivicultura Internacional Difusión Histórica, Análisis Estratégico y Visión Descriptiva; Fundación Caja Rural de Jaén: La Carolina, Spain, 2018; Available online: https://www.researchgate.net/publication/326070772_LA_OLIVICULTURA_INTERNACIONAL_DIFUSION_HISTORICA_ANALISIS_ESTRATEGICO_Y_VISION_DESCRIPTIVA (accessed on 5 September 2022).
- Rosales, R.; Garrido, D.; Ramos, P.; Ramos, J.M. Ethylene can reduce Prays oleae attack in olive trees. Crop Prot. 2006, 25, 140–143. [Google Scholar] [CrossRef]
- Ramos, P.; González, R.; Ramos, J.M. Alternativas naturales al uso de plaguicidas contra la polilla del olivo (Prays oleae Bern.). Oleae 2003, 28, 20–23. [Google Scholar]
- Bento, A.; Torres, L.; Lopes, J.; Pereira, J.A. Avaliação de prejuízos causados pela traça da oliveira, Prays oleae (Bern.) em Trás-os-Montes. 2001. Available online: http://hdl.handle.net/10198/850 (accessed on 5 September 2022).
- Arambourg, Y. Caractéristiques du Peuplement Entomologique de L’olivier Dans le Sahel de Sfax. 1964. Available online: https://www.worldcat.org/title/caracteristiques-du-peuplement-entomologique-de-lolivier-dans-le-sahel-de-sfax/oclc/459406956 (accessed on 5 September 2022).
- Campos, M.; Ramos, P. Some relationships between the number of Prays oleae eggs laid on olive fruits and their predation by Chrysoperla carnea. In Integrated Pesticide Control Olive-Groves Proceedings of the CEC/FAO/IOBC International Joint Meeting, Pisa Italy, 3–6 April 1984; Balkema for CEC: Rotterdam, The Netherlands, 1985; pp. 237–241. [Google Scholar]
- Bento, A.; Lopes, J.; Torres, L.; Passos-Carvalho, P. Biological control of Prays oleae (Bern.) by chrysopids in Trás-os-Montes region (Northeastern Portugal). In Proceedings of the III International Symposium on Olive Growing, Chania, Greece, 22–26 September 1997; Volume 474, pp. 535–540. [Google Scholar]
- Corrales, N.; Campos, M. Populations, longevity, mortality and fecundity of Chrysoperla carnea (Neuroptera, Chrysopidae) from olive-orchards with different agricultural management systems. Chemosphere 2004, 57, 1613–1619. [Google Scholar] [CrossRef]
- González-Ruiz, R.; Al-Asaad, S.; Bozsik, A. Influencia de las masas forestales en la diversidad y abundancia de los crisópidos (Neur.:” Chrysopidae”) del olivar. Cuad. Soc. Esp. Cienc. For. 2008, 26, 33–38. Available online: https://dialnet.unirioja.es/descarga/articulo/4245523.pdf (accessed on 6 September 2022).
- Nave, A.; Gonçalves, F.; Crespí, A.L.; Campos, M.; Torres, L. Evaluation of native plant flower characteristics for conservation biological control of Prays oleae. Bull. Entomol. Res. 2016, 106, 249–257. [Google Scholar] [CrossRef]
- Mahzoum, A.M.; Villa, M.; Benhadi-Marín, J.; Pereira, J.A. Functional response of Chrysoperla carnea (Neuroptera: Chrysopidae) larvae on Saissetia oleae (Olivier) (Hemiptera: Coccidae): Implications for biological control. Agronomy 2020, 10, 1511. [Google Scholar] [CrossRef]
- Principi, M.M.; Canard, M. Feeding habits [Chrysopidae]. Series entomologica. Springer 1984, Volume 27, 76–92. [Google Scholar]
- Ridgway, R.L.; Morrison, R.K.; Badgley, M. Mass rearing a green lacewing. J. Econ. Entomol. 1970, 63, 834–836. [Google Scholar] [CrossRef]
- Koczor, S.; Knudsen, G.K.; Hatleli, L.; Szentkirályi, F.; Tóth, M. Manipulation of oviposition and overwintering site choice of common green lacewings with synthetic lure (Neuroptera: Chrysopidae). J. Appl. Entomol. 2015, 139, 201–206. [Google Scholar] [CrossRef]
- Grafton-Cardwell, E.E.; Hoy, M.A. Intraspecific Variability in Response to Pesticides in the Common Green Lacewing, Chrysoperla carnea (Stephens)(Neuroptera: Chrysopidae). Hilgardia 1985, 53, 1–32. [Google Scholar] [CrossRef] [Green Version]
- Tauber, M.J.; Tauber, C.A.; Daane, K.M.; Hagen, K.S. Commercialization of predators: Recent lessons from green lacewings (Neuroptera: Chrysopidae: Chrosoperla). Am. Entomol. 2000, 46, 26–38. [Google Scholar] [CrossRef]
- Thierry, D.; Cloupeau, R.; Jarry, M.; Canard, M. Discrimination of the West-Palaearctic Chrysoperla Steinmann species of the carnea Stephens group by means of claw morphology (Neuroptera, Chrysopidae). Acta Zool. Fenn. 1998, 209, 255–262. [Google Scholar]
- Henry, C.S.; Brooks, S.J.; Thierry, D.; Duelli, P.; Johnson, J.B. The common green lacewing (Chrysoperla carnea s. lat.) and the sibling species problem. In Lacewings in the Crop Environment; Cambridge University Press: Cambridge, UK, 2001; pp. 29–42. [Google Scholar] [CrossRef]
- Henry, C.S.; Brooks, S.J.; Duelli, P.; Johnson, J.B. Discovering the true Chrysoperla carnea (Insecta: Neuroptera: Chrysopidae) using song analysis, morphology, and ecology. Ann. Entomol. Soc. Am. 2002, 95, 172–191. [Google Scholar] [CrossRef] [Green Version]
- Henry, C.S.; Brooks, S.J.; Duelli, P.; Johnson, J.B. A lacewing with the wanderlust: The European song species ‘Maltese’, Chrysoperla agilis, sp. n., of the carnea group of Chrysoperla (Neuroptera: Chrysopidae). Syst. Entomol. 2003, 28, 131–148. [Google Scholar] [CrossRef]
- Bozsik, A.; González-Ruíz, R.; Lara, B.H. Distribution of the Chysoperla carnea complex in southern Spain (Neuroptera: Chrysopidae). An. Univ. Oradea Fasc. Protecția Mediu. 2009, 14, 60–65. Available online: https://protmed.uoradea.ro/facultate/anale/protectia_mediului/2009/agr/11.Bozsikv%20Andras%202.pdf (accessed on 7 September 2022).
- Bollero, A.L.; Moya, J.H.; Macías, V.V.; Mohedano, D.P.; Moya, J. Introducción al Olivar Ecológico en Andalucía; Instituto de Investigación y Formación Agraria y Pesquera, Junta de Andalucía: Seville, Spain, 2017. [Google Scholar]
- Ehi-Eromosele, C.; Nwinyi, O.C.; Ajani, O.O. Integrated Pest Management. In Weed and Pest Control—Conventional and New Challenges; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef] [Green Version]
- Ehler, L.E. Integrated pest management (IPM): Definition, historical development and implementation, and the other IPM. Pest Manag. Sci. 2006, 62, 787–789. [Google Scholar] [CrossRef]
- Ponzio, C.; Gangatharan, R.; Neri, D. Organic and biodynamic agriculture: A review in relation to sustainability. Int. J. Plant Soil Sci. 2013, 2, 95–110. [Google Scholar] [CrossRef]
- Besson, Y. Une histoire d’exigences: Philosophie et agrobiologie. L’actualité de la pensée des fondateurs de l’agriculture biologique pour son développement contemporain. Innov. Agron. 2009, 4, 329–362. [Google Scholar]
- Gegner, L.; Kuepper, G. Organic Crop Production Overview. Attra Publ. MP 2004, 170, 1–28. [Google Scholar]
- Bengtsson, J.; Ahnström, J.; Weibull, A. The effects of organic agriculture on biodiversity and abundance: A meta-analysis. J. Appl. Ecol. 2005, 42, 261–269. [Google Scholar] [CrossRef]
- Gómez-Guzmán, J.A.; García-Marín, F.J.; Sáinz-Pérez, M.; González-Ruiz, R. Behavioural resistance in insects: Its potential use as bio indicator of organic agriculture. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2017; Volume 95, p. 042038. [Google Scholar]
- Gómez-Guzmán, J.A.; Sainz-Pérez, M.; González-Ruiz, R. Monitoring and Inference of Behavioral Resistance in Beneficial Insects to Insecticides in Two Pest Control Systems: IPM and Organic. Agronomy 2022, 12, 538. [Google Scholar] [CrossRef]
- Scherber, C.; Milcu, A.; Partsch, S.; Scheu, S.; Weisser, W.W. The effects of plant diversity and insect herbivory on performance of individual plant species in experimental grassland. J. Ecol. 2006, 94, 922–931. [Google Scholar] [CrossRef]
- Popp, J.; Hantos, K. The impact of crop protection on agricultural production. Stud. Agric. Econ. 2011, 113, 47–66. [Google Scholar] [CrossRef]
- Colbrant, P.; Fabre, P. Stades Reperes de L’olivier. L’Olivier. Invuflec, Paris 1975, 24–25. Available online: http://afidol.org/wp-content/uploads/2016/04/stades_pheno.pdf (accessed on 7 September 2022).
- Ramos, P.; Ramos, J.M. Veinte años de observaciones sobre la depredación oófaga en Prays oleae Bern. Granada (España), 1970–1989. Boletín Sanid. Veg. Plagas 1990, 16, 119–127. [Google Scholar]
- Razali, N.M.; Wah, Y.B. Power comparisons of shapiro-wilk, kolmogorov-smirnov, lilliefors and anderson-darling tests. J. Stat. Model. Anal. 2011, 2, 21–33. [Google Scholar]
- Bozsik, A.; González-Ruiz, R. First data on the sibling species of the common green lacewings in Spain (Neuroptera: Chrysopidae): (The taxonomic status of the most important cryptic species of Chrysoperla carnea complex in Spain). In Proceedings of the 4th International Plant Protection Symposium at Debrecen University and 11th Trans-Tisza Plant Protection Forum, Debrecen, Hungary, 18–19 October 2006; Debreceni Egyetem, Agrártudományi Centrum, Mezögazdaságtudományi Kar: Debrecen, Hungary, 2006; pp. 3–11. [Google Scholar]
- Pascual, S.; Cobos, G.; Seris, E.; González-Nuñez, M. Field assessment of kaolin as a pest control tool in an olive grove in Madrid. IOBC/WPRS Bull. 2010, 53, 109–113. [Google Scholar]
- Bengochea, P.; Saelices, R.; Amor, F.; Adán, Á.; Budia, F.; del Estal, P.; Viñuela, E.; Medina, P. Non-target effects of kaolin and coppers applied on olive trees for the predatory lacewing Chrysoperla carnea. Biocontrol Sci. Technol. 2014, 24, 625–640. [Google Scholar] [CrossRef]
- Medina, P.; Budia, F.; Contreras, G.; Del Estal, P.; González-Núñez, M.; García, M.; Viñuela, E.; Adán, A. Comportamiento reproductor del depredador Chrysoperla carnea (Stephens)(Neuroptera: Chrysopidae) y el parasitoide Psyttalia concolor (Szèpligeti) (Hymenoptera: Braconidae) en superficies cubiertas con caolín. In V Congreso Nacional de Entomología Aplicada; SEdE Aplicada: Cartagena, Spain, 2007; pp. 22–26. [Google Scholar]
- Pascual, S.; Cobos, G.; Seris, E.; González-Núñez, M. Effects of processed kaolin on pests and non-target arthropods in a Spanish olive grove. J. Pest Sci. 2010, 83, 121–133. [Google Scholar] [CrossRef]
- Marko, V.; Blommers, L.; Bogya, S.; Helsen, H. Kaolin particle films suppress many apple pests, disrupt natural enemies and promote woolly apple aphid. J. Appl. Entomol. 2008, 132, 26–35. [Google Scholar] [CrossRef]
- Crowder, D.W.; Northfield, T.D.; Strand, M.R.; Snyder, W.E. Organic agriculture promotes evenness and natural pest control. Nature 2010, 466, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Berg, H.; Maneas, G.; Salguero Engström, A. A comparison between organic and conventional olive farming in Messenia, Greece. Horticulturae 2018, 4, 15. [Google Scholar] [CrossRef]
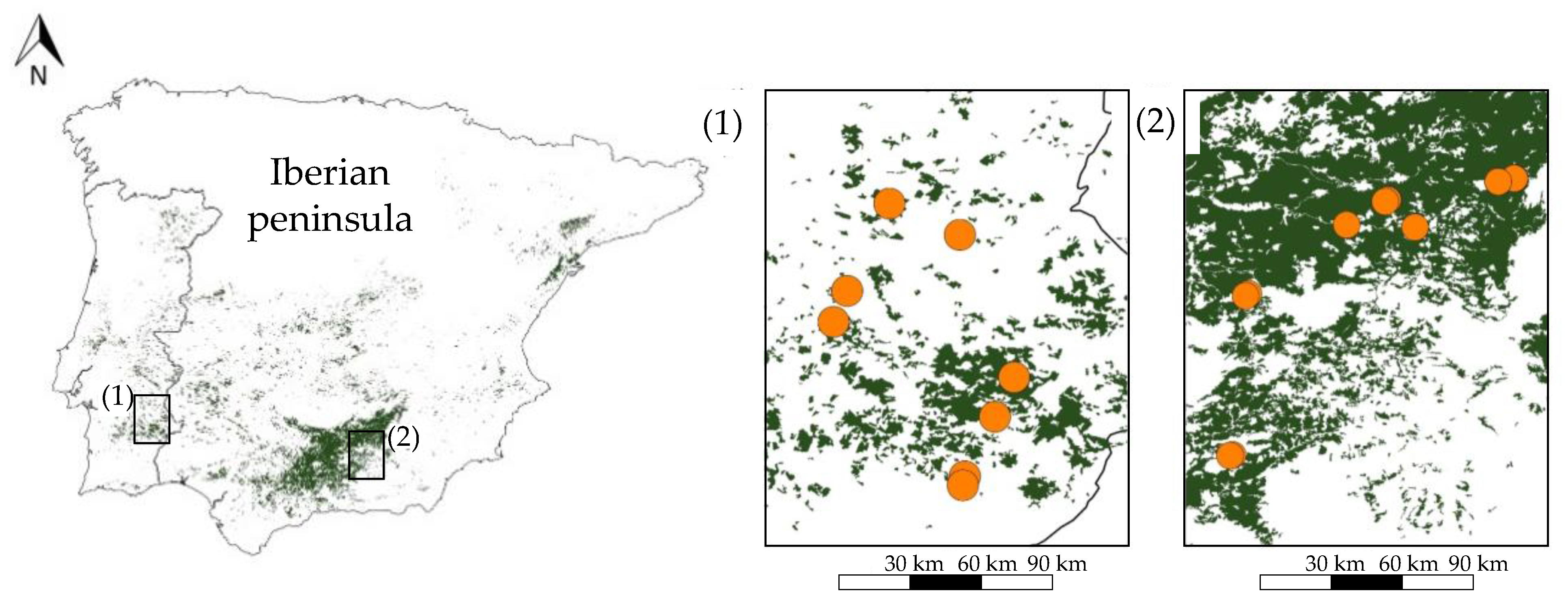

| Herbicide | Insecticide (Olive Moth) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variety | Area (ha) | Age (Years) | Density (Trees × ha) | Active Principle (Dose); Date | Anth. Generation | ||||
| SPAIN | IPM | Baeza | 37°55′29.9″ N 3°24′10.9″ W | “Picual” | 47.9 | 150 | 100 | Glyphosate 36% (1.5 L/ha) + Flazasulfuron 25% (0.06 kg/ha); March & October | Deltametrin 10% (0.125 L/ha); April |
| Deifontes | 37°23′11.8″ N 3°38′09.9″ W | “Picual” | 15.8 | 30 | 150 | ||||
| Pegalajar | 37°45′03.2″ N 3°37′31.2″ W | “Picual” | 24.3 | 80 | 80 | ||||
| Úbeda | 37°58′57.7″ N 3°19′04.1″ W | “Picual” | 9.4 | 40 | 100 | ||||
| Villacarrillo I | 38°03′05.1″ N 3°01′28.3″ W | “Picual” | 7.1 | 200 | 90 | ||||
| Villacarrillo II | 38°02′31.0″ N 3°03′46.0″ W | “Picual” | 4.8 | 100 | 100 | ||||
| ORGANIC | Baeza | 37°55′40.0″ N 3°14′47.9″ W | “Picual” | 23.6 | 100 | 100 | -- | -- | |
| Deifontes | 37°23′04.8″ N 3°38′30.2″ W | “Picual” | 72.2 | 25 | 150 | ||||
| Pegalajar | 37°45′21.8″ N 3°37′08.2″ W | “Picual” | 15.6 | 40 | 120 | ||||
| Úbeda | 37°59′12.2″ N 3°18′43.9″ W | “Picual” | 58.8 | 40 | 100 | ||||
| Villacarrillo I | 38°03′03.3″ N 3°01′33.0″ W | “Picual” | 5.3 | 200 | 90 | ||||
| Villacarrillo II | 38°02’29.5″ N 3°03′43.6″ W | “Picual” | 1.8 | 100 | 100 |
| Herbicide | Insecticide (Olive Moth) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variety | Area (ha) | Age (Years) | Density (Trees × ha) | Active Principle (Dose); Date | Anth. Generation | Carp. Generation | ||||
| PORTUGAL | IPM | Évora | 38°26′56.0″ N 7°41′22.2″ W | “Arbequina” | 250 | 10 | 1667 | Glyphosate 36% (3 L/ha); March and October | Lambda cihalotrin 15% (1.3 L/ha); April | Lambda cihalotrin 15% (1.3 L/ha); May–June |
| Pias | 38°01′30.8″ N 7°29′43.9″ W | “Cobrançosa” | 140.5 | 20 | 205 | |||||
| Vidigueira I | 38°10′01.5″ N 7°44′23.5″ W | “Cobrançosa” | 130 | 16 | 285 | |||||
| Vidigueira II | 38°11′19.8″ N 7°49′13.7″ W | “Cobrançosa” | 163 | 29 | 200 | |||||
| Vidigueira III | 38°11′01.1″ N 7°47′45.2″ W | “Arbequina” | 163 | 9 | 600 | |||||
| ORGANIC | Portel I | 38°16′26.9″ N 7°46′24.6″ W | “Galega” | 35 | 100 | 139 | -- | -- | Kaolin (35 kg/ha); May–June | |
| Portel II | 38°18′26.9″ N 7°43′39.7″ W | “Galega” + “Blanqueta” | 35 | 100 | 139 | |||||
| Reguengos de Monsaraz | 38°23′10.7″ N 7°32′58.5″ W | “Cobrançosa” | 100 | 13 | 285 | |||||
| Serpa I * | 37°54′08.4″ N 7°32′24.2″ W | “Arbequina” | 200 | 3 | 1770 | |||||
| Serpa II * | 37°53′04.7″ N 7°32′38.9″ W | “Cobrançosa” + ”Arbequina” | 100 | 5 | 300 | |||||
| SPAIN | PORTUGAL | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IPM | ORG | ANOVA | IPM | ORG | ANOVA | |||||||
| Mean | (SD) | Mean | (SD) | F | p-Value | Mean | (SD) | Mean | (SD) | F | p-Value | |
| POP | 142.9 | (35.1) | 176.1 | (46.8) | 12.8 | p < 0.001 | 76.8 | (19.9) | 121.2 | (66.8) | 19.6 | p < 0.05 |
| %PA | 80.2 | (12.3) | 88.8 | (7.3) | 10.1 | p < 0.01 | 58.3 | (11.2) | 71.0 | (19.9) | 12.1 | p < 0.05 |
| %PRED | 68.1 | (3.7) | 85.8 | (3.1) | 80.6 | p < 0.001 | 56.8 | (4.9) | 86.5 | (4.1) | 107.8 | p < 0.001 |
| %FA | 34.0 | (5.5) | 19.7 | (2.7) | 32.5 | p < 0.001 | 22.0 | (8.0) | 9.3 | (4.2) | 9.9 | p < 0.05 |
| %REC | 53.8 | (3.2) | 76.2 | (2.2) | 192.6 | p < 0.001 | 53.8 | (5.5) | 80.2 | (7.2) | 42.8 | p < 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Guzmán, J.A.; Herrera, J.M.; Rivera, V.; Barreiro, S.; Muñoz-Rojas, J.; García-Ruiz, R.; González-Ruiz, R. A Comparison of IPM and Organic Farming Systems Based on the Efficiency of Oophagous Predation on the Olive Moth (Prays oleae Bernard) in Olive Groves of Southern Iberia. Horticulturae 2022, 8, 977. https://doi.org/10.3390/horticulturae8100977
Gómez-Guzmán JA, Herrera JM, Rivera V, Barreiro S, Muñoz-Rojas J, García-Ruiz R, González-Ruiz R. A Comparison of IPM and Organic Farming Systems Based on the Efficiency of Oophagous Predation on the Olive Moth (Prays oleae Bernard) in Olive Groves of Southern Iberia. Horticulturae. 2022; 8(10):977. https://doi.org/10.3390/horticulturae8100977
Chicago/Turabian StyleGómez-Guzmán, José Alfonso, José M. Herrera, Vanesa Rivera, Sílvia Barreiro, José Muñoz-Rojas, Roberto García-Ruiz, and Ramón González-Ruiz. 2022. "A Comparison of IPM and Organic Farming Systems Based on the Efficiency of Oophagous Predation on the Olive Moth (Prays oleae Bernard) in Olive Groves of Southern Iberia" Horticulturae 8, no. 10: 977. https://doi.org/10.3390/horticulturae8100977
APA StyleGómez-Guzmán, J. A., Herrera, J. M., Rivera, V., Barreiro, S., Muñoz-Rojas, J., García-Ruiz, R., & González-Ruiz, R. (2022). A Comparison of IPM and Organic Farming Systems Based on the Efficiency of Oophagous Predation on the Olive Moth (Prays oleae Bernard) in Olive Groves of Southern Iberia. Horticulturae, 8(10), 977. https://doi.org/10.3390/horticulturae8100977






